Growing in phosphorus-impoverished habitats in south-western Australia: How general are phosphorus-acquisition and -allocation strategies among Proteaceae, Fabaceae and Myrtaceae species?
Abstract
Numerous phosphorus (P)-acquisition and -utilisation strategies have evolved in plants growing in severely P-impoverished environments. Although these strategies have been well characterised for certain taxa, like Proteaceae, P-poor habitats are characterised by a high biodiversity, and we know little about how species in other families cope with P scarcity. We compared the P-acquisition and leaf P-allocation strategies of Fabaceae and Myrtaceae with those of Proteaceae growing in the same severely P-impoverished habitat. Myrtaceae and Fabaceae exhibited multiple P-acquisition strategies: P-mining by carboxylates or phosphatases, P uptake facilitated by carboxylate-releasing neighbours, and dependence on the elevated soil P availability after fire. Surprisingly, not all species showed high photosynthetic P-use efficiency (PPUE). Highly P-efficient species showed positive correlations between PPUE and the proportion of metabolite P (enzyme substrates), and negative correlations between PPUE and phospholipids (cellular membranes) and nucleic acid P (mostly ribosomal RNA), while we found no correlations in less P-efficient species. Overall, we found that Myrtaceae and Fabaceae used a wider range of strategies than Proteaceae to cope with P scarcity, at both the rhizosphere and leaf level. This knowledge is pivotal to better understand the mechanisms underlying plant survival in severely nutrient-impoverished biodiverse ecosystems.
1 INTRODUCTION
Soils in many global biodiversity hotspots have an extremely low phosphorus (P) concentration ([P]) due to their parent material, age and extensive weathering without significant rejuvenation (Allsopp et al., 2014; Kooyman et al., 2017; Oliveira et al., 2015). Native plants in these regions have adaptations that allow them to survive in P-impoverished habitats (Guilherme Pereira et al., 2019; Lambers et al., 2022). Studying the diversity of strategies to cope with P scarcity in these habitats not only allows us to better understand the drivers of biodiversity but also could help us develop more P-efficient crops (Cong et al., 2020). South-western Australia is a model for P-impoverished environments, where most P-depauperate soils are dominated by Proteaceae, with a rich diversity of other families, especially Fabaceae and Myrtaceae. While we know a substantial amount about the mineral nutrition of Proteaceae in this environment, we know relatively little about the functioning of co-occurring Fabaceae and Myrtaceae.
One of the main challenges for species growing in P-poor habitats is to access soil P efficiently. To achieve this, some species, so-called P-mobilising species, release root exudates that mine soil P (Lambers et al., 2022). For example, most Proteaceae mine soil P; they typically form specialised cluster roots that comprise hundreds to thousands of determinate lateral roots or rootlets (Shane & Lambers, 2005b). These cluster roots release carboxylates that mobilise insoluble soil-bound inorganic P (Pi) and organic P (Po) into the soil solution (Shane & Lambers, 2005a). Micronutrients, such as manganese (Mn), are often co-solubilised with P. As a result, carboxylate-releasing species tend to have higher leaf Mn concentrations ([Mn]) than species that do not release carboxylates. Therefore, leaf [Mn] can be used as an easily measurable proxy for root carboxylate exudation (Lambers et al., 2015b, 2021). The Proteaceae also exude phosphatases from roots that hydrolyse Po, releasing Pi, the only form of P that can be taken up by plants (Grierson & Comerford, 2000). Other P-mobilising plants, including some Proteaceae, exhibit phosphatase-releasing P-mining strategies without releasing carboxylates (Lambers et al., 2022; Shen et al., 2024; Zhong et al., 2021).
Some non-P-mobilising species that do not release exudates to acquire P are facilitated in their P acquisition by P-mobilising neighbours (Li et al., 2014; Yu et al., 2023). When facilitated, these species tend to have higher leaf [Mn] than species that are not facilitated (Muler et al., 2014; Zhou et al., 2020). Thus, leaf [Mn] can be used as a proxy for the facilitation of P acquisition (Lambers et al., 2021; Yu et al., 2023). Another P-acquisition strategy is to depend on times when more P has become available, for example, after a fire (Lambers et al., 2022; Verboom et al., 2024). In P-impoverished fire-prone ecosystems, soil P availability is increased for a short period Postfire (Brown & Mitchell, 1986). Some plants rapidly access this P in ash and resprout within days to weeks after a fire. Following a period of rapid growth, these plants subsequently grow slowly and recycle P efficiently, and an alternative strategy to recycling P efficiently would be able to go through their lifecycle quickly and leave the seeds in the seed bank till the next fire (Bowen, 1993; Brown & Mitchell, 1986). This strategy is successful in P-impoverished ecosystems where these pyrophytic plants show considerable growth plasticity (Bowen, 1993).
Most plants, including most Myrtaceae and Fabaceae, establish symbiotic associations with mycorrhizal fungi that may increase their P-acquisition capacity in soils with moderately low P availability (Brundrett et al., 1996; Treseder & Allen, 2002). However, when soil P availability is extremely low, mycorrhizas are less effective at acquiring P than on moderately low-P soils (Gille et al., 2024; Parfitt, 1979; Treseder & Allen, 2002). Many studies have shown that mycorrhizal fungi benefit their hosts by defending them against pathogens (Marx, 1972; Sikes et al., 2009) or increasing the uptake of water, nitrogen and micronutrients (Ferrol et al., 2016; Javelle et al., 2003; Marulanda et al., 2003) rather than enhancing P-acquisition (Albornoz et al., 2021). This may be the main benefit of mycorrhizal associations in ecosystems with extremely low soil P availability. Many Myrtaceae and Fabaceae species co-occur with Proteaceae in severely P-impoverished habitats in south-western Australia, but we do not know if other P-acquisition strategies have evolved in them to gain access to sufficient P when it is extremely scarce.
High P-use efficiency is important for plants growing in P-impoverished habitats and is associated with low leaf [P]. Species from a variety of families in P-impoverished habitats, including Proteaceae, Myrtaceae and Fabaceae, have high photosynthetic P-use efficiency (PPUE), regardless of their P-acquisition strategy (Denton et al., 2007a; Guilherme Pereira et al., 2019; Liu et al., 2023). Proteaceae, Myrtaceae and Fabaceae typically also have high photosynthetic nitrogen (N)-use efficiency (PNUE) and a high leaf N:P ratio (higher than 15) in P-impoverished environments (Lambers et al., 2010; Liu et al., 2023). Their typically high leaf N:P ratio suggests that the growth of these plants is not generally limited by N.
Total leaf P comprises five main chemical fractions based on their solubility properties: Pi, three Po fractions, that is, nucleic acids, phospholipids, P-containing metabolites, and a residual fraction that is poorly defined, but may include phosphorylated proteins (Suriyagoda et al., 2023; Veneklaas et al., 2012). The Pi that is metabolically active is located in the cytoplasm, whereas the excess Pi is stored in the vacuole as a buffer that regulates cytoplasmic [Pi] (Bieleski, 1968; Lee & Ratcliffe, 1993). Small P-containing metabolites are low-molecular-weight P-esters, such as nucleotides and sugar phosphates (Veneklaas et al., 2012). In leaves, metabolites that contain P are mainly substrates for enzymes in glycolysis for respiration and the Calvin–Benson cycle for photosynthesis, and higher metabolite [P] might allow the higher activity of these pathways (Lambers, 2022). For example, some Proteaceae contain a high metabolite [P] (enzyme substrates), which would allow them to function at low protein (enzyme) concentrations and achieve rapid metabolic fluxes (Lambers, 2022; Raven, 2018). Nucleic acid P is a major cellular pool of Po, and ribosomal RNA (rRNA) comprises about 85% of the nucleic acids in a leaf (Veneklaas et al., 2012). The levels of leaf rRNA in P-efficient Proteaceae are very low (Sulpice et al., 2014), and the P fraction containing nucleic acids is small (Liu et al., 2023; Yan et al., 2019). Phospholipids are components of cellular membranes, and some Proteaceae also replace phospholipids in membranes with galactolipids and sulfolipids during leaf development, contributing to their high PPUE (Lambers et al., 2012). Like P-efficient Proteaceae, some Myrtaceae and Fabaceae also preferentially allocate P to photosynthetically active mesophyll cells (Guilherme Pereira et al., 2018) on severely P-impoverished soils, but we know very little whether this is accompanied by low investment in phospholipids and nucleic acid P and high investment in metabolite P like in Proteaceae, and if there are any correlations between the investment of P in each P fractions and PPUE.
