Leaf cell wall properties and stomatal density influence oxygen isotope enrichment of leaf water
Patrick Z. Ellsworth and Patrícia V. Ellsworth are co-first authors.
Abstract
Measurements of oxygen isotope enrichment of leaf water above source water (Δ18OLW) can improve our understanding of the interaction between leaf anatomy and physiology on leaf water transport. Models have been developed to predict Δ18OLW such as the string-of-lakes model, which describes the mixing of leaf water pools, and the Péclet effect model, which incorporates transpiration rate and the mixing length between unenriched xylem and enriched mesophyll water in the mesophyll (Lm) or veins (Lv). Here we compare measurements and models of Δ18OLW on two cell wall composition mutants grown under two light intensities and relative humidities to evaluate cell wall properties on leaf water transport. In maize (Zea mays), the compromised ultrastructure of the suberin lamellae in the bundle sheath of the ALIPHATIC SUBERIN FERULOYL TRANSFERASE mutant (Zmasft) reduced barriers to apoplastic water movement, resulting in higher E and, potentially, Lv and, consequently, lower Δ18OLW. The difference in Δ18OLW in cellulose synthase-like F6 (CslF6) mutants and wild-type of rice (Oryza sativa) grown under two light intensities co-varied with stomatal density. These results show that cell wall composition and stomatal density influence Δ18OLW and that stable isotopes can facilitate the development of a physiologically and anatomically explicit water transport model.
1 INTRODUCTION
Plants play a major role in the hydrologic cycle as a major conduit for soil and surface water to return to the atmosphere through the transpiration stream, cycling approximately 1015 L of water annually (Jasechko et al., 2013). Along the soil–plant-atmosphere continuum, the stomata in leaves serve as controllers of water movement, through which nearly all transpired water passes. Much of the research on water movement through the leaf has focused on stomates, while many leaf anatomical and physiological traits influencing and driving water movement within the leaf to the stomata are not well understood (Barbour, 2017). Leaf anatomical traits such as vein size, leaf cell wall properties, degree of suberization of the bundle sheath cells surrounding the vasculature and connectivity of veins with mesophyll cells can influence relative flow rates and resistances to flow through water pathways (Barbour, 2017; Barbour et al., 2021). Therefore, combining physiological measurements of leaf water movement with leaf anatomical traits may provide greater insight into how water moves within the leaf. To address this, we tested the hypothesis that cell wall composition mutants alter leaf anatomy and physiology influencing the pathways, flows, and resistances of water movement within the leaf.
Cell wall composition mutations may modify the relationship between leaf anatomy and hydraulics as well as their relationship with physiology (Barbour et al., 2021; Buckley et al., 2015; Loucos et al., 2015). For example, Zmasft double mutants, which were created in maize (Zea mays L.) by mutating two paralogously duplicated, unlinked maize orthologues of Arabidopsis thaliana ALIPHATIC SUBERIN FERULOYL TRANSFERASE, have a compromised suberin lamellae ultrastructure that increased cell wall elasticity and apoplastic water diffusion (Mertz et al., 2020). The purpose of the suberin lamellae is to provide a barrier (greater resistance) to apoplastic water movement and reduce passive water loss from the vasculature (Danila et al., 2021; Mertz & Brutnell, 2014; Vishwanath et al., 2015). Greater apoplastic diffusion and cell wall elasticity can increase water movement out of the xylem and water available for transpiration, while less effective barriers (less resistance) to water movement can increase the number of water channels and modify the relative importance of each channel (Figure 1a–c). Another example of a gene that influences cell walls is the cellulose synthase-like F6 (CslF6) in rice (Oryza sativa L.), which mediates the biosynthesis of mixed linkage glucan (MLG), a polysaccharide important in cell wall composition (Vega-Sánchez et al., 2012). Loss-of-function mutants do not synthesize MLG (Smith-Moritz et al., 2015; Vega-Sánchez et al., 2012), have lower effective cell wall porosity, and has been shown to decrease the internal conductance of CO2 within the leaf (gm), which may also hinder apoplastic water movement (Figure 1a–c) (Ellsworth et al., 2018). In addition to anatomical, hydraulic, and physiological consequences, reduced apoplastic flow and cell wall porosity may have isotopic consequences to leaf water by influencing the contribution of unenriched xylem water on leaf water (Barbour et al., 2017, 2021). Therefore, these mutants provide a means to evaluate the effect of cell wall composition on water transport, and the analysis of stable isotope composition of leaf water may provide a method to understand this relationship.
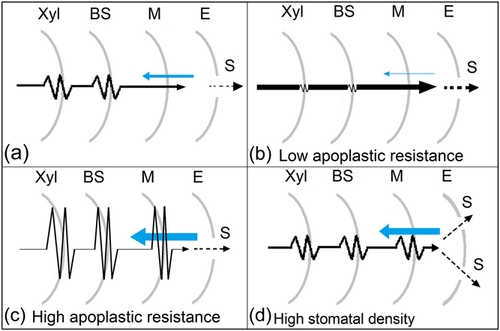
Cell wall mutations that change the ratio of apoplastic to symplastic flow may modify the relative importance and mixing of the two sources of isotopic variation: unenriched source water entering the leaf through the xylem and water undergoing evaporative enrichment at the sites of evaporation, creating detectable isotopic differences in leaf water (Barbour et al., 2021). For example, leaf anatomical traits that vary the ratio of apoplastic to symplastic water transport can lead to variation in the water available for transpiration and the isotopic composition of leaf water because isotopic gradients can develop along apoplastic pathways (Figure 1a–c) (Barbour et al., 2017, 2021). Additionally, stomatal density and pore size influence water movement through and out of the leaf by changing the distance water must move to reach the sites of evaporation and the volume of water undergoing evaporative enrichment. These types of stomatal differences have been shown to influence the isotopic composition of leaf water, where leaves with greater stomatal densities and aperture have more enriched water (Figure 1d) (Larcher et al., 2015; Liang et al., 2018; Sternberg & Manganiello, 2014). Isotopic models have been developed to explain how environmental and physiological processes including water transport control the bulk enrichment of oxygen isotope composition of leaf water above source water (Δ18OLW). These models attempt to explain Δ18OLW as a mixture of unenriched water entering the leaf and water enriched through evaporative enrichment, which is a function of relative humidity (RH), leaf temperature, and oxygen isotopic composition of vapour and source water to the leaf as described in a modified version of the Craig and Gordon model (see Section 2; Craig & Gordon, 1965; Farquhar et al., 1998). Both the two-pool (Roden & Ehleringer, 1999) and Péclet effect models (Barbour et al., 2000, 2004; Farquhar & Lloyd, 1993) can theoretically detect differences in water transport influenced by cell wall composition.
The two-pool model describes bulk leaf water isotopically as two discrete pools: unenriched xylem water and evaporatively enriched at the evaporation sites. In grasses, the two-pool model was modified in the string-of-lakes version to explain the progressive enrichment of water up the leaf blade (Farquhar & Gan, 2003; Helliker & Ehleringer, 2000). Cell wall mutations can influence Δ18OLW if compromised cell walls modify the proportion of water transported apoplastically, potentially altering the proportion of unenriched water supplied to the sites of evaporation and the rate of back diffusion into the xylem. Where Δ18OLW varies with transpiration rate (E), the Péclet effect model describes Δ18OLW as a continuous isotopic gradient from unenriched xylem water to enriched water at the sites of evaporation that is maintained through opposing flows of advection and back-diffusion. The Péclet effect is dependent on the length and tortuosity of the path that water takes through the xylem and mesophyll (L) and transpiration rate (E) (see Section 2; Barbour, Fischer, et al., 2000; Barbour, Schurr et al., 2000). Each of these models have found empirical support under specific conditions and for certain species; however, neither of them can universally explain the isotopic behaviour of leaf water (Barbour & Farquhar, 2000, 2004; Barbour et al., 2000; Helliker & Ehleringer, 2000; Holloway-Phillips et al., 2016; Loucos et al., 2015; Roden & Ehleringer, 1999; Song, Loucos, et al., 2015). Studies using cell wall composition mutants can provide valuable insight into how changes in leaf anatomical properties influence the isotopic composition of leaf water.
