Metagenomic insights into nitrogen cycling functional gene responses to nitrogen fixation and transfer in maize–peanut intercropping
Abstract
The fixation and transfer of biological nitrogen from peanuts to maize in maize–peanut intercropping systems play a pivotal role in maintaining the soil nutrient balance. However, the mechanisms through which root interactions regulate biological nitrogen fixation and transfer remain unclear. This study employed a 15N isotope labelling method to quantify nitrogen fixation and transfer from peanuts to maize, concurrently elucidating key microorganisms and genera in the nitrogen cycle through metagenomic sequencing. The results revealed that biological nitrogen fixation in peanut was 50 mg and transfer to maize was 230 mg when the roots interacted. Moreover, root interactions significantly increased nitrogen content and the activities of protease, dehydrogenase (DHO) and nitrate reductase in the rhizosphere soil. Metagenomic analyses and structural equation modelling indicated that nrfC and nirA genes played important roles in regulating nitrogen fixation and transfer. Bradyrhizobium was affected by soil nitrogen content and DHO, indirectly influencing the efficiency of nitrogen fixation and transfer. Overall, our study identified key bacterial genera and genes associated with nitrogen fixation and transfer, thus advancing our understanding of interspecific interactions and highlighting the pivotal role of soil microorganisms and functional genes in maintaining soil ecosystem stability from a molecular ecological perspective.
Abbreviations
-
- DHO
-
- dehydrogenase
-
- DIM
-
- intercropped maize separated by wooden borders
-
- DIP
-
- intercropped peanut separated by wooden borders
-
- HIM
-
- intercropped maize separated by nylon mesh
-
- HIP
-
- intercropped peanut separated by nylon mesh
-
- IM
-
- intercropped maize
-
- IP
-
- intercropped peanut
-
- NH4+-N
-
- ammonium
-
- NO3−-N
-
- nitrate
-
- NR
-
- nitrate reductase
-
- RRO
-
- protease
-
- TN
-
- total nitrogen
1 INTRODUCTION
With an increasing global population, the importance of food security has intensified. However, the environmental issues of heightened crop production driven by widespread chemical fertilizer use are increasingly evident. These include nutrient imbalances, soil acidification and salinization, which can adversely affect soil fertility and ecological balance. Gramineous and leguminous crops intercropping offers a sustainable, intensive cropping approach that not only promotes yields but also optimizes nutrient utilization, particularly nitrogen uptake and transfer (Kebede, 2021; Liu et al., 2017; Shao et al., 2021), thus ensuring farmland ecosystem stability (Xu et al., 2023). Previous studies have proposed various pathways through which nitrogen uptake can be enhanced in intercropping systems. These pathways include: (1) maize's heightened nitrogen competitiveness, facilitating increased legume nodule formation by alleviating soil nitrogen limitations, and the induction of broad bean nodulation by maize root exudates (Li et al., 2016); (2) signal molecules in adjacent plant root exudates and soil microbial communities stimulating leguminous plant nodulation (Yu et al., 2021); (3) leguminous plants promoting nonleguminous plants through nitrogen transfer processes (Jing, Shi, Liu, et al., 2023; Salgado et al., 2021). Despite the potential benefits of maize and peanut root interactions in nitrogen fixation and transfer, knowledge gaps persist regarding the interplay between biological nitrogen fixation, nitrogen transfer, soil microbial community structure and functional genes attributable to rhizosphere effects.
Intercropping root interactions, particularly between gramineous and leguminous crops, have been reported to enhance nitrogen fixation, distribution and transformation processes in the soil. This interaction significantly expands the effective nitrogen supply range and enhances nitrogen utilization efficiency owing to variations in root distribution (Homulle et al., 2021; Lazali et al., 2021). For example, studies on maize–soybean intercropping systems have indicated that symbiosis among rhizobium, arbuscular mycorrhizal fungi and soybeans can boost soybean nitrogen fixation, transferring approximately 15% of nitrogen to maize via common mycorrhizal networks (Wang et al., 2016). Furthermore, gramineous and leguminous crop intercropping can mitigate nitrogen mineralization and leaching processes in the soil, reducing nitrogen loss through volatilization (Chamkhi et al., 2022; Duchene et al., 2017; Peoples et al., 2015; Rai et al., 2023). These findings suggest the importance of gramineous and leguminous crop intercropping in maintaining soil nutrient balance and promoting sustainable agricultural development.
The utilization of 15N isotope labelling enables the measurement of nitrogen transfer from leguminous to nonleguminous plants (Hao et al., 2022; Shao et al., 2021; Wang et al., 2016; Yong et al., 2015). Studies have suggested that underground nitrogen transfer mechanisms involve mycorrhizal fungi, direct root exudate transfer and indirect transfer via plant material decomposition (Homulle et al., 2021). Recent research has indicated that in systems without root separation, nitrogen transfer from soybeans to cotton reaches 40.41% (Jing, Shi, Liu, et al., 2023; Jing, Shi, Wang, et al., 2023). Additionally, soybeans inoculated with arbuscular mycorrhizal fungi and rhizobium exhibited a significantly increased nitrogen fixation rate of 14.33%–39.09% with complete root interactions, with nitrogen transfer to maize being 1.95–3.48 times greater than that with partial root interactions (Qin et al., 2023; Zhang, Yu, et al., 2023). These findings demonstrate the vital role of interspecific interactions in enhancing nitrogen fixation in leguminous crops and facilitating nitrogen transfer to maize. Consequently, further investigations are necessary to elucidate the impact of interspecific interactions on nitrogen fixation and transfer in leguminous plants.