Soils in south-western Australia have an extremely low [P], and species have a wide range of P-acquisition strategies. However, most of these strategies have only been investigated in Proteaceae (Lambers et al., 2022; Nge et al., 2020; Zhou et al., 2022) and, despite the high plant diversity of these ecosystems, we do not know how other important families, including mycorrhizal species, cope with extremely low soil P concentrations. Here, we studied the P-acquisition and -utilisation strategies of Myrtaceae and Fabaceae that co-occur with Proteaceae in the same severely P-impoverished habitats. We determined the main P-acquisition strategies of selected species, including mycorrhizal colonisation and carboxylate-based strategies. We also quantified traits related to photosynthesis and leaf P fractions, and explored possible correlations between PPUE and each P fraction to better understand leaf P-allocation patterns of co-occurring P-efficient species. We hypothesised that instead of depending only on mycorrhizal symbioses, Myrtaceae and Fabaceae that grow together with Proteaceae on severely P-impoverished soils will also have multiple P-acquisition strategies, either by mining P themselves or absorbing P mined by their neighbours to acquire P. We also hypothesised that Myrtaceae and Fabaceae show high PPUE and that PPUE will be positively correlated with leaf metabolite P and negatively correlated with nucleic acid and lipid P among these potentially P-efficient species.
2 MATERIALS AND METHODS
2.1 Study area and species selection
The fieldwork was conducted in Alison Baird Reserve, 20 km southeast of Perth in Western Australia (32°01'10"S, 115°58'46"E; Supporting Information S1: Figure S1). The reserve contains two-million-year-old dunes of Bassendean sand. Bassendean sands are highly-weathered and severely P-impoverished soils (Turner et al., 2018). The most P-impoverished soils in the reserve are on the top of a tall Bassendean sand dune (Gao et al., 2020). The climate is Mediterranean, with hot dry summers and cool, moist winters. The mean annual rainfall in the Perth region is 737 mm (1994–2022), around 80% of which falls between May and September. The mean daily temperature range is 12.9–24.8°C (1994–2022) (Australian Bureau of Meteorology, http://www.bom.gov.au/climate/data/).
The species selected for this study were the Myrtaceae Melaleuca seriata Lindl, Eremaea pauciflora (Endl.) Druce, and Hypocalymma suave Lindl., the Fabaceae Acacia pulchella R.Br., Bossiaea eriocarpa Benth., Daviesia physodes G. Don, and Jacksonia floribunda Endl., and the Proteaceae Banksia menziesii R.Br., Stirlingia latifolia (R.Br.) Steud. and Adenanthos cygnorum Diels. All plants were sampled on top of the Bassendean sand dune. We used leaf [Mn] to proxy rhizosphere carboxylate concentrations, based on comparison with a positive reference species that is known to release carboxylates to acquire P, and a negative reference species that does not release carboxylates and is not facilitated to acquire P (Zhou et al., 2020). Here, we used B. menziesii as the positive reference (Zhong et al., 2021) and S. latifolia as the negative reference (Lambers et al., 2022).
2.2 Leaf sampling
Leaf samples were collected in March 2019. Five individuals of similar height for each species were selected randomly, predominantly away from the tracks. The youngest fully-expanded undamaged mature leaves were harvested from at least three branches from different parts of each plant, pooled together and wrapped in Al foil, immediately submerged in liquid N2, transported into the lab and stored at −80°C until further use. For A. pulchella, leaf samples from another 17 individual plants were collected separately at various distances from the nearest tall Banksia attenuata to analyse leaf [Mn] to test for facilitation of P acquisition.
2.3 Leaf nutrient analyses
Frozen leaf samples were freeze-dried for 7 days (VirTis Benchtop ‘K’) and stored at room temperature in zip-lock bags with silica gel. Freeze-dried leaf material was finely ground with a grinder using zirconium beads (Geno/Grinder 2010, Spex SamplePrep). To measure total [P] and [Mn], leaf material was digested with concentrated HNO3:HClO4 (3:1) (Zarcinas et al., 1987). The digest was analysed by inductively coupled plasma optical-emission spectrometry (ICP-OES, Model 5300DV, Perkin Elmer). Leaf total [N] was measured using an N analyser (Leco FP628 Analyser).
To analyse leaf [Pi], approximately 25 mg of freeze-dried ground leaf material was extracted in 1 mL of 1% (v/v) acetic acid at 4°C by mechanical shaking (Precellys 24 Tissue Homogenizer, Bertin Instruments) with zirconium beads (2.8 mm) at 2000g for 15 s. This was repeated twice more with a 5 min break on ice between shakings. Debris was removed by centrifuging the homogenate twice at 21,000g for 15 min at 4°C. The extracts were purified using activated charcoal to remove interfering substances (Dayrell et al., 2022). A second aliquot of 50 mg freeze-dried ground leaf material was used to determine nucleic acid P, lipid P, metabolic P (Pi plus metabolite P) and residual P (Hidaka & Kitayama, 2013). Details for the P fractionations can be found in the Supporting Information. The sum of concentrations of the four P fractions was >90% of the total [P] determined by ICP-OES above. Metabolite [P] was determined by subtracting [Pi] from metabolic [P]. Phosphorus concentration in all extracted P fractions including Pi was determined by the malachite green method (Rao et al., 1997). We could not measure [Pi] in H. suave leaves because of an interfering pink colour in the extracts that we were unable to remove with activated charcoal. Thus, metabolic P is shown for this species, but not metabolite P.
2.4 Arbuscular mycorrhizal (AM) colonisation
More than 30 cm of fresh young roots were collected from five individuals of M. seriata, E. pauciflora, H. suave, D. physodes, B. menziesii, S. latifolia and A. cygnorum to check for mycorrhizal colonisation. Mycorrhizal colonisation has already been reported for A. pulchella, B. eriocarpa and J. floribunda growing in similar P-impoverished environments (Abrahão et al., 2018; Png et al., 2017), so we did not check these species in the present study. Freshly collected roots were washed with deionized (DI) water, cut into 20 mm long segments and cleared in 10% (w/v) KOH in a water bath at 90°C for 1 h. Roots of B. menziesii, S. latifolia and A. cygnorum were further cleared for 7 days at room temperature and those of the other species were cleared for a further 3–4 days. Cleared roots were washed with DI water and stained in 5% (v/v) blue ink–vinegar solution for 5 min at 90°C. Stained roots were placed in acidified water for 30 min, followed by rinsing them twice with acidified water. Stained roots were viewed under a microscope (Axioskop optical microscope, El-Einsatz; Carl Zeiss) and photographed (Axiocam digital camera, Carl Zeiss Microscopy, GmbH).