To determine the influence of cell wall properties on Δ18OLW, we tested how the loss of suberin in maize and MLG in rice changed water movement from the xylem to the sites of evaporation under different environmental growth conditions (growth light and RH) that have been demonstrated to influence Δ18OLW. Specifically, we, first, hypothesized that greater cell wall elasticity and transpiration rates in maize Zmasft double mutants would decrease Δ18OLW by increasing the influence of unenriched xylem water on bulk leaf water because water movement out of the xylem would be greater and the back diffusion into the xylem would be relatively less. Second, we hypothesized that the effect of the CslF6 mutation such as decreased cell wall porosity would increase Δ18OLW by reducing the isotopic influence of xylem water on bulk leaf water from lower apoplastic water movement. The data presented here demonstrates that changes in both cell wall properties and stomatal density influenced Δ18OLW through the impact on E and potentially L in the Péclet effect model and altered the effective volume of unenriched xylem water in the string-of-lakes model.
2 THEORY AND CALCULATION OF ISOTOPIC FACTORS
| Variable | |
|---|---|
| Δ18OLW | Leaf water enrichment of oxygen (‰) |
| Δ18Oe | Leaf water enrichment of oxygen (‰) |
| δ18OSW | Oxygen isotopic composition of source water |
| fsw | Fraction of source water in leaf water |
| E | Transpiration rate (mmol m–2 s–1) |
| gs | Stomatal conductance (mol m–2 s–1) |
| Lv | Effective path length in the xylem (mm) |
| Lm | Effective path length in the mesophyll (mm) |
| ϕx | Proportional of xylem water in leaf water |
| Sd | Total stomatal density (no./mm2) |
| Sadaxial | Adaxial stomatal density (no./mm2) |
| Sabaxial | Abaxial stomatal density (no./mm2) |
| Sabaxial length | Abaxial stomatal pore length (μm) |
| Sadaxial length | Adaxial stomatal pore length (μm) |
| gs/Sd | Stomatal conductance per stomate |
| VB | Partial volume of the vascular bundle |
| Vasc | Partial volume of the total vasculature |
| WT | Wild type |
| Zmasft | Maize double mutants (aliphatic suberin feruloyl transferase) |
| CslF6 | Cellulose synthase-like F6 (biosynthesis of mixed linkage glucan [MLG]) |
| PAR | Photosynthetically active radiation |
| LL | Low light (300 μmol photons m–2 s–1 PAR) |
| HL | High light (1000 [rice] and 1200 [maize] μmol photons m–2 s–1 PAR) |
L is related to leaf anatomy because it accounts for the tortuosity of the water pathway and the cross-sectional area perpendicular to water flow relative to the leaf surface area (Barbour & Farquhar, 2004; Cernusak & Kahmen, 2013; Song et al., 2013; Sternberg & Manganiello, 2014).
An important location of the Péclet effect may be the xylem and the magnitude of its influence depends on factors affecting the xylem water pool size and movement of xylem water through the leaf water. For example, the measurable effect of the Péclet effect model in bulk leaf water appears to depend on the relative importance of enriched vein water to bulk leaf water and the interaction between xylem and mesophyll water pools (Holloway-Phillips et al., 2016; Song, Loucos, et al., 2015).
Therefore, the Péclet effect model can be adapted to the species depending on the leaf anatomy and physiology influencing Δ18OLW.
3 MATERIALS AND METHODS
3.1 Plant material
3.1.1 Experiment 1: Maize suberin mutants (Zmasft)
Seeds of maize (Zea mays L.), a C4 Panicoid grass species, were planted in 11.3 L pots filled with the soil substrate, Sunshine Mix LC-1 (Sun Gro Horticulture) and placed in two controlled-environment growth chambers (Biochambers, GRC-36). Five plants of each genotype were grown: wildtype and Zmasft double mutants, which were created by mutating two paralogously duplicated, unlinked maize orthologues of Arabidopsis thaliana aliphatic suberin feruloyl transferase. Plants were watered daily and fertilized every 4 days, initially with 20-20-20 and later with 15-5-15 CalMag (Scotts-Peters Professional). The photoperiod was 16 h and included two 1-h ramps for light and temperature to mimic dawn and dusk. Day/night temperatures were set at 28°C/20°C, respectively. RH in one growth chamber was maintained at 50%, and the other growth chamber was maintained at 80%, and all other environmental conditions were maintained the same between the two chambers. The vapour pressure deficit during the day was approximately 1.89 kPa at 50% RH and 0.76 kPa at 80% RH. Each growth chamber space was isolated from the separate compartment containing light bulbs, which resulted in the chamber temperature being more uniform and better regulated without additional heating from the lights. The pots were distributed in two light treatments even before germination: high light (HL) exposed to 1200 μmol quanta m−2 s−1 at canopy level and low light (LL) exposed to 300 μmol quanta m−2 s−1 at canopy level. The plants in each light treatment were grown simultaneously in the same growth chamber, and shade cloth and the height of the pots were used to maintain the desired light treatments throughout the experiment. At the time of sampling, the plants were 3-week-old. Experiment was conducted from March to May 2015, at Washington State University.
3.1.2 Experiment 2: Rice mixed linkage glucan mutants (CslF6)
The cultivar Nipponbare cv (wild type) and mutants cslf6-1 and cslf6-2, which vary in the position of their transposon insertion within the CslF6 genomic sequence of Nipponbare cv were used in the study (Ellsworth et al., 2018; Vega-Sánchez et al., 2012). Nipponbare rice genome has been sequenced to a high quality and is the reference sequence for rice cultivars in genetic research (Matsumoto et al., 2016). Seeds of rice (O. sativa L.), a C3 grass species, were germinated on wet filter paper in a petri dish. After which nine seedlings of each genotype were transplanted in trays containing Sunshine Mix LC-1 soil (Sun Gro Horticulture) and turface (ratio of 3:1 v/v). Then after a week, they were transplanted into 7.5 L pots and placed in a controlled environment growth chamber (Biochambers, GRC-36). The plants were watered daily and fertilized every 4 days with a nutrient solution containing Sprint 330 iron chelate (1.86 g L−1), magnesium sulphate (0.88 g L−1), Scotts-Peters Professional 10-30-20 compound (3.96 g L−1), and Scott-Peters Soluble Trace Element Mix (10.0 mg L−1; Scotts). The photoperiod was 14 h with a 2 h ramp, reaching 500 μmol photon m−2 s−1 at canopy level. Day/night temperatures were set at 26/22°C, respectively with a 1-h ramp, and RH was maintained at 70%. One week after transplanting, plants were distributed into two light treatments within a single growth chamber where the high light plants (HL) were exposed to 1000 μmol photon m−2 s−1 at canopy level and the low light plants (LL) were under shade cloth to reduce light intensity to 300 μmol photon m−2 s−1 at canopy level. The high light (HL) plants were submitted to a gradual increase in irradiance over 5 days to 1000 μmol photon m−2 s−1 at canopy level, and low light (LL) plants were placed directly under 300 μmol photon m−2 s−1 at canopy level. Plants were grown simultaneously in the same growth chamber, and pot heights were adjusted to maintain the desired light treatments at canopy level. At the time of sampling, the plants were 5-week-old. The experiment was conducted from March to May 2014, at Washington State University.