In recent years, metagenomic sequencing technology has been used to investigate variations in microbial communities and functional genes resulting from environmental changes. In a study on strip intercropping of maize and soybean under different nitrogen levels, significant increases were observed in the abundance and activity of functional genes related to microbial nitrogen fixation (nifH), nitrification (amoA), denitrification (nirS, nirK, nosZ) and degradation (chiA, Yu et al., 2018). Another long-term field experiment suggested that four different crop rotations improved the richness and diversity of key nitrogen cycling functional genes (amoA, amoB, amoC, nasA, norB, nasB, nirS and gdh) and bacterial communities (Liu, Liu, et al., 2023). Moreover, a meta-analysis further confirmed the positive effects of diversified planting patterns on the abundance of nitrogen cycling functional genes (Hao et al., 2022). However, previous research has predominantly focused on alterations in the abundance and diversity of nitrogen cycling functional genes under intercropping patterns (Fan et al., 2023; Yu et al., 2021), with limited exploration of their roles in peanut nitrogen fixation and transfer processes.
In this study, we quantitatively analyzed biological nitrogen fixation in peanuts and nitrogen transfer from peanuts to maize in maize–peanut intercropping systems through applying the 15N isotope labelling. Meanwhile, employing metagenomic approaches, we investigated the interactions between the microbial community composition involved in nitrogen cycling and nitrogen cycling pathways, focusing on the relative abundance and functions of key gene families participating in nitrogen cycling. We hypothesized that comprehensive interactions between maize and peanut roots could increase the expression of nitrogen cycling genes and jointly regulate biological nitrogen fixation and transfer through these genes and essential taxa. To validate these hypotheses, our objectives were to: (1) quantify biological nitrogen fixation in peanuts and nitrogen transfer to maize; (2) understand the response of nitrogen cycling genes to nitrogen cycling pathways under root interaction and (3) assess which crucial taxa and nitrogen cycling genes serve as potential predictors for biological nitrogen fixation and nitrogen transfer.
2 MATERIALS AND METHODS
2.1 Experimental site
The experiment was conducted at the experimental field of Shenyang Agricultural University, located within the Northeast Region Crop Cultivation Science Observation of the Ministry of Agriculture and Rural Affairs (41°82′N, 123°56′E) from 2022 to 2023. The site has a semiarid climate with continental and monsoonal characteristics. During this period, the average monthly temperature was 19.92°C, with an average monthly precipitation of 44.11 mm (Supporting Information S1: Figure 1A). Brown soil obtained from the 0–20 cm layer of long-term field experiments was used. Each pot was filled with 20 kg of soil after air-drying and sieving through a 2 mm mesh. Before the 2022 experiment, the soil contained 14.62 g kg−1 of organic matter, 180.04 mg kg−1 of available nitrogen, 45.78 mg kg−1 of available phosphorus and 202.36 mg kg−1 of available potassium, with a pH of 6.5. Before the 2023 experiment, the soil contained 14.95 g kg−1 of organic matter, 199.97 mg kg−1 of available nitrogen, 59.07 mg kg−1 of available phosphorus and 219.01 mg kg−1 of available potassium, with a pH of 6.5.
2.2 Experimental design
The pot experiment employed a two-factor (A × B), completely randomized design with three repetitions. Factor A comprised two nitrogen fertilizer treatments: no nitrogen fertilizer treatment (0N) and application of 15N urea (S15N, 180 kg hm−2, atom10.15%, Shanghai Research Institute of Chemical Industry Co., Ltd.). Factor B involved three root separation patterns: (1) intercropping of maize and peanuts separated by wooden borders (IMP-D), (2) intercropping of maize and peanuts separated by a 400-nylon mesh (IMP-H) and (3) intercropping of maize and peanuts (IMP, Supporting Information S1: Figure 1B,D). Twenty days after peanut seedlings were planted (25 June 2022 and 27 June 2023), the peanuts underwent stem injections of (15NH4)2SO4 solution using the three root separation patterns. The injections, performed over a 9-day period, utilized a concentration of 88 mol L−1 and a 20 μL microsyringe (Xiao et al., 2004). The maize variety was Liangyu 99 (Dandong Denghai Liangyu Seed Industry Co., Ltd.), and the peanut variety was Nonghua 9 (Peanut Research Institute of Shenyang Agricultural University, Shenyang, Liaoning). Seeding was conducted on 18 May 2022 and 19 May 2023. Three maize and peanut seeds were planted in each pot, retaining one plant per crop during the seedling stage. Additional fertilization (Supporting Information S1: Table 1) ensured a nutrient supply, whereas other cultivation practices adhered to local production standards. Ten days after the final injection (14 July 2022 and 16 July 2023), maize and peanut plant samples along with rhizosphere soil samples were collected for relevant indicator measurements.
2.3 Plant sample collection and chemical analysis
After a 10-day injection period in both 2022 and 2023, one maize and one peanut plant per pot were collected from five pots per treatment, totalling five plants in a single replicate, with three replicates in total. The harvested plants were divided into roots, stems, and leaves, blanched at 105°C for 30 min, and subsequently dried at 80°C until reaching a consistent weight. Dry matter accumulation was determined by weighing the dried plants, followed by sieving through a 0.5 mm mesh before digestion. Total plant nitrogen content was analyzed using the Kjeldahl method (Kjeltec 8400; FOSS). After sieving, plant samples weighing 7 mg were analyzed for 15N abundance using a stable isotope mass spectrometer (Iso ptime100; Elementar).