2.5 Photosynthetic rates
Fresh, youngest, mature undamaged leaves were used to measure photosynthesis and collected for leaf anatomy in March 2019 from the same individuals sampled for leaf P fraction analyses. Leaf gas exchange was measured on the youngest fully-expanded leaves (LI6400XT portable photosynthesis system, Li-Cor Inc.) on sunny days between 9:00 and 11:00 AM to avoid midday depressions (Roessler & Monson 1985). Each measurement was taken at a photosynthetic photon flux density of 1500 µmol photons m–2 s–1 and 400 µmol mol−1 CO2 with ambient humidity and temperature (around 20°C). Leaves that were used to measure photosynthesis rates were harvested immediately after the measurements and stored in ice for the area measurement. The leaf area was measured by scanning leaf segments at 200 dpi resolution (Perfection V800 scanner, Seiko Epson) and analysing the images (WinRHIZO, Regent Instrument). Leaf fresh mass was determined within a few hours of sampling before scanning. Leaf dry mass was determined after drying leaves at 70°C for 72 h. Leaf mass per area (LMA) was calculated by dividing leaf dry mass by leaf area. Leaf dry matter content (LDMC) was calculated as leaf dry mass divided by leaf fresh mass and expressed as a percentage. Photosynthetic P- and N-use efficiencies were calculated as photosynthetic rates on a DW basis divided by leaf total [P] and [N], respectively (Wright et al., 2004). Leaf anatomy was studied for all 10 species to assess the leaf thickness and leaf structures (see Supporting Information for more details).
2.6 Soil sampling
Hypocalymma suave on top of the dune was only found close to a management track. To test for a possible P-enriching influence by the track on the distribution of H. suave, soil total, organic and resin [P] on top of the dune was tested under H. suave plants and in bulk soil further away from the track. Soil samples under H. suave plants growing on the top of the dune were collected in April 2022. We used a trowel to collect soil samples from five points around and within 0.5 m of each plant to a depth of 10 cm and pooled them to make one replicate per plant. Five bulk-soil samples were randomly collected from the top of the dune in open areas lacking plants using the same method.
Phosphatase activity was only measured for M. seriata and E. pauciflora based on leaf [Mn] results as will be explained in the results section. Six replicates of rhizosheath soil were collected from M. seriata and E. pauciflora plants at the end of June 2022. Six bulk soil samples were randomly collected down to 10 cm depth at the same time from an open area without plants on top of the dune.
2.7 Soil nutrient analyses
Soil samples were dried at 40°C for 72 h and sieved (2 mm) to remove roots and debris. The ignition method was used to measure total [P] and organic [P] (Saunders & Williams, 1955), and soil total and organic P were extracted with 1 M HCl. Resin [P], which is considered readily plant-available soil Pi, was collected using anion-exchange membrane (AEM) strips by shaking the strips with 3 g soil in 30 mL DI water for 16 h (Turner & Romero, 2009). Strips were washed with DI water to remove soil particles and incubated in 10 mL 0.5 M HCl with shaking for 1 h to elute bound Pi.
2.8 Phosphatase activity
Phosphatase activity was measured in the rhizosheath of M. seriata and E. pauciflora. We used p-nitrophenol as the substrate to measure acid phosphatase activity in rhizosheath and bulk soils (Tabatabai, 1994). To each 1 g of soil sample, 0.2 mL of toluene and 4 mL of modified universal buffer (pH 6.5) were added. For each sample, 1 mL of 0.05 M p-nitrophenyl phosphate solution was added and mixed using a vortex mixer. Samples were incubated at 37°C for 1 h before adding 1 mL of 0.05 M CaCl2 and 4 mL of 0.5 M NaOH. Samples were mixed, and soil was removed by filtering through a P-free Whatman No. 2 filter paper. The absorbance of the filtered solution was measured spectrophotometrically at 420 nm.
2.9 Statistics
All traits were analysed by analysis of variance (ANOVA) using the one-way non-block experiment ANOVA model in SAS (SAS 8.1). Significant differences among means were determined by the least significant difference (LSD) at p ≤ 0.05. All bar graphs were produced using SigmaPlot 10.0. Linear regression analysis was conducted on the R platform (version 4.3.1) using the package stats followed by post hoc tests (package multcomp).
We characterised the phenotypic space of these 10 species (with species mean traits) using principal component analysis (PCA). Two PCAs were run: one with P fractions expressed as P concentrations (g P kg−1), and the second with P fractions expressed as proportions of total P (%). PCAs were run with the package FactoMineR on log-transformed data (Lê et al., 2008) in R (R Core Team, 2021).
Linear mixed-effects models were tested using the packages nlme and car to explore relationships between PPUE and each P faction (expressed as proportion of total P). In each model, PPUE was the response variable. Species was treated as a random factor. In each model, the considered P fraction (proportion of phospholipid, nucleic acid P and metabolite P) was the first fixed-effect term, family (Fabaceae, Myrtaceae and Proteaceae) was the second fixed-effect term, followed by the interaction between P fraction and family (Pfraction: family) and the interactions between P fraction and species regarding their PPUE classes (i.e., less-P-efficient species, P-efficient species and A. cygnorum; Pfraction: species in the model). The model can be read as PPUE ~ Pfraction + family + Pfraction: PPUE class + Pfraction: family.
3 RESULTS
3.1 Traits related to P-acquisition strategies
We found that the roots of all three Myrtaceae were colonised by AM fungi (Table 1 and Supporting Information S1: Figure S2). They all had weakly developed arbuscules. In contrast, we did not find AM colonisation in roots of any Proteaceae, which are known to be non-mycorrhizal, and also not in D. physodes (Fabaceae) (Table 1 and Supporting Information S1: Figure S2). AM colonisation has previously been found in the roots of Fabaceae, including the studied A. pulchella, B. eriocarpa and J. floribunda (Table 1).
| Family | Species | Arbuscular mycorrhizal colonisation | Cluster roots | P-acquisition strategies | References |
|---|---|---|---|---|---|
| Myrtaceae | Melaleuca seriata | Yes | No | Phosphatases | This study (Figure 2) |
| Possible facilitation involving mycorrhizal symbioses or postfire phosphophile | |||||
| Eremaea pauciflora | Yes | No | Phosphatases | This study (Figure 2) | |
| Possible facilitation involving mycorrhizal symbioses or postfire phosphophile | |||||
| Hypocalymma suave | Yes | No | Growing only on soil with higher resin [P] | This study (Figure 3) | |
| Fabaceae | Acacia pulchella | Yes | No | Seedlings: postfire phosphophile | This study (Figure 1b), Monk et al. (1981), Png et al. (2017) |
| Mature plants: phosphatases; possible facilitation by P-mobilising neighbours | |||||
| Bossiaea eriocarpa | Yes | No | Facilitation by P-mobilising neighbours | Abrahão et al. (2018) | |
| Daviesia physodes | No | Yes | P-mining strategy based on carboxylate release by cluster roots | This study (Figure 1a), Nge et al. (2020) |
|
| Jacksonia floribunda | Yes | No | Phosphatases | Png et al. (2017) | |
| Possible facilitation involving mycorrhizal symbioses or postfire phosphophile | |||||
| Proteaceae | Banksia menziesii | No | Yes | P-mining strategy based on carboxylate release by cluster roots | This study (Figure 1a), Denton et al. (2007b) |
| Stirlingia latifolia | No | Seedlings only | Postfire phosphophile in mature plants | Bowen (1993), Lambers et al. (2022) |
|
| Adenanthos cygnorum | No | Tiny and short-lived | Phosphatases; facilitation by P-mobilising neighbours | Shen et al. (2024) |
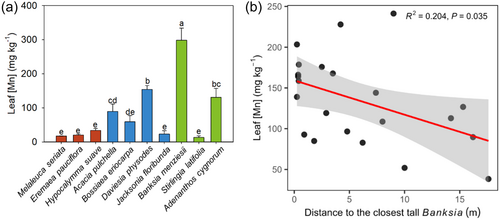
There was a wide variation in leaf [Mn] among the species tested (Figure 1a). Banksia menziesii (Proteaceae) had the highest leaf [Mn] at 299 ± 36 mg Mn kg−1. All three Myrtaceae and J. floribunda and S. latifolia had similarly low leaf [Mn] ranging from 13 to 33 mg Mn kg−1. Acacia pulchella, B. eriocarpa and A. cygnorum showed intermediate leaf [Mn] with a relatively large variation among individuals. A linear correlation was found between leaf [Mn] of A. pulchella and distance to the nearest tall B. attenuata (>1.5 m, Figure 1b).
