Utilising natural diversity of kinases to rationally engineer interactions with the angiosperm immune receptor ZAR1
Abstract
The highly conserved angiosperm immune receptor HOPZ-ACTIVATED RESISTANCE1 (ZAR1) recognises the activity of diverse pathogen effector proteins by monitoring the ZED1-related kinase (ZRK) family. Understanding how ZAR1 achieves interaction specificity for ZRKs may allow for the expansion of the ZAR1-kinase recognition repertoire to achieve novel pathogen recognition outside of model species. We took advantage of the natural diversity of Arabidopsis thaliana kinases to probe the ZAR1-kinase interaction interface and found that A. thaliana ZAR1 (AtZAR1) can interact with most ZRKs, except ZRK7. We found evidence of alternative splicing of ZRK7, resulting in a protein that can interact with AtZAR1. Despite high sequence conservation of ZAR1, interspecific ZAR1-ZRK pairings resulted in the autoactivation of cell death. We showed that ZAR1 interacts with a greater diversity of kinases than previously thought, while still possessing the capacity for specificity in kinase interactions. Finally, using AtZAR1-ZRK interaction data, we rationally increased ZRK10 interaction strength with AtZAR1, demonstrating the feasibility of the rational design of a ZAR1-interacting kinase. Overall, our findings advance our understanding of the rules governing ZAR1 interaction specificity, with promising future directions for expanding ZAR1 immunodiversity.
1 INTRODUCTION
Plant pathogens present a significant threat to global agriculture and food security. Emerging pathogens that evade population-level immunity may require novel approaches to achieve resistance. Plants achieve immunity against pathogens through two interconnected layers of defence (Jones & Dangl, 2006; Ngou et al., 2021; Yuan et al., 2021). Initial resistance occurs at the cell surface, where pattern recognition receptors bind pathogen molecules and initiate pattern-triggered immunity (PTI). Some bacterial pathogens including Pseudomonas syringae and Xanthomonas spp. can secrete proteins known as type III secreted effector proteins (T3SEs) into the plant cell, where they promote virulence and suppress PTI. Plants have intracellular nucleotide-binding leucine-rich repeat receptors (NLRs) that can recognise pathogen effectors, resulting in effector-triggered immunity (ETI). NLRs recognise effectors either through direct NLR-effector interaction or by ‘guarding’ for effector modification of host proteins (Jones et al., 2016). ETI is often associated with a macroscopic programmed cell death known as the hypersensitive response (HR).
HOPZ-ACTIVATED RESISTANCE1 (ZAR1) is a highly conserved NLR capable of recognising diverse T3SEs from several bacterial species (Lewis et al., 2010). The molecular mechanisms of ZAR1-mediated ETI have been explored extensively in Arabidopsis thaliana. ZAR1 recognises several T3SEs through modification of ZAR1-associated receptor-like cytoplasmic kinases (RLCKs). Several members of the RLCK-XII family of pseudokinases, including HopZ-ETI-deficient1 (ZED1), and ZED1-related kinases (ZRKs) interact with A. thaliana ZAR1 (hereafter AtZAR1) in the absence of pathogen effectors (Lewis et al., 2013; Martel et al., 2020; Seto et al., 2017; Wang et al., 2015). Some T3SEs modify kinases in the RLCK-VII family, including PBS1-like kinases (PBLs) to trigger PBL-ZRK interactions and ultimately AtZAR1 activation (Seto et al., 2021; Wang et al., 2015). However, other T3SEs such as HopZ1a modify ZRKs directly leading to activation independent of PBLs (Lewis et al., 2013). Overall, ZAR1 achieves recognition of diverse T3SEs by guarding an initial layer of ZRKs which interact with bacterial T3SEs either directly or through a second layer of PBLs.