- (1)
atom% 15N excess:
where atom%15Nplant represents atom%15N excess of the plant, atom%15Nlabbled is atom%15N excess of the labelled plant and atom%15Nunlabelled denotes atom%15N excess of the unlabelled plant. - (2)
Percentage of plants from 15N fertilizer (%Ndff):
where atom%15Nplant represents atom%15N excess of the plant, and atom%15Nfertilization denotes atom%15N excess of fertilization. - (3)
Amount of 15N absorbed by plants (Ndff, mg):
where N uptake is the total plant nitrogen. - (4)
Percentage of biological nitrogen fixation in peanuts (%Ndfa, %):
where atom%15NIP/HIP/DIP represents atom%15N excess of the intercropped peanut, intercropped peanut separated by nylon mesh, and intercropped peanut separated by wooden borders, respectively; and atom%15NDIM is atom%15N excess of intercropped maize separated by wooden borders. - (5)
Percentage of maize derived from nitrogen fixation transfer from peanuts (%Ntr, %):
2.4 Rhizosphere soil sampling and chemical analysis
When collecting plant samples, maize and peanut roots treated with 0N and S15N were carefully uprooted, shaken to remove loose soil, and closely adhered soil at a depth of 5–15 cm was collected and sifted through a 0.9 mm mesh sieve to obtain clean rhizosphere soil (Supporting Information S1: Figures 1B,D). Composite rhizosphere soil samples were created by mixing soil samples from five pots per treatment (6 treatments × 3 replicates). The sieved rhizosphere soil samples were divided into three parts: one part was air-dried for soil nutrient content analysis, the second part was stored at −4°C for soil enzyme activity evaluation, and the remaining part was stored at −80°C for DNA extraction and metagenomic sequencing. In 2023, rhizosphere soil samples were analyzed for soil total nitrogen (TN) using the Kjeldahl method. Soil ammonium (NH4+-N) and nitrate (NO3−-N) contents were determined using an automated chemical analyzer (Smartchem200; AMS Technologies Ltd.). Soil protease (RRO), dehydrogenase (DHO) and nitrate reductase (NR) activities were determined using ELISA kits (MLBIO). Moreover, metagenomic sequencing was conducted on rhizosphere soil samples from maize and peanuts treated with S15N under different root interaction patterns (IMP and IMP-D) to examine the diversity of nitrogen cycling genes and functional microorganisms in the soil (Supporting Information S1: Figure 1D).
2.5 Metagenomic assembly and annotation
2.5.1 DNA extraction and amplicon sequencing
Total soil DNA was extracted from rhizosphere soil samples (0.5 g) using the anQubit® dsDNA Assay Kit on a Qubit® 2.0 Fluorometer (Life Technologies), following the manufacturer's instructions. DNA concentration and purity were assessed using a Nanodrop 1000 spectrophotometer (Thermo Fisher Scientific), and electrophoresis was performed on 1% (w/v) agarose gels. DNA samples with OD values between 1.8 and 2.0, and contents exceeding 1 μg were used for library construction. A total of 1 μg DNA per sample was adopted as the input material for DNA sample preparation. Sequencing libraries were generated using the NEBNext® Ultra™ DNA Library Prep Kit for Illumina (NEB), following the manufacturer's recommendations and index codes were added to attribute sequences to each sample.
2.5.2 Processing raw paired-end (PE) sequencing data
The Illumina HiSeq high-throughput sequencing platform was utilized alongside the Whole Genome Shotgun strategy to fragment the total DNA of the microbial community into short segments and construct libraries with appropriate insert lengths for PE sequencing. Raw sequencing data were stored in the FASTQ format. Postsequencing processing involved organizing and analyzing sequencing metrics, including the number of reads and proportion of high-quality bases. The reads obtained in DIM, IM, DIP and IP were 91 756 758, 94 913 657.33, 106 310 672.7 and 101 235 663.3, respectively. The corresponding total base pairs acquired were 13 763 513 700, 14 237 048 600, 15 946 600 900 and 15 185 349 500 bp. The Q30 percentages, indicating bases with an accuracy exceeding 99.9%, were 98.02, 97.98, 97.97 and 97.74, respectively. After data screening and filtering, the effective sequence totals were 90 522 486.67, 93 625 957.33, 104 787 898.00 and 99 683 080.00, with corresponding base counts of 13 569 589 777.67, 1 405 864.67, 15 707 265 296.00 and 14 943 541 679.33, respectively.
2.5.3 Identification of nitrogen cycling genes
NCycDB (https://github.com/qichao1984/NCyc) is a meticulously curated database of nitrogen cycling genes that facilitates rapid and precise metagenomic analyses of these genetic components (Tu et al., 2019). It encompasses 68 gene (sub)families across 8 nitrogen cycling processes, with 84 759 and 219 146 representative sequences meeting the 95% and 100% thresholds, respectively. Notably, the database includes 1958 orthologous groups, integrating their respective sequences to mitigate false positive outcomes associated with ‘small databases’.
2.6 Statistical analyses
Plant dry matter, plant N accumulation, rhizosphere soil nutrient content and soil enzyme activities were analyzed using Duncan's multiple comparisons via one-way analysis of variance with SPSS 26.00 (IBM SPSS Inc.). Biological nitrogen fixation in peanuts and nitrogen transfer from peanuts to maize were evaluated using independent sample t tests. A significance level of p < 0.05 was applied. Figures were generated using Origin2023 software (Origin Lab Corporation).
The Shannon index was calculated using the ‘vegan’ package in R and compared between treatments using Student's t test. A circos plot illustrating the species compositional relationships among the samples was constructed using the ‘circlize’ package in R. Linear discriminant analysis (LDA) effect size (Lefse) analyses were performed using the ‘ggtree’ package in R with the LDA threshold specified as 4. Only divergent species with an LDA < 4 were considered biomarker species. A taxonomic cladogram was used to depict the hierarchical distribution of the marker species within each sample group.
The co-occurrence network analysis utilized the SparCC correlation matrix with the WGCNA package in R. The p values of the correlation networks were adjusted using Spearman's correlation analysis. The edges with r < 0.7 and p > 0.01 were eliminated. The resulting network was visualized using Gephi 0.9.4.
Functional units were compared between treatments using the Kruskal–Wallis test for significance analysis and false discovery rate control using the Benjamini-Hochberg method. Fold differences between treatments were obtained for each functional unit and expressed as Log10 (Fold_change_value). Functional units with p < 0.05 were considered to exhibit significant abundance differences.
The ‘linkET’ package for R was utilized to simultaneously depict the connections among the Shannon index, the relative abundance of functional genes related to the nitrogen cycle, and soil physicochemical properties through a combination of correlation heatmaps and network diagrams. This included Pearson correlation analysis of soil physicochemical properties and Mantel test correlation analysis of the Shannon index and the relative abundance of functional genes related to the nitrogen cycle.