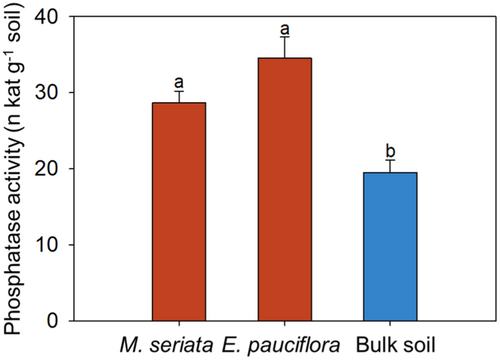
Based on their low leaf [Mn], the potential of Myrtaceae M. seriata and E. pauciflora to exude phosphatases into rhizosheath was explored to investigate their potential P-acquisition strategies. Phosphatase activity in the rhizosheath of both species was about 50% greater than that in the bulk soil (Figure 2).
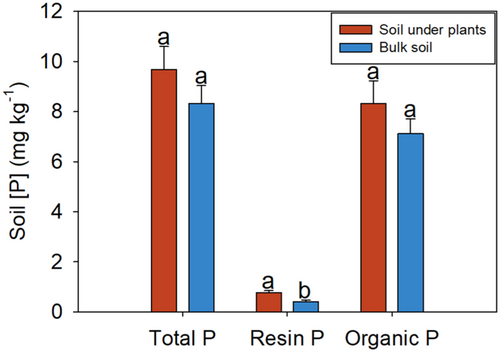
Hypocalymma suave was common at the bottom of the dune, but became scarcer towards the top of the dune. On top of the dune, only six H. suave plants were found, and they all grew near a management track, where the soil is disturbed by human activity. Soil resin [P] under H. suave on top of the dune and near the track was significantly higher than that of the bulk soil on top of the dune about 10 m from the track (Figure 3). Soil total [P] and organic [P] were the same at the two sites.
3.2 Leaf traits
Total leaf [P] of A. pulchella was nearly twice that of any of the other species examined, whereas A. cygnorum had the lowest leaf [P] (Figure 4a). The other species all had a similar leaf [P]. Leaf [N] of all Fabaceae was significantly higher than that of all species from the other two families (Figure 4b). Like leaf [P], the leaf [N] for A. pulchella was at least twice that of any other species examined. Total leaf [N] among Myrtaceae and Proteaceae were indistinguishable. All Fabaceae had a significantly higher leaf N:P ratio than that of all species from the other two families but were indistinguishable among species of Fabaceae (Figure 4c). The N:P ratio of all Myrtaceae and Proteaceae was indistinguishable from each other.
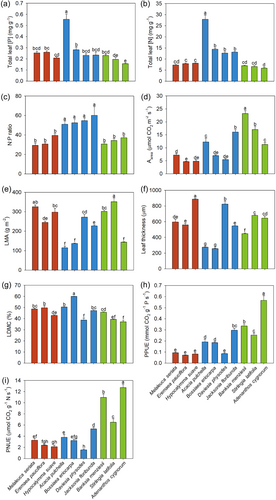
Photosynthetic rates on a leaf area basis (Aarea) varied among species (4.8–23.2 µmol CO2 m−2 s−1; Figure 4d). The Aarea of Proteaceae were significantly faster than those of Myrtaceae, while the rates in Fabaceae overlapped with the rates in the other two families. Myrtaceae leaves had a relatively high and consistent LMA compared with Fabaceae and Proteaceae, where there was more variation in LMA (Figure 4e). All species except the Fabaceae A. pulchella and B. eriocarpa had thick leaves, exceeding 400 µm (Figure 4f). The LDMC (Figure 4g) showed a different pattern from that of the LMA. B. eriocarpa had the highest LDMC; however, in contrast, it had the lowest LMA. Daviesia physodes and A. cygnorum had the lowest LDMC, but their LMA was among the highest. All species had lignified sclerenchyma in their leaves, except for A. pulchella and B. eriocarpa (Supporting Information S1: Figure S3), both of which also had much thinner leaves than the other species (Figure 4f).
A. cygnorum had the greatest PPUE among all species tested (Figure 4h), while the PPUE of the other Proteaceae was significantly higher than that of all the other species except J. floribunda. The PPUE of all Myrtaceae and D. physodes (Fabaceae) were lowest among the species tested. Among the Fabaceae, PPUE of J. floribunda was about 70% higher than that of A. pulchella and B. eriocarpa. Similar to PPUE, the PNUE of the three Proteaceae was significantly higher than that of all the Myrtaceae and Fabaceae examined (Figure 4i).
3.3 Leaf P fractions
The allocation of P to each leaf P-containing chemical fraction was similar among the Myrtaceae but was variable among the Fabaceae (Figure 5a). The concentration of P in each fraction in A. pulchella was significantly higher than that in all other species. The exceptions were that leaf metabolite [P] in E. pauciflora and B. menziesii was similar to that in A. pulchella. The nucleic acid [P] of A. pulchella and B. eriocarpa was significantly greater than that of the three Proteaceae, while nucleic acid [P] in the other two Fabaceae, D. physodes and J. floribunda, was indistinguishable from that of the Proteaceae (Figure 5a). The lipid [P] within leaves of the Myrtaceae and Fabaceae did not differ significantly from each other, except for A. pulchella, in which lipid [P] was about two-fold higher than that of the other three Fabaceae (Figure 5a). Metabolite [P] among all species had a generally similar trend as lipid [P], except that metabolite [P] in A. pulchella was similar to that of E. pauciflora and B. menziesii. Overall, Myrtaceae species tended to accumulate more P in nucleic acids than Proteaceae did, and the high concentrations of each P fraction in Fabaceae species were reflected in their high total [P]. Thus, we analysed leaf P allocation in P-containing chemical fractions and also determined proportions to compare the differences among families.
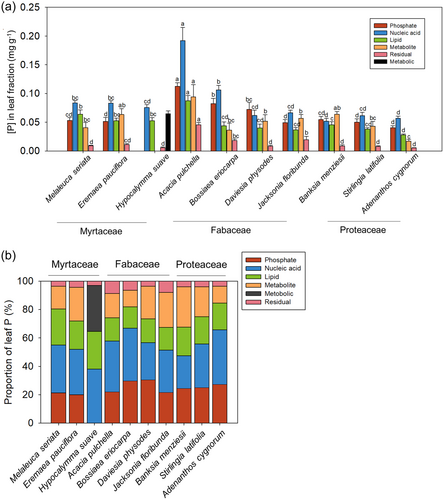
The relative proportion of P allocated to the various P fractions generally differed among species (Figure 5b). However, as for the [P] in each fraction, the relative proportion of P in each fraction among the Myrtaceae was similar, except for the lower proportion of P in lipids in E. pauciflora. The proportion of P present as Pi in leaves was indistinguishable among the species in this study. The proportion of P in nucleic acids in both Fabaceae and Proteaceae varied substantially. In Fabaceae, there was a greater proportion of P in nucleic acids in A. pulchella and B. eriocarpa than in D. physodes and J. floribunda, but it was similar to that in the Proteaceae S. latifolia and A. cygnorum. In Fabaceae, the proportion of P in phospholipids was similar to that in the Proteaceae, but generally lower than that in the Myrtaceae, except for D. physodes. The proportion of P in the metabolite pool was greatest in the Fabaceae A. pulchella, B. eriocarpa and J. floribunda. The other seven species had a similarly low proportion of P in the metabolite pool.