Several NLRs ‘guard’ host proteins to detect modification by T3SEs (Ade et al., 2007; Axtell & Staskawicz, 2003; Chung et al., 2011; Gutierrez et al., 2010; Liu et al., 2011; Mackey et al., 2002). For example, the A. thaliana NLR RPS5 guards AVRPPHB SUSCEPTIBLE 1 (PBS1), and triggers ETI when PBS1 is cleaved by P. syringae effector AvrPphB (Ade et al., 2007). However, unlike many NLRs, ZAR1 can respond to a unique breadth of T3SEs that have different enzymatic functions and that come from several bacterial species. ZAR1 immunodiversity is facilitated through the guarding of multiple ZRKs (Lewis et al., 2013; Martel et al., 2020). In A. thaliana, the ZRK family consists of 13 members with ZED1, RKS1, ZRK2 and ZRK3 having a demonstrated role in effector recognition (Lewis et al., 2013; Martel et al., 2020; Seto et al., 2017; Wang et al., 2015). The P. syringae T3SE HopZ1a directly interacts with and acetylates ZED1, triggering ETI though AtZAR1 (Lewis et al., 2013). The P. syringae T3SE HopX1i is also recognised through ZED1, but requires an additional RLCK-VII, suppressor of ZED1-D (SZE1) for AtZAR1-mediated ETI (Martel et al., 2020). AtZAR1 also mediates ETI through ZED1 in response to P. syringae T3SE HopZ5, through ZRK3 for HopF1r, HopO1c, HopO1b and HopO2a and through ZRK2 for HopBA1 (Martel et al., 2020, 2021; Zheng et al., 2022). While PBL27 has been shown to be required for HopF1r recognition, no PBLs have been identified for recognition of other P. syringae T3SEs (Seto et al., 2021). In addition to diverse P. syringae effectors, AtZAR1 can recognise the Xanthomonas campestris T3SE AvrAC, which uridylates PBL2, triggering an interaction between PBL2 and RKS1 and the activation of AtZAR1 (Wang et al., 2015). While ZED1, RKS1 and ZRK3 have been shown to interact directly with AtZAR1, no direct interaction between ZRK2 and AtZAR1 has been observed (Lewis et al., 2013; Seto et al., 2017; Wang et al., 2015). Although they have no known role in effector recognition, ZRK6 and ZRK15 have also been shown to interact with AtZAR1 (Wang et al., 2015). A recent review of the ZAR1 effector recognition system highlights these effector recognition pathways in additional detail (Breit-McNally et al., 2022).
In addition to mediating extensive immunodiversity within A. thaliana, ZAR1 is functionally conserved across angiosperms (Baudin et al., 2017; Schultink et al., 2019). We have previously shown that the Nicotiana benthamiana ZAR1 ortholog (hereafter NbZAR1) can recognise HopZ1a when A. thaliana ZED1 is transiently expressed in N. benthamiana, and that silencing of NbZAR1 abrogates recognition of HopZ1a (Baudin et al., 2017). Recognition of HopZ1a by NbZAR1 requires the catalytic activity of HopZ1a, as observed in A. thaliana (Baudin et al., 2017; Lewis et al., 2008, 2010). A forward genetic screen in N. benthamiana identified a ZRK homologue and showed that NbZAR1 is critical for mediating the recognition of X. perforans effector XopJ4 (a HopZ family member) (Schultink et al., 2019), which further supports the conservation of ZAR1 function. This suggests that ZAR1-mediated guarding of ZRKs is conserved across angiosperms. Transient overexpression of RKS1 with ZAR1 orthologs from multiple eudicot species can result in autoactive cell death of A. thaliana protoplasts (Gong et al., 2022), suggesting that coevolution of specific pairs within a species regulates inadvertent activation.