The random forest model evaluated the significant predictors affecting biological nitrogen fixation and nitrogen transfer, including soil physicochemical properties, Top10 bacterial genera and key functional genes of the nitrogen cycle. These analyses were conducted using the Random Forest software package. Model significance and predictor importance were verified using the rfUtilities and rfPermute packages in R software, respectively.
Structural equation modelling (SEM) was conducted using the R ‘piecewiseSEM’ package to evaluate the direct and indirect relationships among biological nitrogen fixation, nitrogen transfer, key nitrogen cycling genes (nrfC and nirA), key bacterial genus (Bradyrhizobium) and soil physicochemical properties (NH4+-N, NO3−-N and DHO).
3 RESULTS
3.1 Comparison of dry matter and nitrogen accumulation in maize and peanut plants under different root separation patterns
Regardless of nitrogen fertilization or root separation patterns, both exerted a positive impact on the accumulation of dry matter and nitrogen in maize and peanuts. Increasing nitrogen fertilizer resulted in higher dry matter and nitrogen accumulation in the roots and aboveground parts (stems and leaves) of maize and peanuts, with significant differences observed between years (Supporting Information S1: Tables 2 and 3). Overall, regardless of nitrogen application, maize and peanuts exhibited higher dry matter and nitrogen accumulation in the roots and aboveground parts in the order of IM > HIM > DIM and IP > HIP > DIP. Dry matter in roots and aboveground parts significantly increased under S15N treatment compared to DIM and DIP, with increases of 18.82% and 64.34% for IM and 55.00% and 70.61% for IP, respectively. Nitrogen accumulation in roots and aboveground parts significantly increased under S15N treatment compared to DIM and DIP, with increases of 81.97% and 150% for IM and 59.68% and 71.36% for IP, respectively. These findings demonstrated the critical role of nitrogen levels and root interactions in nutrient competition and facilitation within intercropping systems.
3.2 Nitrogen uptake in maize and peanut intercropping: Understanding nitrogen fixation and transfer
The 15N stem injection labelling experiments revealed that 15N absorption increased with heightened root interactions in maize and peanut plants. The absorption order was IP > HIP > DIP and IM > HIM > DIM (Figure 1a). Similarly, biological nitrogen fixation in peanut increased with the intensity of root interactions, measured at 33.75, 19.23 and 5.64 mg/pot for IP, HIP and DIP, respectively. These values were 6.0 and 3.4 times higher for IP and HIP compared with DIP, respectively (averaged of 2 years, Figure 1b). In the IMP-D, IMP-H and IMP treatments, nitrogen transferred from peanut plants to maize accounted for 0.00%, 10.33% and 17.39%, respectively, with amounts of 0.00, 143.41 and 331.14 mg/pot, respectively (averaged of 2 years, Figure 1b). Therefore, as the root interaction intensity increased, nitrogen transfer from peanuts to maize increased, indicating that root contact primarily facilitated nitrogen transfer.
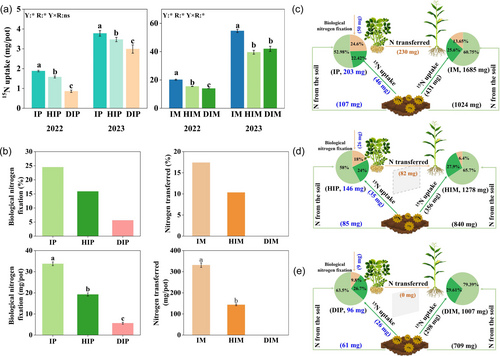
The 15N-labelled soil experiments revealed that, in maize plants, the source of fertilizers and soil nitrogen content ranked as IM > HIM > DIM (Figure 1c–e). The nitrogen derived from peanut nitrogen fixation transfer in the IM was three times higher than that in the HIM (Figure 1c,d). Notably, the amount of nitrogen in the DIM derived from peanut nitrogen fixation transfer was 0 mg (Figure 1e). Similarly, in peanut plants, the source of fertilizers and soil nitrogen content followed the order of IP > HIP > DIP (Figure 1c–e). Peanut's biological nitrogen fixation increased with stronger root interactions, with increments of 17 mg and 41 mg/pot for HIP and IP, respectively, compared to DIP (averaged of 2 years, Figure 1c–e).
3.3 Comparison of nutrient contents and enzyme activities in maize and peanut rhizosphere soil under different root separation patterns
Nitrogen fertilizers and root separation patterns affected TN, NH4+-N, and NO3−-N contents, as well as RRO, DHO and NR activities in maize and peanut rhizosphere soils. The interaction between nitrogen fertilizer and root separation methods significantly affected TN content and activities of RRO, DHO and NR in the rhizosphere soil (Figure 2). Generally, nitrogen content followed IM > HIM > DIM and IP < HIP < DIP (Figure 2a–c), whereas soil enzyme activities followed IM > HIM > DIM and IP > HIP > DIP (Figure 2d–f). Under the S15N treatment, compared with DIM, TN, NH4+-N and NO3−-N contents in IM significantly increased by 14.37%, 26.54% and 61.76%, respectively, and HIM increased by 6.32%, 1.96% and 57.35%, respectively (Figure 2a–c). Conversely, compared to DIP, TN, NH4+-N and NO3−-N contents in IP decreased by 26.83%, 27.48% and 30.00%, respectively, and HIP decreased by 9.97%, 4.21% and 9.75%, respectively (Figure 2a–c).