3.4 Main trends of variation and correlations among traits
The first two principal components (PCs) describing the phenotypic space of the 10 species explained 58% of the total variance (Figure 6a and Supporting Information S1: Table S1). The PC1 described a leaf economics axis, separating species with high foliar nutrient concentrations (leaf [N], [P]) and LDMC from species with high LMA and leaf thickness. Interestingly, those species with high foliar nutrient concentrations allocated less P to the phospholipid fraction than other species. The PC2 described a continuum that separated species with high Aarea, PPUE, PNUE and leaf [Mn] from other species. Those species with high Aarea, PPUE, PNUE and leaf [Mn] tended to allocate less P to both the phospholipid and nucleic acid P fractions than other species, although lipid P was slightly better represented by PC1, and nucleic acid P slightly better by PC3 and opposed to metabolite P. Inorganic P did not contribute strongly to either PC1 or PC2 and was independent of the other traits (PC4) (Supporting Information S1: Table S1). Finally, residual P was associated with the PC1, with more acquisitive species. Species within a family clustered in the same zone in the PCA, except for the Fabaceae D. physodes, which clustered in the same zone as Myrtaceae and had a similarly low PPUE as the Myrtaceae (Figure 6a). Here, we define species with PPUE higher than 0.1 mmol CO2 g−1 P s−1 (global plant mean PPUE under field conditions; Wright et al., 2004) as P-efficient species which was the case for six species (A. pulchella, B. eriocarpa, D. physodes, A. cygnorum, B. menziesii and S. latifolia), and the other four species as less P-efficient species (D. physodes, E. pauciflora, H. suave and M. seriata). The P-efficient species clustered in the same zone in the PCA that separated them from less-P-efficient species (Figure 6a). Another PCA was also performed for leaf [P] fractions; however, the leaf [P] in each fraction clustered in the same direction as total [P], indicating only a low level of independence of [P] within a fraction from the total leaf [P] (Supporting Information S1: Figure S4 and Table S2).
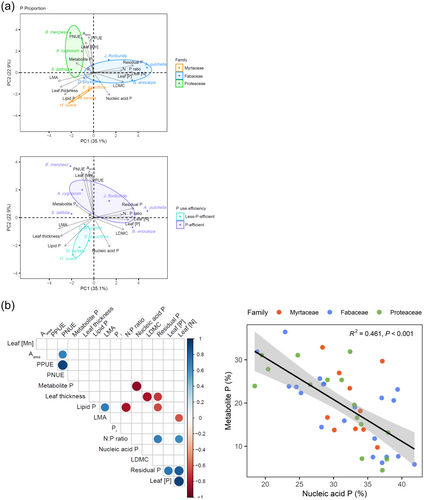
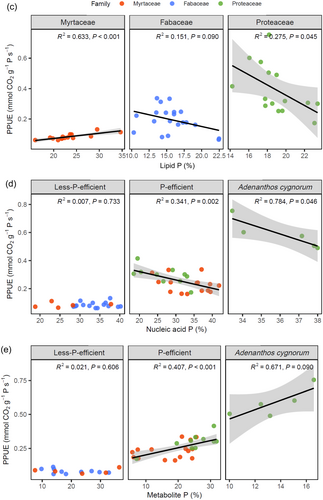
There was no correlation between mean PPUE and mean of any of the leaf P fractions when all species were tested as a group (Figure 6b). Nucleic acid P was negatively correlated with metabolite P among the 10 species (Figure 6b). Lipid P was negatively correlated with the N:P ratio and positively correlated with LMA (Figure 6b). Based on the results of the PCA (Figure 6a), we further explored if there were any correlations within families or P-efficient species between PPUE and leaf P fractions using linear mixed-effect models. The PCA using P proportions showed that species grouped by families and P-efficiency classes. However, the PPUE of A. cygnorum was significantly higher than any other species. When all the P-efficient species were placed in one group, there were no correlations between PPUE and P fractions. However, correlations were apparent when A. cygnorum was excluded from this group. From this analysis, we separated the species into three PPUE classes: less-P-efficient species, P-efficient species and A. cygnorum. Thus, we considered the effects of P fractions on PPUE with the interactions of family and P efficiency class (Table 2). Phospholipid P was negatively correlated with PPUE, and family and P efficiency class had effects on that negative correlation. When compared by families, phospholipid P was positively correlated with PPUE in Myrtaceae, while it was negatively correlated in Proteaceae (Figure 6c). Among the Fabaceae, phospholipid was weakly correlated with PPUE (p = 0.09). Nucleic acid P had a highly significant effect on PPUE, and this effect was significantly different among families and species (Table 2). Nucleic acid P was negatively correlated with PPUE among the highly P-efficient class of species when A. cygnorum was excluded because of its extremely high PPUE (Figure 6d), although nucleic acid P was also negatively correlated with PPUE in A. cygnorum. Thus, nucleic acid P was negatively correlated with PPUE among high-PPUE species. Metabolite P had similar effects on PPUE to those of nucleic acid P; however, the effect of metabolite P on PPUE was only marginally different among families (Table 2). In contrast to nucleic acid P, metabolite P was positively correlated with PPUE among P-efficient species (Figure 6e). However, there was no correlation between nucleic acid P or metabolite P and PPUE among less-P-efficient species.
| Source of variation | χ² | p |
|---|---|---|
| Lipid P | 8.94 | 0.025 |
| Lipid P: Class | 10.55 | <0.001 |
| Lipid P: Family | 8.14 | <0.001 |
| Nucleic acid P | 19.04 | <0.001 |
| Nucleic acid P: Class | 26.11 | <0.001 |
| Nucleic acid P: Family | 7.96 | 0.019 |
| Metabolite P | 0.88 | <0.001 |
| Metabolite P: Class | 18.47 | <0.001 |
| Metabolite P: Family | 2.55 | 0.078 |
- Note: We treated species as a random factor.
4 DISCUSSION
We conclude that species of Myrtaceae and Fabaceae exhibited a variety of P-acquisition strategies in an extremely P-impoverished habitat. They either mined P by themselves as suggested by their high leaf [Mn] (Lambers et al., 2015b; Zhou et al., 2020) and/or rhizosheath phosphatase activity, or acquired P mined by their neighbours, in addition to depending on mycorrhizal symbioses. In contrast to our hypotheses, not all species growing in an extremely P-impoverished environment had a high PPUE. In particular, all three species of Myrtaceae and D. physodes (Fabaceae) had a PPUE lower than 0.1 mmol CO2 g−1 P s−1. All P-efficient species in this study had negative correlations between the lipid and nucleic acid P proportions and PPUE, and positive correlations between the metabolite P proportion and PPUE, whereas no correlations were found among less-P-efficient species. For the first time, this study, demonstrates correlations between investment of P in leaf P fractions and PPUE class, and that differences among species, such as families/P efficiency class, should be considered when comparing correlations between PPUE and individual P factions. Plants from each family allocated P differently, and variation in the allocation of leaf P to chemical fractions was greater among families growing in the same habitat than within each family.
4.1 Phosphorus-acquisition strategies
On highly weathered and extremely P-impoverished Bassendean sand, we found multiple P-acquisition strategies that allowed Myrtaceae and Fabaceae to acquire P (Table 1), including P-mining, facilitation of P acquisition and postfire P-acquisition strategies, rather than solely depending on a mycorrhizal scavenging strategy. AM fungi may enhance plant P acquisition in Fabaceae and Myrtaceae because of the abundant colonisation of the roots of these family members (Brundrett et al., 1996; Png et al., 2017). However, while mycorrhizas are effective in enhancing plant P-acquisition on moderately P-impoverished soils (5–13 mg P kg−1 plant-available P), they are likely less effective and tend to be suppressed on severely P-impoverished soils (<5 mg P kg−1) in different ecosystems (Lambers et al., 2022; Lekberg et al., 2024; Treseder & Allen, 2002). In Alison Baird Reserve where this research was carried out, the readily-available soil P concentration was less than 5 mg P kg−1; therefore, mycorrhizas are unlikely to support P acquisition there, except where P was made available by P-mining neighbours.