3.2 Anatomical analysis
For measurements of partial volume of total vasculature, vascular bundles (both including and excluding the bundle sheath), and xylem, cross sections were made on leaf 5 at the point of maximum lamina width. The same leaves that were used for gas exchange measurements and isotope analysis were also sampled for microscopy. Transverse sections were collected after the gas exchange measurements and before they were sampled for isotope analysis. The freehand transverse sections were immersed in water and imaged under UV light filter (with excitation wavelength range 350–520 nm) using cell wall autofluorescence to maximize the contrast between the bundle sheath cell wall and the surrounding mesophyll cells. Images were captured using Leitz Epi-Fluorescent Microscope with Leica DFC425C Camera at ×100 for the leaf blade and ×250 for the midrib. Additional images were taken at ×40 magnification for the leaf blade and ×25 for the midrib to calculate cross sectional leaf area. The cross-sectional area of all vascular bundles (including and excluding the bundle sheath) was measured. For xylem area, diameter of each xylem element was measured twice perpendicular to each other, and mean diameter was used to calculate xylem area as the area of a circle. All measurements were made in ImageJ (Schneider et al., 2012). The total vasculature was calculated as the area of the vascular bundles in the leaf blade plus the entire midrib. Partial volume of each was calculated as the total cross-sectional area of each respective anatomical feature relative to the total leaf cross-sectional area (blade area + midrib area).
The surface of three fresh leaves per genotype per treatment was analyzed under the low vacuum mode of FEI Scanning Electron Microscope (SEM) Quanta 200F (FEI Company, Field Emission Instruments). The leaves were the same or similar to those used for gas exchange measurements and anatomical analyses. Images of the adaxial and abaxial epidermal surfaces were randomly captured across the entire leaf blade avoiding the central vein (minimum 10 images per leaf) to determine the number of stomata per mm2 of leaf surface (stomatal density) and axis length of stomatal pores.
3.3 Gas exchange measurements
Gas exchange measurements were performed inside the growth chamber, and light intensity, temperature, and RH in a 2 × 3 cm leaf cuvette connected to the LI-COR 6400XT were set to mimic growth chamber conditions under each treatment to ensure similar conditions for the entire leaf (LI-COR Biosciences). Gas exchange measurements were made between 12:00 and 14:00. Both light treatments were maintained in the same growth chamber to minimize differences in other environmental conditions, and within a light treatment all individuals of each genotype were positioned next to each other. The gas exchange cuvette was set to match the temperature and RH of the growth chamber. Within each treatment positions of plants were randomized daily. The youngest, fully expanded leaves (leaf 5 in maize and typically leaves 3, 4 and 5 for rice were placed side by side in the cuvette) were used throughout the experiment when plants were all the same height and age (3-week-old in maize and 5-week-old in rice) during early vegetative growth well before flowering or antithesis. This sampling protocol minimized differences in ambient conditions for all measured leaves, including temperature. Although leaf temperature may vary along the blade, the strong air flow in the chamber constantly moved the leaves and minimized long term shading on a given section of the leaf, reducing temperature variation along the leaf blade. The growth chamber lighting was separated from the chamber by a glass divider to reduce the heat on the plants generated by the light bulbs. Flow rate maintained at 300 μmol air s−1, and oxygen partial pressure was 19.3 kPa (21%). Gas exchange measurements were quick, lasting only a few minutes, so that the process of making gas exchange measurements would minimize any isotopic impact on leaf water.
3.4 Leaf trait measurements
The entire fully expanded leaf blade selected for transpiration measurements (section above) was collected immediately after gas exchange measurements and photographed to calculate area using ImageJ (Schneider et al., 2012) before placing into preweighed glass tubes. After measuring the fresh weight, leaf water was extracted according to Vendramini and Sternberg Lda (2007) for δ18OLW measurements. Issues with oxygen isotope fractionation increases in samples of less than 0.6 mL water (Chen et al., 2020; Diao et al., 2022), but this was not a problem in this experiment because samples had 1.5–2.0 mL of water. The extraction was conducted over 18–24 h to minimize the isotopic depletion that can occur in extractions of less than 8 h (Vendramini & Sternberg Lda, 2007). The dried leaf material was weighed to calculate leaf water content (LWC) and leaf dry mass per area (LMA). LWC was calculated as the difference between fresh and dry leaf weight per leaf area (g H2O/cm2 leaf), and LMA was calculated as dry leaf weight per leaf area (g leaf/cm2 leaf).
Root crowns were collected after gas exchange measurements and placed in a separate glass tube for water extraction (Vendramini & Sternberg Lda, 2007) and oxygen isotope analysis, so that source water was characterized for each plant to calculate leaf water oxygen isotope enrichment (Δ18OLW). Vapour was collected from the growth chamber through the collection port at approximately canopy height near the plants every 30 min during transpiration measurements, while the chamber was closed. The vapour was sealed in 5 L inert foil gas sampling bags (Supelco Analytical #30228-U) that restrict exchange of water vapour or CO2 exchange with ambient air, and after collection the vapour samples were measured immediately (details below).
3.5 Oxygen isotope analysis
Root crown and leaf water oxygen isotope ratios (δ18OLW) were analyzed by isotope ratio mass spectrometer (IRMS). A 0.3% CO2/He gas mixture was flushed through the vials and equilibrated with water for 48 h on a ThermoFinnigan GasBench II. The equilibrated CO2 gas was separated at 40°C with a 25 × 0.32 mm ID GC column (Varian, poraplot Q) and analyzed with a continuous flow isotope ratio mass spectrometer (Delta PlusXP, ThermoFinnigan) (Brenna et al., 1997; Qi et al., 2003) to derive the oxygen isotope ratios of the water samples. Standards (SLAP and Puerto Rico water) were analyzed alongside the samples. Final delta values were the mean of five sample peaks reported relative to Vienna Standard Mean Ocean Water (V-SMOW).
For calculation of effective leaf water mixing length, some parameters were measured, and others were calculated. Steady state condition (SS) was assumed for all calculations. Water isotope enrichment at the sites of evaporation (Δ18Oe) was calculated for each leaf (Craig & Gordon, 1965; Dongmann et al., 1974) relative to source water (Equation 2). Source water was root crown water. Leaf temperature and RH used in the calculation of δ18Oe were determined from the LI-COR leaf cuvette, which was set to match the temperature and RH within the growth chamber. Δ18Ov is the atmospheric water vapour enrichment above plant water source and was from the atmosphere, as only about 6.5% of the leaf was inside the cuvette during gas exchange measurements for about 3–5 min. ea/ei was the ratio of vapour pressure external to the leaf to internal vapour pressure, ε* was the temperature-dependent (°K) equilibrium fractionation factor between vapour and water inside the leaf (Equation 5; Majoube, 1971), and εk was the kinetic fractionation during diffusion of vapour from the leaf to the atmosphere (Equation 6; Farquhar et al., 1989).
3.6 Statistical analysis
All statistical analyses and graphing were performed in R software (version 3.2.2) (R_Core_Team, 2021). The number of replicates varied depending on the measured parameters. Leaf anatomy study was performed in 3–5 replicates. The remaining measurements were performed on five replicates. If the dependent factor was not normal or homogeneous, the data were transformed before using a two-way ANOVA. Two-way ANOVA followed by contrasts was selected to estimate mean differences (LL plants subtracted from HL plants) for each genotype separately for individual parameters. Genotypes were combined for the ANCOVA followed by contrast analysis to determine if changes in leaf water oxygen isotope ratios co-varied with changes in other physiological and anatomical traits (in terms of mean differences of HL and LL growth treatment). Contrast analysis was considered the appropriate statistical approach to interpret mean differences according to personal consultation provided by the statistical facility at WSU.