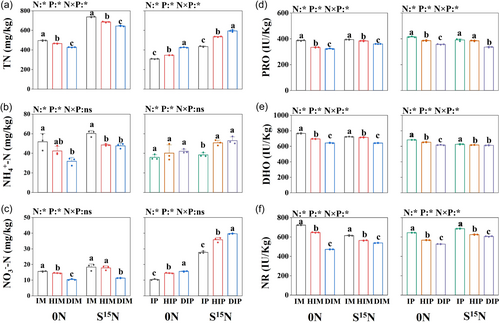
In the S15N treatment, compared with DIM, RRO, DHO and NR activities in the IM rhizosphere soil increased significantly by 9.44%, 12.64% and 14.14%, respectively. In the HIM, these activities increased by 6.57%, 11.40% and 4.74%, respectively, compared with DIM (Figure 2d–f). Compared with DIP, RRO, DHO and NR activities in the IP significantly increased by 16.39%, 2.21% and 13.22%, respectively. In the HIP, RRO and NR activities increased by 14.50% and 3.09%, respectively. These results indicated a significant regulatory effect of root interactions on the physicochemical properties of the rhizosphere soil.
3.4 Comparison of microbial community relative abundance and diversity in maize and peanut rhizosphere soil under different root separation patterns
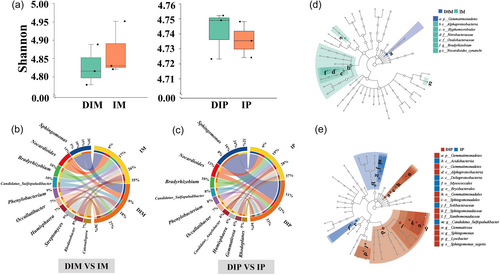
Alpha diversity analyses demonstrated higher microbial diversity in the IM than in the DIM, whereas microbial diversity in the IP was lower than that in the DIP (Figure 3a). In the maize rhizosphere soil, the dominant genera were Sphingomonas (20%), Nocardioide (14%), Bradyrhizobium (10%) and Candidatus_Sulfopaludibacter (10%, Figure 3b). Compared with DIM, IM exhibited an increased relative abundance of Sphingomonas, Nocardioide, Bradyrhizobium, Phenylobacterium and Occallatibacter. In the peanut rhizosphere soil, the dominant genera were Sphingomonas (32%), followed by Nocardioide (13%) and Bradyrhizobium (10%, Figure 3c). Compared with DIP, IP showed an increased relative abundance of Bradyrhizobium, Candidatus_Sulfopaludibacter, Phenylobacterium, Occallatibacter, Candidatus_Angelobacter and Humisphaera. The Lefse analysis highlighted significant differences in bacteria between DIM and IM, particularly in Bradyrhizobium (Figure 3d), and between DIP and IP, particularly in Sphingomonas, Candidatus_Sulfopaludibacte and Gemmatirosa (Figure 3e). These findings suggested that root interactions affected the growth and relative abundance of specific bacterial genera. Bradyrhizobium and Candidatus_Sulfopaludibacte were positively correlated with soil RRO, DHO and NR, whereas Gemmatirosa and Sphingomonas exhibited positive correlations with soil NR and significant negative correlations with soil RRO and DHO. Overall, NO3−-N, DHO and NR accounted for 68.59%, 68.4% and 68.2% of the bacterial community, respectively (Supporting Information S1: Figure 2).
3.5 Nitrogen cycling genes identified and screened
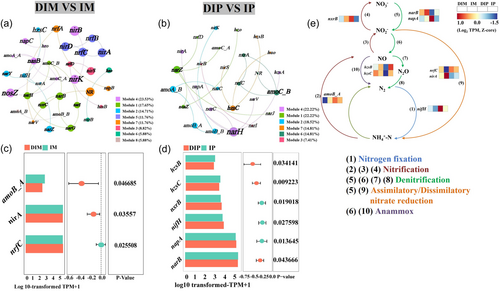
Through sequencing, we identified 43 genes involved in nitrogen cycling, encompassing pathways such as anammox, assimilatory nitrate reduction, denitrification, dissimilatory nitrate reduction, nitrification and nitrogen fixation (Supporting Information S1: Table 4). The co-occurrence network analysis revealed greater complexity in the DIM VS IM network than in the DIP VS IP network. DIM VS IM comprised eight modules featuring genes such as nirK, nasB, narB, narG and nifH, which interacted with multiple functional genes (Figure 4a), whereas DIP VS IP contained six modules with genes such as narH, narZ, amoA_B, hzsC and amoC_B interacting similarly (Figure 4b). Differential functional genes implicated in dissimilatory nitrate reduction (nrfC), assimilatory nitrate reduction (nirA) and nitrification (amoB_A) were detected in DIM VS IM (Figure 4c), whereas ammonia oxidation (hzsB and hzsC), nitrification (nxrB), nitrogen fixation (nifH), assimilatory nitrate reduction (narB) and denitrification/dissimilatory nitrate reduction (napA) were identified in DIP VS IP (Figure 4d). These nine differentially expressed genes were designated as key genes and their relative abundances across various samples were investigated. Notably, variations in the relative abundances of genes associated with the same nitrogen cycling pathways were observed among the samples (Figure 4e), indicating a significant impact of root interactions on the complexity and stability of nitrogen cycling gene networks. Soil physicochemical factors such as TN, PRO and NH4+-N were identified to explain variations of 29.81%, 22.15% and 18.59% of functional genes, respectively. Genes related to pathways such as nitrification, denitrification, nitrate reduction and nitrogen fixation exhibited significant positive correlations with NH4+-N and NO3−-N and negative correlations with TN, PRO, DHO and NR (Supporting Information S1: Figure 3).
3.6 Nitrogen fixation and transfer depend on nitrogen cycling genes and microbial diversity
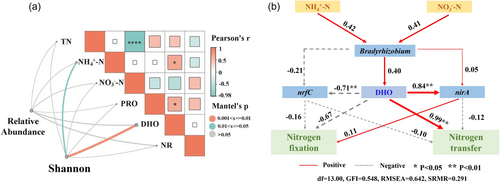
Root interactions can induce nitrogen fixation and transfer, which are closely correlated with soil physicochemical properties, functional microbes and nitrogen cycling genes. The Mantel tests revealed a significant positive correlation between microbial diversity (Shannon index) and soil NH4+-N and DHO (Figure 5a), indicating the notable impact of soil environmental factors on microbial communities. The random forest model identified DHO, Bradyrhizobium, nrfC and nirA as key factors in predicting biological nitrogen fixation and transfer (Supporting Information S1: Figure 4). The SEM analysis demonstrated that the primary factors affecting Bradyrhizobium in maize–peanut intercropping were NH4+-N and NO3−-N (Figure 5b). Among the assessed factors, DHO exerted a significantly positive direct impact on nirA, and a significantly negative direct impact on nrfC. Bradyrhizobium exhibited significant indirect positive effects on nitrogen transfer through DHO, with the greatest negative indirect effect on nitrogen fixation. Bradyrhizobium also had an indirect positive effect on nitrogen fixation through nirA and an indirect negative effect on nitrogen fixation and transfer through nrfC (Figure 5b).