Mycorrhizal fungi provide benefits beyond P uptake for the host plants (Albornoz et al., 2021). Water uptake of Acacia caven (Mol.) is enhanced by mycorrhizal fungi (Poca et al., 2019). Mycorrhizal fungi also protect plants against metal toxicity (Shi et al., 2019). In plants growing in extremely nutrient-impoverished habitats, they are very likely to provide physical barriers in roots against soil pathogens (Albornoz et al., 2017). In this study, the Fabaceae A. pulchella and B. eriocarpa showed relatively high leaf [P] compared with the other species. Even though the relatively high leaf [P] might suggest that the AM strategy might be successful for them, the mycorrhizal colonisation rates of these two species are low (Abrahão et al., 2018; Png et al., 2017). Abrahão et al. (2018) compared the mycorrhizal colonisation rates of B. eriocarpa grown in a glasshouse pot experiment in soil with a range of P supplies and in the field along a soil chronosequence in south-western Australia. The chronosequence comprises different soil developmental stages from young high-P Quindalup sand to very old severely P-impoverished Bassendean sand (the same dune sand as in this study), as previously described by Turner et al. (2018). They found that mycorrhizal colonisation rates did not depend on the plant-available soil [P] which suggests that the mycorrhizal fungi do not enhance P acquisition in this species. Instead, B. eriocarpa exhibits other P-acquisition strategies (Abrahão et al., 2018; Png et al., 2017), which are discussed below.
4.2 Phosphorus mining based on carboxylate or phosphatase release
Banksia menziesii and D. physodes most likely released carboxylates for P acquisition in this study, as evidenced by their high leaf [Mn]. Banksia menziesii is a typical Proteaceae that makes functional compound cluster roots that strongly exude carboxylates (Denton et al., 2007b) and it is, therefore, not surprising that it exhibited a higher leaf [Mn] than any other species in the present study. Non-mycorrhizal D. physodes is a Fabaceae that also makes cluster roots (Nge et al., 2020). Its high leaf [Mn] suggests that it also released carboxylates to acquire P.
Many species in the present study that probably do not release carboxylates, based on their leaf [Mn], released phosphatases, which hydrolyse less-tightly-bound soil Po to release Pi in the rhizosheath (Tarafdar & Claassen, 1988). For example, the atypical Proteaceae A. cygnorum, which produces poorly functional cluster roots, releases phosphatases to acquire P at the base of the dune studied here, where soil P, including Po, is higher than that at the top of the dune (Shen et al., 2024). Legumes tend to have a greater investment in root phosphatases than non-legumes in P-impoverished soils (Houlton et al., 2008; Olde Venterink, 2011; Png et al., 2017). Growing in soils with very low P availability, N-fixing legumes, including A. pulchella and J. floribunda, have high root phosphatase activity but are not abundantly colonised by mycorrhizal fungi (Png et al., 2017). Melaleuca seriata and E. pauciflora (Myrtaceae) had low leaf [Mn], but the significantly higher phosphatase activity in the rhizosheath soil than in the bulk soil indicated that they also release phosphatases. We acknowledge that rhizosphere microorganisms may also contribute to the higher rhizosheath phosphatase activity, but the relative contribution of plants and microorganisms to the soil phosphatase activity would be extremely difficult to determine. The higher rhizosheath phosphatase activity would provide these plants with the potential to hydrolyse less-tightly-bound soil Po to release Pi without depending on large amounts of carboxylates, an acquisition strategy similar to that of Xylomelum occidentale (Zhong et al., 2021) and A. cygnorum (Shen et al., 2024).
4.3 Acquiring P mined by neighbouring plants
Some species cannot mine P by themselves; instead, they acquire P mined by neighbouring plants. For example, P acquisition in Cleistogenes squarrosa (Poaceae) and Bromus inermis (Poaceae) in steppe vegetation in Inner Mongolia is facilitated by neighbouring P-mobilising species (Yu et al., 2020). In agroecosystems, P-mobilising Cicer arietinum (chickpea, Fabaceae) facilitates the P uptake of non-P-mobilising wheat and maize when intercropped (Li et al., 2003, 2004). The A. cygnorum on top of the dune in Alison Baird Reserve shows moderately high leaf [Mn]. Even though A. cygnorum is a Proteaceae, it only produces small short-lived cluster roots. Instead, its P acquisition on top of the dune is facilitated by neighbouring large Banksia spp. (Shen et al.,2024). This finding suggests that using Po alone on top of the dune is likely insufficient for the phosphatase-based P-acquisition strategy of A. cygnorum to support its growth there. The large variation among individuals in A. pulchella, B. eriocarpa and A. cygnorum in the present study also indicates facilitation by a carboxylate-exuding neighbour (Lambers et al., 2021). Since facilitation of P acquisition has not been reported for A. pulchella, we investigated this species more deeply. Similar to A. cygnorum, the linear correlation between leaf [Mn] of A. pulchella and distance to the closest tall B. attenuata suggests that P acquisition in this species was facilitated by B. attenuata (Figure 1). Both A. cygnorum and A. pulchella (as discussed below), therefore, have multiple P-acquisition strategies in severely P-impoverished soils. The B. eriocarpa (Fabaceae) provides another example in this system of P acquisition by a non-cluster-root-forming plant facilitated by a cluster-root-forming neighbour (Abrahão et al., 2018; Staudinger et al., 2024).
Soil Mn availability is quite homogeneous at the study site, namely about 50 ± 3 mg Mn kg−1 (Shen et al., 2024). Based on their low leaf [Mn], suggesting the absence of a carboxylate-based P-acquisition strategy, other P-acquisition strategies may have evolved in M. seriata, E. pauciflora and J. floribunda. We envisage three potential P-acquisition strategies for these species. First, these three species may be facilitated by surrounding P-mobilising plants through AM associations. Although AM fungi are poorly effective at mining P directly from these extremely P-impoverished soils, mycorrhizal symbionts of these three species might transfer P from the rhizosphere of P-mobilising neighbours to the roots of these three species. The mycorrhizal fungi would intercept carboxylate-mobilised Mn (Bethlenfalvay & Franson, 1989; Kothari et al., 1991) but transfer P to the host plant (Smith & Read, 2008) to facilitate plant P uptake and growth. This scenario would explain the low leaf [Mn] in these three species. Second, plant growth rates are negatively correlated with LMA (Poorter et al., 2009), and LMA of facilitated A. cygnorum was lower than that of M. seriata, E. pauciflora and J. floribunda, suggesting that it grows faster than these other species. Unlike A. cygnorum, which is killed by fire (Lamont & Witkowski, 2020), these latter three species resprout after fire (Hobbs & Atkins, 1990). Therefore, another possible P-acquisition strategy is that the P that can be acquired after fire is sufficient for the growth of the three presumably slower-growing species, but not for the presumably faster-growing A. cygnorum. Like S. latifolia (Bowen & Pate, 2004), these three slower-growing species possibly recycle the P acquired after a fire within the plant until the next fire. Third, compared with individual A. cygnorum plants, which grow to about 2–3 m in height, M. seriata, E. pauciflora and J. floribunda individuals are much smaller at about 1 m at the study site, so they would need less P to grow to maturity than A. cygnorum does. Thus, even though soil Po was not enough at the study site to satisfy the needs of A. cygnorum, it might be sufficient for M. seriata, E. pauciflora and J. floribunda. This would eliminate their need for other P-acquisition strategies to survive on top of the Bassendean sand dune. Better understanding of the mechanisms of facilitation of P-acquisition in natural ecosystems may provide more possibilities for intercropping P-mobilising crops with non-P-mobilising species.