AtZAR1 is a canonical NLR comprised of an N-terminal coiled-coil domain (CC, hereafter AtZAR1CC), a central nucleotide-binding domain (NBD, hereafter AtZAR1NBD) and a C-terminal leucine-rich repeat domain (LRR, hereafter AtZAR1LRR; Baudin et al., 2019; Lewis et al., 2010). The cryo-electron microscopy (EM) structure of AtZAR1 during three stages of activation upon AvrAC recognition was a breakthrough for our understanding of ZAR1-ZRK interactions and ZAR1 molecular function. In the absence of X. campestris effector AvrAC, RKS1 interacts with AtZAR1 primarily through AtZAR1LRR (Wang, Hu, et al., 2019; Wang, Wang, et al., 2019). We have previously demonstrated that AtZAR1LRR is required and sufficient for ZED1 interaction in planta (Baudin et al., 2017), which supports the importance of ZAR1LRR for ZRK interaction. In addition, we have previously shown that a weakened AtZAR1LRR-ZED1 interaction is correlated with a loss of HR triggered by HopZ1a (Baudin et al., 2021). When PBL2 is modified by AvrAC, a PBL2-RKS1-AtZAR1 complex forms, resulting in stabilisation of the RKS1 activation loop which then allosterically clashes with AtZAR1NBD (Wang, Wang, et al., 2019). This clash triggers extensive conformational changes, causing AtZAR1 to oligomerize with other activated AtZAR1 complexes to form a wheel-like pentamer, termed the ‘resistosome’ (Wang, Hu, et al., 2019). In the resistosome conformation, RKS1 interacts with AtZAR1 exclusively through AtZAR1LRR (Wang, Hu, et al., 2019). While the AtZAR1 structure is invaluable for understanding the AtZAR1-RKS1 interaction, the determinants of ZAR1 interaction specificity for guardees is not well understood.
Plant kinase repertoires are generally larger than those in other Eukaryotes and are common targets of T3SEs (Rufián et al., 2021; Schreiber et al., 2021; Shao et al., 2003; Zhang et al., 2010). The A. thaliana proteome is made up of almost 5% kinases (Lehti-Shiu et al., 2012), and yet AtZAR1 appears to achieve specificity for a small subset of the A. thaliana kinase repertoire (Lewis et al., 2014). Understanding the determinants of ZAR1 specificity for kinase guardees may allow for the identification of additional ZAR1-mediated pathogen recognition components, as well as the rational design of a ZAR1 guardee as a first step toward generating a novel ZAR1-mediated effector recognition pathway. Investigating ZAR1 interaction specificity may also provide insight into the evolution of the ZAR1-ZRK pathogen recognition system. Here, we show that ZAR1 can achieve interaction specificity for kinases both within and outside of the A. thaliana ZRK family (hereafter ZRKs). We also show that interspecific pairing of specific ZRKs with NbZAR1 results in an autoactive HR-like phenotype. Using AtZAR1-kinase interaction data, we demonstrate the feasibility of rational guardee design by increasing the interaction strength between AtZAR1LRR and ZRK10. Overall, we identify key determinants of ZAR1-kinase interaction specificity using natural diversity of kinases.
2 MATERIALS AND METHODS
2.1 Molecular cloning
All gene fragments used in cloning were amplified with Phusion polymerase (New England Biolabs), and all constructs were confirmed by sequencing. All ZRKs, in addition to PBL2, and WAKL2 were amplified with a 5′XhoI site. The pMac16 vector was modified from pBD to contain a C-terminal 3xFlag tag between the StuI and SpeI sites and has been previously described (Baudin et al., 2019). The pMac16 vector was digested with XhoI and StuI to insert ZRKs, WAKL2 and PBL2. BIK1 was amplified to contain attB1 and attB2 sites, then cloned into a pDonr207 vector through a BP reaction. We generated a Gateway-compatible pMac16 vector (hereafter, pMac16-GW) using the Gateway Conversion Kit (Invitrogen) to insert a Gateway cassette into StuI digested pMac16. We then used the LR reaction to clone BIK1 into this vector. The LRR domain of NbZAR1 (hereafter NbZAR1LRR) was amplified with a 5′XhoI site and cloned into pMac15, a vector modified from pBD to contain a C-terminal 5xMyc tag between the StuI and SpeI sites (Baudin et al., 2017). The ZED1, AtZAR1, AtZAR1LRR, NbZAR1, At5g48620 and virus-induced gene silencing (VIGS) constructs were described in previous studies (Baudin et al., 2017, 2021). The dexamethasone-inducible HopZ1a construct was described previously (Lewis et al., 2008). For site-directed mutagenesis, ZRK10 was amplified to contain attB1 and attB2 and cloned into pDonr207 using the BP reaction. Mutations were introduced using the Q5 Site-directed Mutagenesis Kit (New England Biolabs). ZRK10 point mutants were cloned into pMac16-GW using the LR reaction. The AtZAR1 constructs used for the genetic transformation of A. thaliana were designed by amplifying a genomic fragment of AtZAR1 containing 1 kb of the promoter region and the coding sequence, or the coding sequence and a 3xFlag tag (Baudin et al., 2021). The fragments were amplified by PCR to add a 5′SalI site and an attB1 site, and a 3′NotI site and an attB2 site. The resulting promoter-AtZAR1-3xFlag or promoter-AtZAR1 products were first cloned into pBS in a three-body ligation, followed by a BP reaction into pDONR207 and an LR reaction into pEarleyGate303 (Earley et al., 2006).