According to the Péclet effect theory, the extent to which the Péclet effect is occurring varies with leaf water pool, so the relative importance of each Péclet effect is dependent on its strength and water pool size (Farquhar & Gan, 2003; Holloway-Phillips et al., 2016). The two Péclet models fitted to the fsw versus E data were the xylem and the mesophyll and xylem only Péclet models where the unknowns were Lm, Lv and ϕx for the mesophyll and xylem Péclet model and Lv and ϕx in the xylem only Péclet model. The knowns were fsw (1-Δ18OLW/Δ18Oe) and E. Model fitting was done with the Levenberg-Marquardt Nonlinear Least-Squares Algorithm in R using the minpack.lm package (Elzhov et al., 2022). Starting values were adjusted until the algorithm was able solve for all unknowns.
4 RESULTS
4.1 Maize Zmasft double mutant versus wildtype
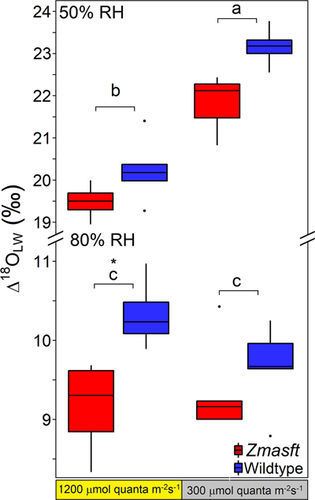
Maize Zmasft double mutants were less enriched in leaf water oxygen isotope enrichment above source water (Δ18OLW) than wildtype in every treatment with a mean difference of 0.8 ± 0.3‰ (Table 2; Figure 2). RH drove differences in Δ18OLW more than light intensity where Δ18OLW was 11.65 ± 0.65‰ lower at 80% than at 50% RH. Differences in the light treatment was only significant at 50% RH, where Δ18OLW was greater under low light. Alternatively, the difference in Δ18OLW was 2.8 ± 0.5 and −0.4 ± 0.4‰ between 300 and 1200 μmol photons m−2 s−1 at 50% and 80% RH, respectively (Table 2, Supporting Information: Table S1; Figure 2). The calculated Δ18Oe values did not significantly differ with genotype but trended lower in wildtype, showing that differences in Δ18Oe were not driving the genotypic differences in Δ18OLW. The calculated Δ18Oe values were much greater at 50% RH than 80% RH, and within each RH they were greater at 1200 PAR than at 300 PAR by 4.3 ± 0.3 at 50% RH and by 2.2 ± 0.4 at 80% RH.
| RH 50% | RH 80% | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Light intensity 300 | Light intensity 1200 | Light intensity 300 | Light intensity 1200 | ||||||||
| Genotype | Genotype | Genotype | Genotype | ANOVA | |||||||
| Trait | WT | Zmasft | WT | Zmasft | WT | Zmasft | WT | Zmasft | RH | PAR | Gen |
| Δ18OLW (‰) | 23.2 ± 0.2 | 22.2 ± 0.5 | 20.2 ± 0.3 | 19.6 ± 0.2 | 9.7 ± 0.2 | 9.2 ± 0.4 | 10.4 ± 0.2 | 9.3 ± 0.3 | *** | ns | *** |
| Δ18Oe (‰)a | 23.1 ± 0.1 | 23.7 ± 0.5 | 27.5 ± 0.1 | 27.9 ± 0.2 | 11.2 ± 0.2 | 11.4 ± 0.2 | 13.9 ± 0.4 | 13.0 ± 0.6 | *** | *** | ns |
| δ18OSW (‰) | –13.8 ± 0.2 | –13.5 ± 0.1 | –15.0 ± 0.1 | –15.2 ± 0.1 | –15.6 ± 0.1 | –14.8 ± 0.1 | –14.9 ± 0.2 | –15.6 ± 0.1 | *** | *** | ns |
| fsw | 0.0 ± 0.01 | 0.06 ± 0.03 | 0.26 ± 0.01 | 0.30 ± 0.004 | 0.14 ± 0.01 | 0.19 ± 0.03 | 0.25 ± 0.03 | 0.28 ± 0.04 | *** | *** | ** |
| E (mmol m–2 s–1) | 1.20 ± 0.07c | 1.22 ± 0.03c | 2.37 ± 0.15a | 3.20 ± 0.21b | 0.69 ± 0.04c | 0.80 ± 0.03c | 2.26 ± 0.09a | 3.04 ± 0.11b | *** | *** | *** |
| gs (mol m–2 s–1) | 0.059 ± 0.00c4 | 0.057 ± 0.003c | 0.107 ± 0.008d | 0.147 ± 0.011c | 0.061 ± 0.004c | 0.069 ± 0.002c | 0.210 ± 0.01b | 0.322 ± 0.008a | *** | *** | *** |
| Lv (mm; Vasc)b | 0 ± 3 | 15.1 ± 6.6 | 43 ± 3 | 40 ± 3 | 63 ± 5 | 87 ± 16 | 43 ± 7 | 39 ± 9 | *** | ns | * |
| Lv (mm; VB)c | 0.2 ± 5.5 | 32 ± 14 | 155 ± NA | NA | 163 ± 18 | 298 ± 86 | 74 ± NA | 75.3 ± 0.04 | ** | ns | . |
| Sd (no./mm2) | NA | NA | NA | NA | 84.8 ± 2.8 | 93.6 ± 1.6 | 116.6 ± 1.9 | 117.8 ± 5.5 | NA | *** | ** |
| Sdadaxial (no./mm2) | NA | NA | NA | NA | 33.6 ± 2.0 | 39.1 ± 1.2 | 51.6 ± 2.3 | 52.3 ± 1.8 | NA | *** | ns |
| Sdabaxial (no./mm2) | NA | NA | NA | NA | 51.12 ± 1.3 | 54.5 ± 1.6 | 65.0 ± 3.0 | 65.5 ± 3.7 | NA | *** | ns |
| Sabaxial length (μm) | NA | NA | NA | NA | 33.3 ± 3.0a | 25.7 ± 1.1b | 26.0 ± 0.6b | 26.6 ± 0.4b | NA | ns | * |
| Sadaxial length (μm) | NA | NA | NA | NA | 34.5 ± 2.8 | 27.2 ± 2.1 | 29.6 ± ± 1.0 | 29.2 ± 0.5 | NA | ns | ns |
| gs/Sd | NA | NA | NA | NA | 0.71 ± 0.04 | 0.69 ± 0.03 | 1.31 ± 0.16 | 2.00 ± 0.29 | NA | *** | ns |
- Note: Stomatal measurements were on plants grown at 80% RH. Results of the main effects of a three-way ANOVA are included, except the stomatal traits. Stomatal traits such as total stomatal density (Sd), abaxial and adaxial stomatal density (Sabaxial and Sadaxial, respectively) and length (Sabaxial length and Sadaxial length, respectively) were analyzed using a two-way ANOVA because they were only measured in plants grown under 80% RH. Significance was shown as ns, *, ** and *** for not significant, p < 0.1, p < 0.05, p < 0.01 and p < 0.001, respectively. For each trait except for gs and E, all interactions between factors in the three-way ANOVAs other than RH x PAR were not significant (Supporting Information: Table S1).
- a Δ18Oe was calculated using Equation (2).
- b Partial volume of leaf total vasculature including all midrib tissue was used to calculate the effective path length in the xylem (Lv) and was 0.50 and 0.49 for WT and Zmasft double mutants, respectively. There was no significant difference between genotypes (p = 0.72), but there was a significant interaction between genotype and light intensity (p < 0.05), where Lv was higher in the Zmasft double mutant only under 300 μmol m–2 s–1. There was a significant interaction between RH and light intensity (p < 0.0001), where Lv was higher under 300 μmol m–2 s–1 at 50% RH than at 80% RH.
- c Partial volume of vascular bundles in the leaf (including the bundle sheath) was used to calculate the effective path length in the xylem (Lv) and was 0.25 and 0.26 for WT and Zmasft double mutants, respectively. Genotypes were marginally different in Lv (p = 0.06), and there was a significant interaction between genotype and light intensity (p < 0.05), where Lv under 300 μmol m–2 s–1 was higher in the Zmasft double mutant.