4.4 Other P-acquisition strategies
Stirlingia latifolia exhibits a postfire P-acquisition strategy (Bowen & Pate, 2004) and stores P in its roots (Lambers et al., 2022). This species rapidly accumulates biomass after a fire and then stops increasing in size (Bowen & Pate, 2004). In contrast to most other Proteaceae, S. latifolia only produces cluster roots at its seedling stage (Lambers et al., 2021). Adult plants make so-called ‘bubble roots’, which likely contribute to its plasticity by providing storage space for starch (Bowen, 1993) as well as P, albeit at a similar [P] as in non-bubble roots (Lambers et al., 2022). Acacia pulchella also expresses a postfire P-acquisition strategy and germinates massively after a fire, so its seedlings can acquire enough P within a short time to support their rapid growth (Monk et al., 1981). Mature plants may be able to supplement the P acquired from ash immediately after a fire as seedlings by releasing phosphatases to hydrolyse Po (Png et al., 2017), or they may be facilitated by neighbouring P-mobilising species (Figure 5b). Postfire P-acquisition strategies only occur in extremely P-depleted habitats, such as that of Itasina filifolia (Apiaceae) and some legumes in the fynbos of the cape region of South Africa. These species resprout fast after fire but die within 3–5 years without other P-acquisition strategies (Power et al., 2010; Rutherford et al., 2011).
Hypocalymma suave was only found on soil with higher plant-available soil [P] than the surrounding soil. On top of the dune, we found only a few plants of this species, and they always grew next to a track with higher soil resin [P] than the bulk soil further away from the track. Most individuals of this species grew at the bottom of the dune where soil total and resin [P] are significantly higher than those on top of the dune (Shen et al., 2024). Thus, the P-acquisition strategy of this plant is likely through mycorrhizal associations which restricts its distribution to areas where soil [P] is high enough for mycorrhizal associations to be effective (Treseder & Allen, 2002).
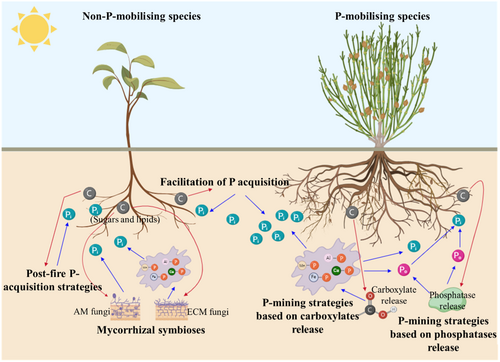
To better understand the mechanisms of how plants acquire P from P-impoverished environments, we summarised the costs and benefits of getting different soil P compounds with the studied P-acquisition strategies (Figure 7). Plants were divided into P-mobilising species, which release exudates that mine P, and non-P-mobilising species that do not release exudates and are unable to acquire tightly-bound P or Po from the environment. All plants can acquire soluble Pi in the soil solution without needing root exudates, and they only require carbon to provide energy to take up Pi (Raven, 2018). However, to survive in extremely P-impoverished soils, plants incur additional costs associated with the various soil P compounds they access (Figure 7). The P-mobilising plants exhibited P-mining strategies based on carboxylate release to access insoluble Pi and mobilise Po from the soil; in addition, some P-mobilising plants released phosphatases, thus accessing Po through hydrolysis to Pi (B. menziesii, D. physodes). However, some P-mobilising species that were not effective at releasing carboxylates, but instead had P-mining strategies based only on phosphatase release incurred costs associated with the release of phosphatases that hydrolyse less tightly-bound soluble Po (A. cygnorum, E. pauciflora, M. seriata, J. floribunda). For species that cannot acquire sufficient P by themselves, mycorrhizal plant species may transfer sugars and lipids to support mycorrhizas that access soluble Pi, insoluble Pi and Po (Smith et al., 2015). AM fungi only access soluble Pi, while ectomycorrhizal (ECM) fungi can also release low-molecular-weight carboxylates such as oxalate (Arvieu et al., 2003) and citrate (Tuason & Arocena, 2009). They also exhibit phosphatases in their hyphosphere that access Po (Dighton, 1983; Smith et al., 2015), although these enzymes may actually be released by microbes associated with their hyphae (Yuan et al., 2024). A. cygnorum, A. pulchella and B. eriocarpa were unable to acquire sufficient soluble Pi themselves, but depended on their P-mobilising neighbours and, therefore, used facilitation for their P acquisition strategy. In addition, some species can only access soluble Pi from the soil. These species either only survive in soils with sufficient Pi that can support their growth or show a pyrophytic P-acquisition strategy and then succumb a few years after a fire if they express no other P-acquisition strategies (H. suave, S. latifolia) (Verboom et al., 2024).
4.5 Leaf economic traits
Species that used P efficiently for their photosynthesis also used N efficiently (Figures 4h,I and 6b), as these nutrients are tightly linked via rRNA (Veneklaas et al., 2012). Proteaceae in south-western Australia also use N efficiently, which is reflected by their PNUE of around 6.9 µmol CO2 g−1 N s−1 (Wright et al., 2004), and a high PNUE was also found for the Proteaceae in this study. The N-efficient plants can function at low amounts of protein, and thus, a low amount of P is required for the low amount of rRNA needed to produce that protein (Sulpice et al., 2014). The global plant mean PPUE under field conditions is 0.10 mmol CO2 g−1 P s−1 (Wright et al., 2004), whereas the average PPUE for Proteaceae in south-western Australia is 0.2 mmol CO2 g−1 P s−1 (Guilherme Pereira et al., 2019). The Proteaceae also showed a high PPUE in this study; however, three Myrtaceae species and D. physodes (Fabaceae) had a PPUE lower than 0.10 mmol CO2 g−1 P s−1.
Interestingly, leaf thickness was negatively correlated with the LDMC (Figure 6b). A greater LDMC indicates greater investment in non-productive cell types such as sclerenchyma (Witkowski & Lamont, 1991), and LMA is often positively correlated with LDMC (Vile et al., 2005). However, the species in this study, except A. pulchella and B. eriocarpa, have scleromorphic leaves with different amounts of sclerenchyma (Supporting Information S1: Figure S1), which might explain why LDMC was negatively correlated with leaf thickness but not with LMA. The sclerenchyma had many air spaces (Supporting Information S1: Figure S3), and thus, when the space between top and bottom mesophyll cells in the same area varied, the thickness differed but the leaf mass may not differ. Leaf mass area depends on leaf thickness, while LDMC is independent of leaf thickness, so LMA and LDMC do not need to correlate.
4.6 Leaf P allocation patterns in plants growing in a severely P-impoverished habitat
Allocation of P to leaf P fractions varies among terrestrial vascular plants, with variability among families > species in a family > within regions > plant life forms (Suriyagoda et al., 2023; Tsujii et al., 2023, 2024). Although all species co-occurred at the same site, there was no convergence in P-allocation patterns among the 10 species. When further explored, species were grouped as families in the PCA, and also grouped as P-efficient and less-P-efficient species that showed different correlations (Figure 6a and Supporting Information S1: Table S1). We found that within the leaf P fractions, both P concentration and proportion of leaf P in a fraction varied more among families than among species within a family. However, Pi expressed as a proportion of total P was independent of all other traits in the PCA, reflecting the severely P-impoverished soils in Alison Baird Reserve (Shen et al., 2024). Leaf [Pi] depends on soil P availability (Veneklaas et al., 2012) and all species were collected in the same habitat. Moreover, the proportion of leaf P in Pi for Fabaceae and Myrtaceae was low and indistinguishable from that of Proteaceae. In severely P-impoverished environments, most leaf P is in the organic form (Veneklaas et al., 2012) and there is presumably very little Pi in vacuoles. Similar results were also found among 35 Australian woody species, showing that plants growing in low-P sites had a lower [Pi] than plants growing in soils with more available P (Tsujii et al., 2024). Fabaceae, Myrtaceae and Proteaceae in extremely low-P habitats allocate their P preferentially to photosynthetically active mesophyll cells instead of photosynthetically inactive epidermal cells (Guilherme Pereira et al., 2018; Hayes et al., 2018). Thus, preferential P allocation to mesophyll cells reflects a species-dependent trait that has been selected for by soil conditions and contributes to the high PPUE of species from severely P-impoverished habitats.