2.2 Plant material and growth conditions
N. benthamiana and A. thaliana plants were grown as previously described (Baudin et al., 2017). N. benthamiana seeds were sterilised in 10% bleach and germinated on soil. A. thaliana seeds were vapour sterilised and germinated on 0.5X Murashige and Skoog (MS) 0.8% agar media. N. benthamiana seedlings were transplanted on Sunshine #1 (Sun Gro Horticulture) soil supplemented with 14:14:14 Osmocote and grown in a growth chamber at 22°C with 9 h of light (∼130 µEm−2 s−1). For A. thaliana, Sunshine #1 soil (lacking RSi) was used and supplemented with 20:20:20 fertiliser (Hassan et al., 2018).
2.3 Generation and validation of transgenic lines
A. thaliana zar1-1 mutants (Lewis et al., 2010) were transformed with native promoter-driven AtZAR1 carrying an in-frame 3xFlag epitope tag or a native promoter-driven AtZAR1 construct lacking the 3xFlag tag. Transformations were conducted via the floral dip method using A. tumefaciens GV3101. T1 individuals were selected by Basta resistance and confirmed to be zar1-1 by PCR. Candidate T1 lines were screened for restoration of the HR when plants were pressure-infiltrated with P. syringae pathovar tomato DC3000 carrying hopZ1a (Lewis et al., 2010). The number of insertions in the T2 generation was determined based on the percentage of plants that survived growth on 0.5X MS containing 6 mg/L bialaphos, and T2 lines with one insertion were selected. Homozygous lines in the T3 generation were identified based on the percentage of plants surviving on 0.5X MS containing 6 mg/L bialaphos. Protein expression in T3 lines was checked via anti-Flag western blot.
2.4 N. benthamiana transient expression, VIGS and HR assays
For transient gene expression, A. tumefaciens GV2260 was grown overnight at 28°C in LB containing appropriate antibiotics. Bacteria were resuspended in induction media (10 mM MES pH 5.6 and 200 μM of acetosyringone) and incubated at room temperature in the dark for approximately 4 h. The optical density at 600 nm (OD600) of each culture was adjusted to 0.25. The leaves of 4–7-week N. benthamiana plants were infiltrated with bacteria using a needle-less syringe. All constructs used for N. benthamiana transient expression were inducible with dexamethasone as previously described (Baudin et al., 2017). Twenty-four hours post infiltration, 40 μM dexamethasone was sprayed on leaves. To assay for the HR, plants were scored 24 h after dexamethasone induction based on four categories (Figure 3a). The scores were defined as no HR, weak HR (<25% of leaf area), medium HR (<75% of leaf area) or strong HR (75%–100% of leaf area). N. benthamiana plants silenced for NbZAR1 or GUS were prepared as previously described (Baudin et al., 2017). Briefly, A. tumefaciens was prepared as described for transient assays. The fourth and fifth leaves of the 3-week-old N. benthamiana plant were infiltrated completely with bacteria using a needle-less syringe. Plants were used 3 weeks after initial VIGS infiltrations.