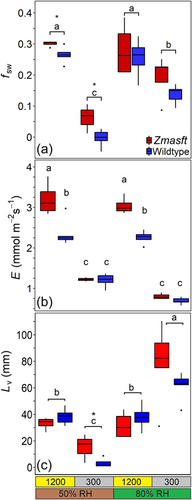
The fractional contribution of source water (unenriched xylem water; fsw) was significantly greater in Zmasft mutants (0.22 ± 0.03) than wild type (0.16 ± 0.03). This difference was most apparent under low light where Zmasft mutants had higher fsw at both 50% and 80% RH (Table 2; Figure 3a). Alternatively, for both genotypes fsw was greater under 1200 compared to 300 µmol quanta m−2 s−1 (0.27 ± 0.1 and 0.1 ± 0.02, respectively), and RH increased fsw under 300 but not under 1200 µmol quanta m−2 s−1 (Table 2; Figure 3a). Transpiration rate (E; mol H2O m−2 s−1) was greater in the Zmasft double mutants than in wildtype across treatments (p < 0.0001), and this difference was most apparent under high light intensity. At high light intensity, E was higher than at low light intensity, and RH only affected E at low light intensity (Table 2; Figure 3b). Stomatal conductance (gs; mol H2O m−2 s−1) was 44% greater in Zmasft double mutant, and this genotypic difference was most apparent at high light intensity (Table 2). Differences in Lv were found between growth light intensities, but the influence of light intensity on Lv was not consistent where Lv was smaller at low light than high light in 50% RH, but the opposite was observed at 80% RH (Figure 3c).
4.2 Rice mixed linkage glucan mutants (cslf6-1 and cslf6-2) versus wildtype
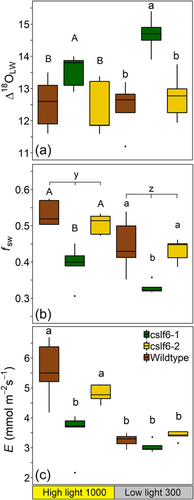
Δ18OLW was significantly higher in cslf6-1 than wild type and cslf6-2 mutants, but this did not appear to be due to higher Δ18Oe, which was lower in cslf6-1 than in wild type and cslf6-2 (Table 3; Figure 4a). Calculated Δ18Oe was greater at 1000 PAR in wild type and cslf6-2 relative to all other light intensity × genotype combinations. Therefore, calculated Δ18Oe values were lower in cslf6-1 compared to cslf6-2 and wild type, contrary to what is expected if differences in Δ18Oe were driving the genotypic differences in Δ18OLW. There was a greater proportion of enriched than unenriched water or lower fsw in cslf6-1 plants (0.36 ± 0.02) compared to wildtype (0.49 ± 0.02) and cslf6-2 (0.48 ± 0.02) mutant (Table 3; mean across all treatments in parentheses). In all genotypes, fsw was greater under high light than low light (0.48 ± 0.02 and 0.40 ± 0.02, respectively; Table 3; Figure 4b). E and gs were greater in wild type and cslf6-2 than cslf6-1 under high light only by 40% and 37%, respectively (Table 3; Figure 4c). Lv was shorter in cslf6-1 when the total vasculature was used as the proportion of leaf water in the xylem (ϕx; Table 3). E and gs were significantly lower under low light compared to high light in wild type and cslf6-2 but were not in cslf6-1 (Table 3; Figure 4c).
| Growth light condition | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1000 | 300 | ANOVA | |||||||
| WT | cslf6-1 | cslf6-2 | WT | cslf6-1 | cslf6-2 | P | G | PxG | |
| Δ18OLW (‰) | 12.5 ± 0.4b | 13.5 ± 0.2a | 12.4 ± 0.4b | 12.1 ± 0.5b | 14.7 ± 0.2a | 12.7 ± 0.3b | ns | *** | ns |
| Δ18Oe (‰)a | 27.02 ± 1.02a | 22.36 ± 0.57b | 24.97 ± 0.17a | 22.01 ± 0.54a | 21.88 ± 0.19b | 22.42 ± 0.21a | *** | *** | ns |
| δ18OSW (‰) | –15.78 ± 0.1 | –15.36 ± 0.14 | –15.76 ± 0.15 | –15.04 ± 0.11 | –15.16 ± 0.19 | –14.87 ± 0.15 | *** | ns | ns |
| fsw | 0.53 ± 0.02a | 0.39 ± 0.02b | 0.5 ± 0.01a | 0.45 ± 0.03a | 0.33 ± 0.01b | 0.43 ± 0.01a | *** | *** | ns |
| E (mmol H2O m–2 s–1) | 5.6 ± 0.4a | 3.5 ± 0.3b | 4.8 ± 0.1a | 3.2 ± 0.1b | 3 ± 0.1b | 3.4 ± 0.1b | *** | *** | ** |
| gs (mol H2O m–2 s–1) | 0.55 ± 0.06a | 0.36 ± 0.04b | 0.5 ± 0.02a | 0.32 ± 0b | 0.3 ± 0.01b | 0.34 ± 0.01b | *** | ** | * |
| gs/Sd (nmols s–1) | 0.74 ± 0.07a | 0.53 ± 0.02b | 0.65 ± 0.02a | 0.65 ± 0.04a | 0.52 ± 0.01b | 0.64 ± 0.04a | ns | ** | ns |
| Lv (mm; Vasc)b | 103 ± 9a | 62 ± 4b | 108 ± 1a | 81 ± 18a | 39 ± 2b | 64 ± 8a | ** | ** | ns |
| Prop. vasculaturec | 0.52 ± 0.04 | 0.50 ± 0.04 | 0.48 ± 0.01 | 0.55 ± 0.04 | 0.57 ± 0.05 | 0.54 ± 0.02 | ns | ns | ns |
| Prop. veins + BSd | 0.23 ± 0.01 | 0.18 ± 0.01 | 0.20 ± 0.01 | 0.21 ± 0.004 | 0.19 ± 0.01 | 0.18 ± 0.01 | ns | * | ns |
| Prop. veins – BS | 0.08 ± 0.002 | 0.07 ± 0.002 | 0.08 ± 0.005 | 0.09 ± 0.005 | 0.08 ± 0.004 | 0.08 ± 0.001 | ns | ns | ns |
| Prop. Xylem | 0.011 ± 0.002 | 0.012 ± 0.001 | 0.015 ± 0.003 | 0.015 ± 0.001 | 0.013 ± 0.004 | 0.015 ± 0.003 | ns | ns | ns |
| Sd (no./mm2) | 745 ± 42 | 735 ± 7 | 730 ± 57 | 503 ± 32 | 557 ± 12 | 524 ± 50 | *** | ns | ns |
| Sdabaxial (no./mm2) | 387 ± 26 | 381 ± 5 | 401 ± 22 | 267 ± 15 | 284 ± 5 | 279 ± 20 | *** | ns | ns |
| Sdadaxial (no./mm2) | 358 ± 24 | 354 ± 2 | 329 ± 23 | 236 ± 15 | 273 ± 10 | 245 ± 18 | *** | ns | ns |
| Sabaxial length (μm) | 13.3 ± 0.4 | 11.9 ± 0.8 | 13.1 ± 0.4 | 14.5 ± 0.6 | 12.4 ± 0.2 | 13.3 ± 0.4 | ns | ns | ns |
| Sadaxial length (μm) | 13.5 ± 0.7 | 12.2 ± 0.7 | 12.9 ± 0.4 | 15.2 ± 0.9 | 13.2 ± 0.3 | 13.3 ± 0.9 | ns | ns | ns |
- Note: fsw is the proportion of unenriched water in bulk leaf water. The calculation of Lv made by using the partial volume of vascular tissue including the entire midrib as θx. A two-way ANOVA was performed to test significance: *p < 0.05, **p < 0.01, ***p < 0.001. Tukey post hoc test represented when PxG interaction was significant. Values with the same superscripted letters are not significantly different. Growth light condition is represented by P (PAR) and genotype by G.