The Proteaceae that have been examined replace most of their phospholipids with galactolipids and sulfolipids during leaf development as an adaptation to their P-impoverished habitat (Lambers et al., 2012). In this study, plants that showed higher PPUE also tended to allocate less P to phospholipids (Figure 6c) than species with lower PPUE, thus saving P. Similar results were found in tropical tree species on Mount Kinabalu, Borneo and in a study about rice varieties in Japan, showing that low investment in phospholipids is associated with high PPUE (Hayes et al., 2022; Hidaka & Kitayama, 2013). Like most Proteaceae, Myrtaceae also had a low leaf total [P], but their relatively high lipid [P] indicates that they do not replace their phospholipids with other lipids to the same extent as Proteaceae do, and thus did not use P as efficiently as more P-efficient species. In a wide range of species, the endoplasmic reticulum accounts for over 60% of the lipid-P mass (Lagace & Ridgway, 2013), and a reduced allocation of P to lipids might indicate a reduced amount of endoplasmic reticulum, which is needed to support protein synthesis by bound ribosomes. Protein concentrations can be approximated by total [N]. As [N] was positively correlated with [P], more lipid P in Myrtaceae could explain the negative correlation between lipid P and leaf N:P ratio (Figure 6b).
Low investment in nucleic acid P and high investment in metabolite P was associated with a high PPUE in extremely P-impoverished environments (Figure 6). However, unlike findings from previous studies (Lambers, 2022), nucleic acid and metabolite P only correlated with PPUE in P-efficient species including A. cygnorum, but not in less-P-efficient species. As found in previous studies (Lambers, 2022), our finding of a negative correlation between nucleic acid P and metabolite P suggests a trade-off between these two fractions. The operation of metabolism at low rRNA abundance, which is the main component of the nucleic acid P fraction, is a P-saving strategy (Sulpice et al., 2014). A low metabolite [P] would require higher enzyme concentrations to achieve the same rate of metabolism (Lambers et al., 2015a). Instead, lower enzyme levels would require less ribosomes and rRNA, and thus lower leaf [P] and [N], so it would contribute to the efficiency of both P and N use (Veneklaas et al., 2012). Also, being predominantly substrates for enzymes in glycolysis and the Calvin–Benson cycle (Lambers, 2022; Nelson & Millar, 2015), decreasing the investment in metabolite P might decrease the activity of these pathways. Thus, P-efficient species tend to allocate less P to nucleic acids, thus reducing enzyme levels and showing a trade-off of the metabolite P (enzyme substrate) and nucleic acid P (enzyme) concentrations in achieving rapid metabolic fluxes. We found no significant correlation between nucleic acid P proportion and PPUE among low-PPUE species; that might be explained by their relatively high nucleic acid P proportion. The range for PPUE was too low to vary, and these less-P-efficient species tended to invest a large amount of nucleic acid P with low PPUE that reached a plateau and left no space for them to show a negative correlation. Similar reasons might also explain the relatively low metabolite P proportion for non-P-efficient species, which invested a small amount of P in metabolite P with similar low PPUE that reached a plateau. Previous studies used to focus on the leaf P-allocation patterns of P-efficient species, and the correlations between each fraction with PPUE of plants growing in severely P-impoverished environments in or outside Australia (Liu et al., 2023; Suriyagoda et al., 2023; Tsujii et al., 2024; Yan et al., 2019). Similar to findings from previous studies, we found the correlations between nucleic acid/metabolite P and PPUE in P-efficient species. However, unlike previous studies, we further discovered that there was no correlation of nucleic acid/metabolite P concentrations with PPUE when compared among the less-P-efficient species.
This study was conducted in south-western Australia in extremely P-impoverished habitats. It is worthwhile to do similar work in other P-impoverished regions across the world to better understand plant P-utilisation strategies. In a tropical lowland forest in southern China, plants reduce the allocation of P to non-metabolic P fractions while maintaining high photosynthetic capacity when growing in low-P soils (Mo et al., 2019). Those tropical plants in Mo et al. (2019) also exhibit species-dependent leaf P-allocation strategies, and this indicates that plants that are restricted to P-impoverished habitats may have more P-efficient adaptations than co-occurring species that have a wider distribution. Phospholipid concentration is negatively correlated with PPUE in two invasive species in a subtropical P-impoverished habitat (Zhang et al., 2021). Tropical tree species on Mount Kinabalu, Borneo exhibit significantly greater proportions of nucleic acid P and phospholipid P in P-rich soil than species in P-poor soils, and these tropical trees allocate a greater proportion of P to leaves among plant organs in response to P deficiency which shows the potential for high PPUE (Tsujii et al., 2017, 2020). In eastern Australia, 35 studied woody species all show relatively high PPUE (greater than 0.1 mmol CO2 g−1 P s−1), and the proportion of P in phospholipid is negatively correlated with PPUE in low-P soil, high-P soil and disturbed soil, while the proportion of nucleic acid P is negatively correlated with PPUE only in low-P soil with no correlations in other soils (Tsujii et al., 2023, 2024). Studies in P-impoverished regions outside south-western Australia including tropical and subtropical forests suggest that P-efficient plants allocate a greater proportion of P to metabolites and less to phospholipids and nucleic acids than less-P-efficient plants growing in P-rich regions. A wide range of species from the same regions should be compared as variability in leaf P-allocations among families > species in a family > within regions > plant life forms (Suriyagoda et al., 2023). Future studies should also focus on the correlation between PPUE and different P fractions in other P-impoverished regions comparing plant species with different natural distribution and life forms to explore the possibility of convergence among P-efficient species.
5 CONCLUSION
Instead of depending solely on mycorrhizal symbioses, Myrtaceae and Fabaceae species growing in severely P-impoverished environments acquired P by mining it themselves or absorbing P mined in the environment by neighbour plants or released by fire. Not all species growing in the P-impoverished environments used P efficiently, and the three Myrtaceae studied and D. physodes (Fabaceae) did not have a high PPUE. For the first time, we found that the correlations between PPUE and each P fraction varied depending on their P-use efficiency. In P-efficient species, nucleic acid P was negatively correlated with PPUE, and metabolite P was positively correlated with PPUE, whereas no correlations were shown among less-P-efficient species. Also, a trade-off was found between the allocation of P to nucleic acids and to the pool of phosphorylated metabolites among all species. New knowledge of the P-acquisition and leaf P-allocation strategies revealed by this study provides a better understanding of the mechanisms leading to high PPUE and contributes to a better understanding of how diverse P-acquisition and P-utilisation strategies coexist and interact in nutrient-impoverished biodiverse ecosystems. Most studies about leaf P-allocation strategies have been conducted in highly P-efficient species in Australia and south-east Asia and more studies in other ecosystems with different plant species are needed to further understand the mechanisms underlying P-utilisation strategies. Genetic analysis and a method to measure the chemical nature of P-containing metabolites are needed to further explore the evolutionary relationships among different plant species and identify the affected metabolites and the replaced membranes in future studies. Exploring leaf P fractions in detail will provide a better understanding of both P-efficient crops and plants growing in natural habitats.
ACKNOWLEDGEMENTS
Qi Shen was supported by a scholarship from the China Scholarship Council (CSC) and a top-up scholarship from the Kwongan Foundation. Funding was provided by the Australian Research Council Discovery Grant DP200101013 to Hans Lambers and Patrick M. Finnegan. Félix de Tombeur was supported by the EU Horizon 2020 Research and Innovation Program under Marie Skłodowska-Curie grant agreement 101021641 (project SiliConomic). We thank Haylee Gooding, Toby Bird, Hongtao Zhong, Shu Tong Liu, Clément Gille and Benjamin Nestor for help with sample collection. We are grateful to Todd Buters for the drone map and Joanna Kotula for help with the P-fractionation experiment. Open access publishing facilitated by The University of Western Australia, as part of the Wiley - The University of Western Australia agreement via the Council of Australian University Librarians.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