2.5 Liquid chromatography-tandem mass spectroscopy (LC-MS-MS)
For each pAtZAR1:AtZAR1-3xFlag or pAtZAR1:AtZAR1 line, 6–7-week-old plants were sprayed with PtoDC3000 carrying hopZ1a. Bacteria for spray inoculation were resuspended in 10 mM MgCl2 with 0.02% Silwet L-77, adjusted to OD600 of 0.8, and sprayed until leaves were wet, as previously described (Hassan et al., 2018). The total protein was extracted from 3 g of leaf tissue harvested 3 days after spray inoculation, and the mixture was incubated with magnetic beads coated with anti-Flag antibodies. The beads were washed three times with low salt buffer (50 mM HEPES, 50 mM NaCl, 10 mM ethylene diamine tetraacetic acid [EDTA], pH 7.5), three times with high salt buffer (50 mM HEPES, 150 mM NaCl, 10 mM EDTA, pH 7.5) and twice with phosphate buffer on a magnetic stand. The low salt and high salt buffers were supplemented with a homemade proteinase inhibitor cocktail (1 mM phenylmethylsulfonyl fluoride, 1 mg/mL leupeptin [Sigma-Aldrich L2023], 1 mg/mL aprotinin [Sigma-Aldrich A6191], 1 mg/mL antipain [Sigma-Aldrich A1153], 1 mg/mL chymostatin [Sigma-Aldrich C7268] and 1 mg/mL pepstatin [Sigma-Aldrich P5315]). The beads were resuspended in 20 µL of ammonium bicarbonate buffer with 5% of acetonitrile. Next, 10 µL of 15 mM dithiothreitol (DTT) was added and the reaction was incubated for 1 h at 37°C, followed by the addition of 40 µL of 22 mM iodoacetamide and another 1 h incubation in the dark. Last, 20 µL of 22 mM DTT and 10 µL of 100 ng/µL trypsin (Promega) were added, and the reaction was incubated overnight at 37°C. The tryptic digest was centrifuged to pellet the magnetic beads. The peptide-containing supernatant was removed and placed into a 96-well plate.
The peptides were analysed using a Thermo Scientific, Orbitrap Elite mass spectrometer (Thermo Fisher Scientific). Proteins were identified with Mascot Server (Matrix Science Inc.) while Scaffold (version Scaffold_4.5.1, Proteome Software Inc.) was used to validate MS/MS-based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 95% probability. For additional details on mass spectrometry and data analysis, see supplemental materials and methods.
2.6 Coimmunoprecipitation experiments
Tissue samples were harvested from at least two separate N. benthamiana plants 48 h after A. tumefaciens infiltration and 24 h after dexamethasone induction. For co-IP experiments, 3 cm2 of tissue was collected. For experiments involving RKS1 and ZRK13, NbZAR1-VIGS plants were used to avoid cell death.
Tissue samples were ground using liquid nitrogen and homogenised in 1 mL IP1 buffer (50 mM HEPES, 50 mM NaCl, 10 mM ethylene diamine tetraacetic acid (EDTA), 0.2% Triton X-100 and 0.1 mg mL−1 Dextran [Sigma-Aldrich D1037], pH 7.5). Samples were incubated with 5 µL Myc-Trap magnetic beads (ChromoTek) for 2.5 h on a spinning wheel at 4°C. Beads were subjected to four quick washes (tubes inverted six times) followed by two long washes (5 min on a spinning wheel) with IP2 (50 mM HEPES, 250 mM NaCl for ZAR1LRR or 450 mM NaCl for ZAR1, 10 mM EDTA and 0.1% Triton X-100, pH 7.5). Both IP1 and IP2 were supplemented with the same homemade protease inhibitor cocktail as for the mass spectrometry experiment. The beads and input fraction were boiled with Laemmli buffer, and each fraction was separated on a 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis. Proteins were detected using an anti-Myc antibody (Abcam ab32072), or an anti-Flag antibody (Sigma-Aldrich F1804) followed by a secondary antibody coupled with horseradish peroxidase.
2.7 Homology modelling, binding affinity, phylogenetic and selection analysis
Chimera was utilised to identify contacts between RKS1 and AtZAR1 based on the inactive AtZAR1-RKS1 cryo-EM structure (PDB:6j5w; Pettersen et al., 2004; Wang, Hu, et al., 2019; Wang, Wang, et al., 2019). MutaBind2 was used to predict the change in binding affinity (ΔΔG) resulting from every possible mutation in RKS1 in the inactive AtZAR1-RKS1 cryo-EM structure (Zhang, Chen, et al., 2020). Results were converted into a heatmap using the ggplot2 package in R (R Core Team, 2019; Wickham, 2016). To test for selection, kinase protein sequences were aligned using MUSCLE, and columns with gaps were removed with Belvu (Edgar, 2004; Sonnhammer & Hollich, 2005). To generate a codon alignment, nucleotide sequences were aligned to the corresponding protein alignments using PAL2NAL (Suyama et al., 2006). The Datamonkey web interface was used to test for selection within codon alignments using adaptive Branch Site REL (aBSREL), and contrast fixed effects likelihood (Contrast-FEL) hyphy models (Kosakovsky Pond & Frost, 2005; Kosakovsky Pond et al., 2020; Smith et al., 2015; Weaver et al., 2018).