- a Δ18Oe was calculated using Equation (2).
- b Calculation of Lv made by using the partial volume of vascular tissue including the entire midrib as θx.
- c Partial volume of vascular tissue including the entire midrib.
- d WT and cslf6-2 were significantly greater than cslf6-1, and 1000 μmol photons m–2 s–1 was significantly greater than 300 μmol photons m–2 s–1. Proportion of leaf volume found in the vessels and midrib was used for the proportion of xylem water (θx) in the xylem Péclet model.
4.3 Anatomical measurements in maize and rice
The partial volume of the leaf represented by vasculature (midrib and vascular bundles in the blade), veins (both including and excluding the bundle sheath cells) and xylem were not significantly different across genotypes in either maize or rice, except for the partial volume of veins including the bundle sheath in rice (Tables 2 and 3). In rice, the partial volume of veins including the bundle sheath was slightly greater in WT (0.22 ± 0.02) than cslf6-1 (0.18 ± 0.01) and cslf6-2 (0.19 ± 0.01), but there was no difference in anatomy with growth light intensity (Table 3). The mean partial volume of total vasculature (0.50 ± 0.01), veins including the bundle sheath (0.25 ± 0.01), and the veins excluding the bundle sheath (0.11 ± 0.01), and xylem (0.03 ± 0.01) in maize and in rice (0.53 ± 0.04, 0.20 ± 0.03, 0.08 ± 0.01, 0.01 ± 0.02, respectively) showed that about half of the leaf was vasculature tissue, and less than half of the cells associated with vasculature appears to be part of the unenriched water pool (Tables 3 and 4).
| Mean fsw | Mean E (mmol m–2s–1) | Equation (11) Lv (mm)a | Equation (11) ϕxa | Part. vol. total vasculature | Lv (mm) | Prop. V + BS | Lv (mm)b | Part. vol. V-BSc | Part. vol. xylemc | |
|---|---|---|---|---|---|---|---|---|---|---|
| All maize genotypes | 0.19 ± 0.02 | 1.80 ± 0.16 | 57 ± 28 | 0.40 ± 0.11 | 0.50 ± 0.01 | 43 ± 5 | 0.26 ± 0.01 | 111 ± 49 | 0.11 ± 0.005 | 0.03 ± 0.001 |
| Wild type | 0.16 ± 0.03 | 1.59 ± 0.17 | 70 ± 40 | 0.37 ± 0.12 | 0.49 ± 0.01a | 37 ± 6b | 0.26 ± 0.01a | 85 ± 24a | 0.11 ± 0.005a | 0.03 ± 0.004a |
| Zmasft | 0.22 ± 0.03 | 2.04 ± 0.29 | 68 ± 44 | 0.36 ± 0.11 | 0.50 ± 0.01a | 50 ± 9a | 0.25 ± 0.01a | 177 ± 56a | 0.11 ± 0.01a | 0.03 ± 0.001a |
| All rice genotypes | 0.44 ± 0.01 | 3.93 ± 0.20 | 36 ± 8 | 0.68 ± 0.08 | 0.53 ± 0.02 | 53 ± 1 | 0.20 ± 0.01 | N/A | 0.08 ± 0.002 | 0.013 ± 0.001 |
| Wild type | 0.49 ± 0.02 | 4.42 ± 0.45 | 57 ± 15 | 0.60 ± 0.06 | 0.53 ± 0.02a | 87 ± 11a | 0.22 ± 0.01a | N/A | 0.084 ± 0.003a | 0.013 ± 0.001a |
| cslf6-1 | 0.36 ± 0.02 | 3.27 ± 0.18 | 27 ± 16 | 0.76 ± 0.32 | 0.53 ± 0.03a | 50 ± 4b | 0.18 ± 0.01b | N/A | 0.077 ± 0.003a | 0.013 ± 0.002a |
| cslf6-2 | 0.47 ± 0.02 | 4.11 ± 0.24 | 52 ± 16 | 0.59 ± 0.07 | 0.51 ± 0.02a | 77 ± 7a | 0.19 ± 0.01b | N/A | 0.081 ± 0.002a | 0.013 ± 0.002a |
- Note: Values are ± SE. Lv was calculated when ϕx was considered the partial volume of either total vasculature, veins including the bundle sheath (V + BS) or excluding the sheath (V – BS). Means within each species and each column followed by the same letter are not significantly different.
- a The xylem Péclet model converged on values of ϕx and Lv when three negative values of fsw were removed in maize.
- b The xylem Péclet model failed to calculate Lv for rice when ϕx euqalled the partial volume of bundle sheath and veins.
- c The model failed to calculate Lv using partial volume of the veins excluding the bundle sheath cells and the partial volume of the xylem elements as ϕx in both maize and rice.
In maize and rice, stomatal density (Sd) differed with growth light intensity where plants under high light had greater Sd (117.2 ± 1.5 and 737 ± 35, respectively) than those grown under low light (88.7 ± 1.4 and 528 ± 31, respectively) (Tables 2 and 3). The Sd was slightly higher in leaves of Zmasft double mutants (105.7 ± 3.4) than wild type (99.8 ± 3.9), but there were no differences across rice genotypes. Sd on both adaxial and abaxial sides of the leaf was significantly higher under high light, but neither was significantly different across genotypes (Table 2, Supporting Information: Table S1). In maize, abaxial stomata subsidiary cells length was greater in wildtype grown under low light intensity (34.5 µm ± 2.0) than all other conditions (25.7–26.6 µm; Table 2, Supporting Information: Table S1). Abaxial and adaxial pore length did not differ across rice genotypes (Table 3).
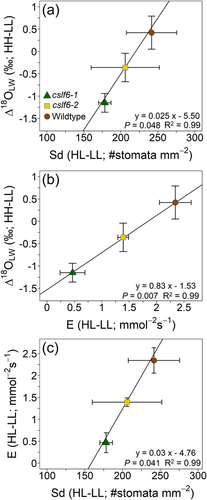
Changes in Δ18OLW between plants grown in high and low light (HL-LL) co-varied with changes in Lv, E, Sd, Sdabaxial and partial volume of veins excluding the bundle sheath (p < 0.05) and partial volume of the total vasculature and gs (p = 0.05; Supporting Information: Table S2). The mean genotypic difference (HL-LL) in Sd and E formed significant linear relationships with Δ18OLW (Figure 5a,b), while E and Sd also formed a linear relationship (Figure 5c). In contrast, the change in Δ18OLW between plants grown at high and low light did not co-vary with changes in Δ18Oe, mesophyll and bundle sheath cell wall thickness (bordering the IAS), leaf thickness, mesophyll cell layers, volume fraction of intercellular air spaces (IAS) per mesophyll area, mesophyll surface area exposed to IAS per unit leaf area (Smes), surface area of chloroplasts exposed to IAS per unit of leaf area (Sc) and leaf dry mass per area (Supporting Information: Table S2, these data were previously published in Ellsworth et al., 2018).