4 DISCUSSION
4.1 Root interactions enhanced peanut biological nitrogen fixation and nitrogen transfer
This study revealed that intensified root interactions in intercropping systems enhanced dry matter and nitrogen accumulation in maize and peanut plants (Supporting Information S1: Tables 2 and 3). This indicated that root interactions were conducive to dry matter and nitrogen accumulation. This improvement suggests that intercropping promotes nitrogen exchange and symbiotic nitrogen fixation, thereby improving nitrogen utilization efficiency (Raza et al., 2023; Salgado et al., 2021; Shao et al., 2021). For instance, a study on chickpea-flax intercropping demonstrated that flax obtained 8%–22% of its fixed nitrogen from chickpeas (Reid et al., 2023). Similarly, in a maize-alfalfa intercropping study, alfalfa contributed 28% of the biological nitrogen fixed in maize without nitrogen fertilization but only 7% with 112 or 224 kg ha−1 nitrogen fertilizer (Osterholz et al., 2023). The 15N stem injection labelling experiment revealed that increased root interactions led to increased 15N uptake and nitrogen fixation in maize and peanut plants, validating the nitrogen transfer from peanuts to maize (Figure 1a,b). Previous studies have confirmed the efficacy of 15N steam injection in labelling plant stalks (Yasmin et al., 2006). Moreover, Götz and Herzog (2006) observed that during the pod filling stage, root 15N concentration in cowpeas was only 2%, whereas that in stems, leaves, and pods was 24%, 11% and 62%, respectively. Furthermore, Xiao et al. (2004) confirmed nitrogen transfer from fababean to wheat through 15N petiole injection labelling in wheat-faba bean intercropping, noting a minimal possibility of nitrogen secretion from the roots into the transfer pathway, leading to potential underestimation. Further, Wang et al. (2020) quantified rhizosphere N deposition as 25, 51, 20 and 63 kg N ha−1 for four legume crops (peanut, soybean, mungbean and adzuki bean) using the cotton-wick method. They found that wheat utilized 13%–85% of the rhizosphere N deposition from legumes and contributed to 4%–20% of the total nitrogen uptake of wheat. However, in this study, although the 15N stem injection labelling method provided valuable insights, there remains a risk of underestimation of the estimated values due to nitrogen absorption primarily by plant roots before transfer to stems. Therefore, the actual contribution of roots to nitrogen absorption and transfer may be higher than the current estimates, warranting further research to elucidate their precise role in these processes.
The 15N labelling soil experiment was conducted to identify nitrogen changes in the soil, fertilizer, and atmosphere, thereby deeply exploring the effects of root interactions on nitrogen cycling in maize–peanut intercropping. The findings revealed that across various root separation patterns, the nitrogen content from fertilizer and soil in maize followed the trend IM > HIM > DIM (Figure 1c–e, aim 1). Notably, the nitrogen content in maize from peanuts was three times higher in the IM than in the HIM. Similarly, the nitrogen content in peanuts from the soil, fertilizer, and atmosphere followed the sequence IP > HIP > DIP (Figure 1c–e, aim 1). Moreover, the biological nitrogen fixation increased by 17 mg and 41 mg/pot in HIP and IP, respectively, compared with DIP. This indicates that nitrogen transfer was promoted to a higher level when the root systems were fully interactive. This phenomenon may be attributed to the enhanced nitrogen transfer facilitated by the types and quantities of root exudates coupled with the increased diversity and abundance of rhizosphere microorganisms. For example, organic acids can facilitate nitrogen transfer from alfalfa to maize, with 15.4%–21.5% of alfalfa-fixed nitrogen being translocated to maize (Wang et al., 2019; Zhang et al., 2019). Hu et al. (2021) observed that maize root exudates induced enrichment of faba bean rhizosphere nitrogen-fixing bacteria (Agromyces, Arthrobacter, Bacillus, Lysobacter and Paenibacillus), promoting nitrogen fixation in faba bean. A recent study elucidated that plant root contact in maize–peanut intercropping systems modulates the rhizosphere microbiome which enriched Pseudomonas with its siderophore (pyoverdine) to improve iron nutrition in intercropped peanuts (Wang et al., 2024). These findings highlight that root interactions can indirectly influence nitrogen fixation and transfer in intercropping systems by optimizing the composition and structure of the rhizosphere microbial communities. Overall, root interactions may alter soil microorganism composition and metabolism, enhance soil nitrogen flow, and facilitate plant nitrogen uptake (Lu et al., 2024; Zou et al., 2023). In addition, agronomic management and crop varieties affect soil nutrient status (Gao et al., 2019; Jiang et al., 2022; Wang et al., 2022). The ‘apparent transfer’ phenomenon may pose a risk of overestimating nitrogen transfer when utilizing 15N-labelled urea in the soil, complicating the distinction between non-labelled nitrogen transfer and nitrogen fixation from peanuts (Cheng et al., 2015; Davidson et al., 2018; Xiao et al., 2004; Xie et al., 2015). Considering these findings, future studies should focus on optimizing isotope-labelling techniques to precisely quantify nitrogen transfer. These findings are important for unravelling nitrogen transfer mechanisms in soil-plant systems and improving agricultural sustainability.