4.4 Mesophyll and xylem Péclet models in maize and rice
All genotypes of maize and rice formed significant positive relationships between fsw and transpiration rate (E), suggesting a Péclet effect in these genotypes (Figure 6a,b). Isotopic steady state was not verified but assumed because the plants were maintained under constant conditions and were not removed for gas exchange measurements. The relationship between fsw and E was not as apparent in the low light treatment alone where fsw but not E varied substantially. When fsw and E were fitted to the mesophyll and xylem Péclet model (Equation 10) to solve for the following three variables: mesophyll L (Lm), xylem L (Lv) and the proportion of xylem water (ϕx), the model in maize and rice converged, but the estimated Lm was essentially 0 (1 × 10−12 to 2 × 10−17; Table 4). Considering that Lm was estimated as ~0, it was assumed that the mesophyll Péclet effect was insignificant, and the Péclet effect was only in the xylem (Equation 11) for maize and rice. In maize, the xylem Péclet model only converged on significant values of Lv and ϕx when the three negative fsw values were removed. Wildtype and Zmasft did not differ in Lv (68 ± 44 mm) from wildtype (70 ± 40 mm; Table 4; Figure 6a). In rice, Lv calculated from the xylem Péclet only model was smaller in cslf6-1 than wild type and cslf6-2. In each species, estimates of ϕx were similar across genotypes, but ϕx was two times larger in rice than in maize (Table 4). In both maize and rice, the xylem Peclet model fit the data better than the other Peclet models (Figure 6a,b).
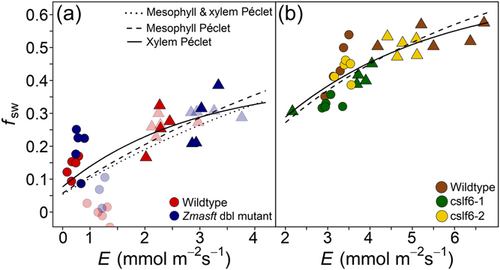
The partial volume of the total vasculature and that of veins including and excluding BS were used to represent ϕx in the xylem Péclet model to calculate Lv. When the partial volume of the total leaf vasculature was used as ϕx in the xylem Péclet model, estimates of Lv were greater for Zmasft double mutants than WT, and this difference was most apparent at low light intensity (Tables 2 and 4). In rice, Lv was lower in cslf6-1 than WT and cslf6-2. These results were similar to modelled Lv (solving for both Lv and ϕx) in rice (Table 4). When the partial volume of the vascular bundles including the bundle sheath was used as ϕx, estimates of Lv were able to be calculated in maize only. A sensitivity analysis of the interaction between Lv and ϕx in the xylem Péclet model showed that ϕx in rice was greater than in maize and that Lv had a large effect on ϕx until it reached its minimum threshold in both maize and rice at approximately 75 mm (Supporting Information: Figure S1).
5 DISCUSSION
Both the string-of-lakes and Péclet effect models of Δ18OLW consider leaf anatomical traits that influence water movement within the leaf and can provide insights into how changes in cell wall properties influence this water movement. Within the string-of-lakes model, reduction in channelization or barriers to water movement can affect the separation of the enriched and unenriched water pools, thereby influencing the relative contribution of the unenriched xylem water pool to Δ18OLW. In this respect, unenriched xylem water pool size may not be restricted by anatomical structures alone but by how the cell wall properties influence water movement through those anatomical structures. Alternatively, the Péclet effect model is largely dependent on E and leaf anatomical traits that influence the effective mixing between unenriched xylem water and enriched water from the sites of evaporation. The observed positive relationship between the fractional contribution of source water (unenriched xylem water; fsw) and transpiration rate (E) shown here in all genotypes of maize and rice indicates a Péclet effect, assuming the leaves were in isotopic steady state. While we did not explicitly verify that the leaves were in isotopic steady state, the plants were grown under constant growth chamber conditions that were maintained during the brief gas exchange measurements, which suggests that the leaves were at an isotopic steady state (Song, Loucos, et al., 2015; Song, Simonin, et al., 2015). However, it is important to note that leaves may not maintain isotopic steady state under all growing conditions (Simonin et al., 2013). Nonetheless, it is reasonable to evaluate the Péclet effect theory to better understand the relationship between fsw and E in cell wall mutants.
According to Farquhar and Gan (2003), Δ18OLW is the accumulation of the Péclet effects of each water pool such as mesophyll and xylem pools relative to the fraction of total leaf water pool present in each pool. However, several studies have found that the xylem water pool is the primary location of the Péclet effect, especially in grasses (Farquhar & Gan, 2003; Gan et al., 2002, 2003; Helliker & Ehleringer, 2000; Holloway-Phillips et al., 2016). When we modelled the Péclet effect for both the mesophyll and xylem and xylem only, the Lm estimates were nearly 0, indicating that the mesophyll Péclet effect did not describe the fsw versus E relationship. Therefore, the primary location of the Péclet effect for both the maize and rice data presented here was in the xylem and not the mesophyll. Additionally, for both maize and rice, the cell wall modifications influenced Lv more than ϕx, which indicates that these mutations affect the water path and flow rather than xylem water volume. Similarly in a water limitation study, L apparently changed more than ϕx when fsw was estimated from leaf anatomy (Timofeeva et al., 2020). However, because anatomy and physiology can influence water movement within the leaf, care must be taken when estimating what anatomical features reflect fsw.
Xylem pool size as a function of leaf anatomy and physiology may explain differences in fsw values observed between C3 rice and C4 maize and may also affect the interpretation of the string-of-lakes model. The partial volume of the total vasculature was similar between C4 maize and C3 rice, yet measured fsw and modelled ϕx were two times higher in C3 rice than in C4 maize. This difference may be because C3 species have greater water movement outside the xylem as a result of higher outside xylem conductivity and increased number of sites of evaporation (Buckley et al., 2011; Sonawane & Cousins, 2020). In rice, greater outward movement of water from the xylem means that the isotopic signature of the xylem water extended beyond the xylem and into the surrounding ground tissue and reduced the enrichment of xylem water along the leaf blade. In contrast, C4 species generally have greater suberization of the bundle sheath and lower bundle sheath surface area adjacent to intercellular airspace than C3 species, and these anatomical features reduce water movement from the vasculature (Mertz & Brutnell, 2014; Sonawane & Cousins, 2020). These features in C4 maize restricted flow of unenriched water, reducing its influence on surrounding tissue and, ultimately, Δ18OLW. The consequence of this is that the proportion of unenriched water in maize leaves is more closely related to the partial volume of the veins and bundle sheath, while in C3 rice the influence of unenriched water is more related to the partial volume of total vasculature. Previous studies have speculated on the contribution of ground tissue to the effective xylem pool size and its dependency on anatomic and physiological features of a given species (Gan et al., 2003; Holloway-Phillips et al., 2016; Song, Loucos, et al., 2015). Our data suggests that the lower Δ18OLW in the C3 grass species compared to the C4 species may depend as much on the facility of water movement out of the xylem and outside xylem conductivity as on anatomical features such as interveinal distance (vein density) that affect xylem water pool size and water exchange between the xylem and mesophyll. The two-pool model has been using to study 18O ecohydrology in C3 grasslands (Hirl et al., 2019), but care should be taken when modelling mixed C3/C4 plant communities because fsw can differ substantially between C3 and C4 photosynthetic groups.
5.1 Suberin mutation and Δ18OLW
In the Zmasft maize double mutants, E, fsw and potentially Lv were affected by the compromised ultrastructure of the bundle sheath cells and increased apoplastic permeability that occurred at the interface of suberin lamellae and adjoining polysaccharide cell walls (Mertz et al., 2020). Suberin is considered essential as a functional barrier to unrestricted diffusion of water across cell walls (Botha et al., 1982; Mertz & Brutnell, 2014; Schönherr, 1982). Consequently, overall greater water flow out of the xylem in Zmasft double mutants increased the influence of xylem water on Δ18OLW (higher fsw values and lower Δ18OLW), and this translated into more water available for E under high light (and higher VPD) (Figure 7a,b). The influence of xylem water on Δ18OLW was a function of more than leaf anatomy alone but rather the interaction of leaf anatomy and physiology, as was seen with effect of the interaction between RH and light on Δ18OLW and fsw. This is problematic for the string-of-lakes model, which assumes that the xylem pool size (fsw) is a fixed parameter dependent only on leaf anatomy and independent of changes in water movement out of the xylem and E.
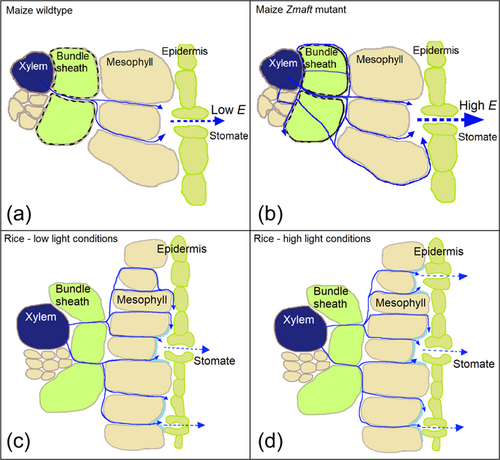
According to the Péclet effect theory, Lv increased along with E in the Zmasft double mutants. This could be due to the increased apoplastic permeability and less restricted water flow in the mutants that allowed water to move down other longer and more tortuous paths that were unavailable in the WT plants. This mutation appears to have created a water flow condition similar to C3 grass species where greater outside xylem conductivity increased through greater flow out of xylem and more available water channels, leading to greater Lv (Sonawane & Cousins, 2020). Nevertheless, it is not known if greater E and fsw in the Zmasft double mutants are both the consequence of greater water flow out of the xylem or if the cell wall changes lead to a greater E that subsequently increased fsw. For example, under low light E did not change much yet fsw differed between treatments and genotypes, showing some uncoupling between E and fsw. As has been seen previously, suberin and suberin lamellae play a role in the plant's ability to regulate transpiration (Schreiber, 2010; Vishwanath et al., 2015), and our results further demonstrate that suberin changes cell wall properties and influences Δ18OLW. In other studies, levels of suberization on the endodermis increased with needle age, and in three studies young needles had higher E and lower Δ18OLW than in older needles (Allison et al., 1985; Barnard et al., 2007; Shu et al., 2008). This is expected if the degree of suberization negatively affected E, such that low suberization increases the influence of unenriched xylem water on surrounding leaf tissues, lowering Δ18OLW. In contrast, another study found that young needles were less suberized and had higher E and Δ18OLW but much shorter L (Roden et al., 2015). In this study, low suberization may have allowed for more direct water movement within the leaf. This would have reduced Lv and coupled with low E led to greater back diffusion of mesophyll water into the xylem, which reduced the influence of unenriched xylem water on Δ18OLW.
5.2 Mixed linkage glucan (MLG) and Δ18OLW
The data presented here suggests that cslf6-1 and cslf6-2 genotypes varied in their physiological and isotopic response to growth light intensity. Although these genotypes are transposon insertion lines created in the same Nipponbare background, they differed in many traits such as the concentration of various amino acids and stem and seedling cellulose content (Upadhyaya et al., 2006; Vega-Sánchez et al., 2012). Genotype cslf6-1 had lower gs, E, and both Amax and Jmax on a per gram basis than cslf6-2 (Ellsworth et al., 2018). In this study the mutant cslf6-1 was smaller, more fragile with thinner leaf blades than cslf6-2, and Lv and Δ18OLW were lower in cslf6-1 than cslf6-2. Because all plants were grown simultaneously in the same growth chamber, these differences between cslf6-1 and cslf6-2 genotypes could be attributed to pleiotropic effects that resulted from either subtle differences in cell wall composition or secondary effects of the mutation (Vega-Sánchez et al., 2012). Genetic background effects such as the differences observed between Nipponbare and the isogenic WT siblings of these different lines could have contributed to the observed differences between cslf6-1 and cslf6-2 (Vega-Sánchez et al., 2012). For example, shorter Lv in cslf6-1 than in cslf6-2 was not expected based on the results that gm was much lower in both cslf6 mutants, which suggests that the effective path length of gaseous CO2 is different than that of liquid water (Ellsworth et al., 2018). The effective path length may be shorter in cslf6-1 because it had thinner leaf blades and lower E (and related water flow out of the xylem) that increased mesophyll and xylem water exchange, which reduced the mixing length between enriched mesophyll water and unenriched water in the xylem.
The genotypic response of Δ18OLW to growth light intensity can be explained, in part, by the corresponding response in stomatal density (Sd). This is because, according to the Péclet effect theory, greater Sd reduces the Péclet number because the distance from the xylem to the stomatal cavity (L) is reduced, subsequently increasing Δ18OLW. This relationship can, however, be masked if greater Sd increases E as well (Liang et al., 2018; Sternberg & Manganiello, 2014). Nonetheless, differences in Sd and pore length were sufficient to drive species-level differences in Δ18OLW between mangrove and nearby freshwater species and across Arabidopsis stomatal density lines (Larcher et al., 2015; Liang et al., 2018; Rosado et al., 2013; Sternberg & Manganiello, 2014). In this study, it was the magnitude of the differences in Sd and not the absolute Sd values that were proportional to the response in Δ18OLW and E (Figure 7c,d). Therefore, changes in Sd determined the relative differences observed in Δ18OLW of these rice genotypes, but Sd alone cannot be used to predict the magnitude of Δ18OLW because it is one of several factors influencing Δ18OLW. Conversely, the elaborated string-of-lakes model can explain these results as well because an increase in Sd (and increase in inter-stomatal cavity volume) would provide more evaporative surface area and potentially increase the leaf water volume directly affected by the sites of evaporation and influenced by Δ18Oe (Figure 7c,d). In this case, it is not the effect of the changes in L or E that govern the relative influence of Δ18Oe on Δ18OLW but rather the increase in the volume of water pool size that is influenced by evaporative enrichment from a greater evaporative surface area. In any case, the fact that the partial volume of the veins along with Sd, Lv and E co-varied with Δ18OLW suggests that Δ18OLW was significantly influenced by both the path of water transport from the xylem to the stomates and the water flow rate out of the xylem.
6 CONCLUSIONS
Leaf cell wall and other anatomical properties can affect Δ18OLW altering water flow out of the xylem to the stomata and, ultimately, E. Leaf properties that limit water flow out of the vasculature in C4 species likely are a major influence on the difference in Δ18OLW between C3 and C4 grass species. Water movement out of the xylem also influences the application of the string-of-lakes and Péclet effect models because the xylem water pool becomes a function of not only the vascular volume based on anatomy but factors influencing water movement out of the xylem. Stable isotope analysis and models of Δ18OLW can potentially be used to better understand how leaf traits influence water movement in the leaf. For example, when studying genes associated with Sd, differences in water movement may not be discernable from measures of Sd per se, but nevertheless differences in Sd can influence Δ18OLW and indicate a subtle response of Sd to genotype or environmental conditions. Further, integration between leaf water transport through and outside the xylem with stable isotope theory will facilitate the development of a physiologically and anatomically explicit water transport model.
ACKNOWLEDGEMENTS
Our research is supported by the Office of Biological and Environmental Research in the DOE Office of Science (DE-SC0008769) and by the Russian Science Foundation (16-16-00089) for N. K. (leaf anatomy study and anatomical traits analysis). We are grateful to the Core Facility Center “Cell and Molecular Technologies in Plant Science” of Komarov Botanical Institute and Franceschi Microscopy and Imaging Center of Washington State University for use of their facilities. Seed Oryza sativa, Nipponbare cultivar (wild type) and mutants cslf6-1 and cslf6-2 were kindly provided by Drs Miguel E. Vega-Sánchez and Pamela C. Ronald, Joint BioEnergy Institute, Emeryville, California 94608 and UC Davis Genome Center and Plant Pathology, University of California, Davis, California 95616.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.