4.2 Root interactions enhanced nutrient contents and enzyme activities in maize and peanut rhizosphere soils
In our study, we observed a significant increase in the contents of TN, NH4+-N, and NO3−-N in maize rhizosphere soil as the intensity of root interactions increased, whereas these parameters notably decreased in the peanut rhizosphere soil (Figure 2a–c). Additionally, the activities of RRO, DHO and NR in the maize and peanut rhizosphere soils increased (Figure 2d–f). Notably, the nitrogen levels in strip intercropping patterns, such as maize/wheat, maize/fava bean and wheat/fava bean, were higher than those in sole cropping practices (Cong et al., 2014). Correspondingly, studies on maize–soybean strip intercropping (Shen et al., 2023) and sugarcane–soybean intercropping have confirmed this trend, presenting the role of intercropping in enhancing soil fertility by optimizing nutrient utilization through mutual complementation and competition (Zhang, Tang, et al., 2023). The intercropping system of proso millet/mung beans enhanced the activities of conversion enzymes, urease, and catalase in the proso millet rhizosphere soil (Gong et al., 2019). Similarly, maize/soybean intercropping contributed to increased activities of urease, conversion enzymes, acid phosphatase, and catalase in maize and soybean rhizosphere soils (Lu et al., 2023). These results corroborate the benefits of root interactions in improving rhizosphere soil nutrient and enzyme activities. However, previous studies have indicated a shift in factors affecting rhizosphere soil physicochemical properties from crop varieties (Yu et al., 2018) to root exudates (Sun et al., 2019), rhizosphere soil microbial community composition (Lin et al., 2022), and expression levels of functional genes in biogeochemical cycles (Duan et al., 2023; Li et al., 2023). Overall, this underscores the importance of further investigation of the impact of diverse root interaction patterns on the expression of crucial functional genes in nitrogen cycling and the role of soil microbes in nitrogen transformation processes, contributing to a comprehensive understanding of nitrogen cycling. In conclusion, these findings lay the groundwork for exploring the potential genes involved in nitrogen cycling using molecular biology and bioinformatics tools.
4.3 Root interactions promote nitrogen fixation and transfer by recruiting nitrogen-cycling functional microorganisms
This study highlights the significant impact of root interactions on bacterial communities, particularly on the relative abundance and composition of bacterial genera. First, alpha diversity analysis revealed increased diversity in the IM rhizosphere soil and decreased diversity in the IP rhizosphere soil (Figure 3a), suggesting that root interactions and resource sharing enhanced soil microbial diversity. However, reduced diversity in intercropped peanut rhizosphere soils may result from interspecific competition (Duchene et al., 2017). For example, Zhang et al. (2024) suggested the importance of intercropping for enhancing crop productivity, stress mitigation, soil-borne disease control and erosion reduction. Furthermore, interspecies interactions in maize–soybean intercropping systems can alter the abundance of saprotrophic fungi and improve network stability and complexity, thereby improving nutrient cycling in maize (Zhang, Yang, et al., 2023). Research on various crop rotation patterns has demonstrated that root interactions can affect the diversity of soil bacteria in the rhizosphere, potentially influencing the soil ecosystem function (Koskey et al., 2023; Liu, Schroeder, et al., 2023; Liu et al., 2024). Furthermore, changes in abundance and composition revealed an increase in Sphingomonas and Bradyrhizobium in the IM and peanut rhizosphere soils (Figure 3b,c), and Lefse analysis results confirmed significant variations in the distribution of specific bacterial genera under root-interaction conditions (Figure 3d,e). Hence, plant interactions can influence the growth of specific bacterial genera in the soil, thereby altering community composition and ultimately affecting plant growth, development, and nitrogen cycling. Previous studies have highlighted the crucial roles of Bradyrhizobium (Correa et al., 2023; Msaddak et al., 2023) and Sphingomonas (Mazoyon et al., 2023; Su et al., 2024) in nitrogen cycling, providing plants with readily available nitrogen. Bradyrhizobium secretes indole acetic acid, which stimulates root development, enhances nutrient uptake, and promotes plant growth. Sphingomonas, a potential soil nitrifying and denitrifying bacterium, provides plants with accessible nitrogen. This finding highlights the pivotal role of root interactions in promoting microbial diversity and optimizing microbial community composition. This process recruits the enrichment of key microorganisms, such as Bradyrhizobium and Sphingomonas, involved in the nitrogen cycle, thereby promoting nitrogen fixation and transfer. This study laid the foundation for investigating the molecular regulatory networks of specific bacterial genera that interact with the root system and elucidating key signalling molecules and pathways affecting the nitrogen cycle.
4.4 Root interactions induce functional gene expression for nitrogen cycling in peanut rhizosphere soil
This study identified 43 nitrogen cycling functional genes in maize and peanut rhizosphere soils that participate in six nitrogen metabolic pathways (Supporting Information S1: Table 4). Efficient annotation of metagenomic functions demonstrated the importance of these genes in critical processes, such as ammonia and nitrate reduction and nitrogen fixation (Culligan & Sleator, 2016; Kim et al., 2024; Navgire et al., 2022). The co-occurrence networks revealed variations in functional gene interactions in maize and peanut rhizosphere soils, indicating different effects of root interactions on nitrogen cycling regulation (Figure 4a,b). The presence and expression changes in these differentially functional genes among different crops reflect the differential response mechanisms of crops to the nitrogen cycle. In maize rhizosphere soil, nrfC and nirA genes were identified as key players in nitrate reduction, facilitating the conversion of nitrate to ammonia, thereby supplying essential nitrogen resources to plants (Figure 4c, Yu et al., 2021). Additionally, amoB_A played a crucial role in the ammonia oxidation process by converting ammonia to nitrite and contributing to nitrification, thereby facilitating nitrogen absorption and utilization by plants (Figure 4c, Bossolani et al., 2023). The narB and nxrB genes facilitated nitrate reduction and nitrification, thereby enhancing nitrogen utilization and transformation in the soil (Figure 4d, Grassmann et al., 2022). The nifH gene helped convert atmospheric nitrogen into ammonia nitrogen, thereby increasing the soil nitrogen content (Zhang et al., 2006; Zhao et al., 2017). However, the abundance of nifH in the intercropped peanut rhizosphere soil reduced (Figure 4d). The following factors may contribute to this. (1) Competition from maize for soil resources, such as nutrients and water can lead to decreased expression of the nitrogen fixation nifH gene in peanuts (Benchuan et al., 2022; Yang et al., 2022; Yu et al., 2022; Zhang et al., 2022). (2) Rhizosphere effects, where root interactions result in differences in the composition of the rhizosphere microbiota, can influence the expression of nitrogen cycling functional genes and their regulatory mechanisms (Dijkstra et al., 2010). Nevertheless, a greater number of genes associated with ammonia, nitrification, nitrogen fixation, and nitrate reduction were identified in the peanut rhizosphere soil (Figure 4d), indicating a more complex nitrogen cycling and transformation process. Previous studies have demonstrated that the composition and concentration of plant root exudates influence the growth and metabolic activity of different microbial communities, thereby affecting nitrogen cycling genes in the rhizosphere soil (Ulbrich et al., 2022; Yue et al., 2023; Zhou et al., 2023). Based on this, we hypothesized that root interactions recruit the enrichment of nitrogen-cycling microorganisms. These microorganisms activate the expression of functional nitrogen-cycling genes in the peanut rhizosphere soil through various pathways, including ammonia oxidation, nitrification, nitrogen fixation, and nitrate reduction. This process enhances biological nitrogen fixation in peanuts and facilitates nitrogen transfer from maize (aim 2). These functional genes provide novel avenues for investigating nitrogen fixation and transfer between crops at the molecular level. However, owing to the limited sample size and experimental constraints, further research can be necessary across different crop species, seasons, and soil types to comprehensively understand these processes.
4.5 Nitrogen cycling functional microorganisms and functional genes co-drive mechanisms of nitrogen fixation and transfer
Biological nitrogen fixation and transfer are jointly influenced by various factors including soil physicochemical properties, microbial communities, and functional genes. In particular, a close relationship exists between the soil physicochemical properties, microbial community composition, and functional genes. Mantel tests revealed a significant positive correlation between microbial diversity and soil NH4+-N and DHO (Figure 5a), indicating the role of soil environmental factors in shaping microbial community diversity. Specifically, the NH4+-N content and DHO in the soil may create more favourable habitat conditions for certain microorganisms, thereby influencing microbial diversity and composition (Cuartero et al., 2022; Duan et al., 2023; Zhang, Zhu, et al., 2023). For instance, soil pH, NH4+-N, available phosphorus, and total potassium significantly affect the composition and structure of microbial communities in maize-soybean strip intercropping systems (Yu et al., 2018). Soil properties, such as TN, organic matter and pH, greatly influenced nitrogen cycling genes, explaining up to 86.5% of the variability (Zhang & Lv, 2021). The random forest model predictions identified factors such as the bacterial genus Bradyrhizobium and the key functional genes of the nitrogen cycle, nrfC and nirA, as potentially significant influencers of biological nitrogen fixation and transfer in peanuts (Figure S2B–S2D). This underscores the close correlation between functional microorganisms and key nitrogen cycle genes that are jointly involved in soil nitrogen fixation and transfer processes. SEM modelling further explored the potential mechanisms of these microorganisms and genes in soil nitrogen cycling (Figure 5b). The results showed that soil NH4+-N and NO3−-N contents significantly affected the relative abundance of Bradyrhizobium (Figure 5b), suggesting that soil chemical properties regulate soil microbial community composition, thereby influencing nitrogen fixation and transfer processes. We speculate that nrfC and nirA play crucial roles in the regulation of nitrogen fixation and transfer. Moreover, Bradyrhizobium's role in this process, influenced by soil nitrogen content and DHO, indirectly affected nitrogen fixation and transfer efficiency (Figure 5b, aim 3). These findings highlight the intricate interplay between nitrogen-cycling microorganisms and nitrogen-cycling functional genes in regulating nitrogen fixation and transfer, suggesting the need for further investigation of the molecular mechanisms of these genes in nitrogen-cycling regulation.
5 CONCLUSIONS
Nitrogen transfer from peanuts to maize is pivotal for augmenting nitrogen accumulation in IM. Maize and peanut intercropping increased nitrogen fixation in peanuts and transferred nitrogen to maize, reaching 50 mg and 230 mg, respectively. This study emphasized the beneficial impact of root interaction on nitrogen content in the maize rhizosphere and enzyme activities (RRO, DHO and NR) in peanut rhizosphere soil. Additionally, root interactions promoted the growth of beneficial microbes in the rhizosphere, particularly enhancing the abundance of nitrogen-fixing bacteria such as Sphingomonas and Bradyrhizobium. Root interactions played a vital role in upregulating the expression of nitrogen cycling genes, facilitating better nitrogen utilization by plants. Specifically, a greater number of genes related to ammonia (hzsB/C), nitrification (nxrB), nitrogen fixation (nifH) and nitrate reduction (narB and napA) were detected in the intercropped peanut rhizosphere soils. In summary, these underground interactions influenced soil physicochemical properties, shaped microbial communities in the rhizosphere, activated nitrogen cycling genes, and promoted nitrogen fixation and transfer, thus laying a scholarly foundation for enhancing nitrogen efficiency in maize–peanut intercropping systems.
ACKNOWLEDGEMENTS
The authors would like to thank the China Agricultural Research System (CARS-13) and Liaoning Province Science and Technology Plan Project (2022-MS-263) for supporting the work. They would also like to thank the editors and reviewers for their comments and constructive suggestions, which have been invaluable for improving the quality of the manuscript. Atlast, they would like to thank Dr. Jing Wang for her assistance in the manuscript revision process. This work was supported by the earmarked fund for CARS-13 and Liaoning Province Science and Technology Plan Project (2022-MS-263).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. Data will be made available on request.