The A. thaliana kinase phylogeny was generated by searching the A. thaliana proteome in Uniprot using the Pfam protein kinase Hidden Markov Model (PF00069). Significant matches were aligned using MUSCLE, and columns and sequences with excessive gaps were removed using Belvu (Edgar, 2004; Sonnhammer and Hollich, 2005). A maximum likelihood tree was constructed using RAxML and the tree was viewed in iTOL (Letunic & Bork, 2021; Stamatakis, 2014).
Homology models of AtZAR1 in complex with BIK1, PBL2 and WAKL2KIN were generated in Swiss-model with the AtZAR1-RKS1 structure as a template for modelling (PDB: 6j5w) (Wang, Wang, et al., 2019; Waterhouse et al., 2018). Homology models were uploaded to the PRODIGY web server, and binding affinity (ΔG; kcal/mol) and dissociation constant (Kd; M) were predicted at 25°C (Xue et al., 2016).
3 RESULTS
3.1 AtZAR1 interacts with multiple ZRKs under native conditions in A. thaliana
To determine which ZRKs interact with AtZAR1 during immune activation, we transformed A. thaliana zar1-1 plants with a native promoter-driven AtZAR1 construct carrying an in-frame 3xFlag epitope tag (hereafter pAtZAR1:AtZAR1-3xFlag). We also generated control lines with a native promoter driven AtZAR1 construct lacking the 3xFlag tag (hereafter pAtZAR1:AtZAR1). We screened T1 and T2 plants for Basta resistance (Supporting Information: Figure S1), presence of the transgene, the zar1-1 genotype, protein expression in the tagged lines and complementation of HopZ1a recognition when plants were pressure-infiltrated with P. syringae pv. tomato DC3000 (PtoDC3000) carrying hopZ1a. We confirmed that AtZAR1 protein expression was maintained in the T3 lines and showed that the lines were still complemented for the HopZ1a-triggered HR (Figure 1a).
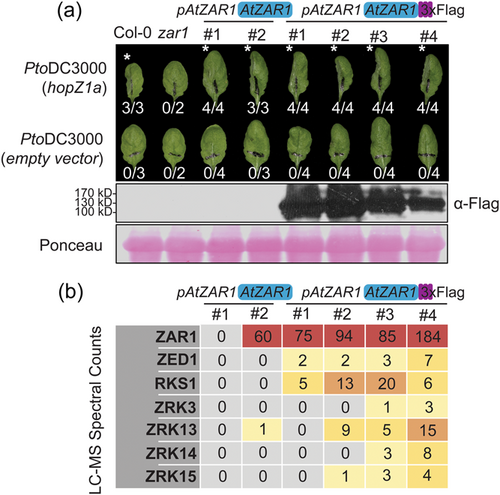
Next, we selected four pAtZAR1:AtZAR1-3xFlag lines and two untagged pAtZAR1:AtZAR1 control lines and collected tissue samples from 6 to 7-week-old plants, 3 days after spray inoculation with PtoDC3000 (hopZ1a). We conducted immunoprecipitation followed by liquid chromatography mass spectrometry (LC-MS). We searched the spectral counts for kinases, and found an enrichment of ZED1, RKS1, ZRK3, ZRK13, ZRK14 and ZRK15 in pAtZAR1:AtZAR1-3xFlag lines compared to pAtZAR1:AtZAR1 lines (Figure 1b). Our results indicate that ZRK13 and ZRK14 can interact with AtZAR1, suggesting that AtZAR1 can interact with additional ZRKs beyond those already reported (Lewis et al., 2013; Seto et al., 2017; Wang et al., 2015).
3.2 AtZAR1 displays interaction specificity within the ZRK family
To further investigate whether AtZAR1 displays interaction specificity for kinases within the ZRK family, we cloned all ZRKs (Figure 2a) with C-terminal 3x-Flag fusions and transiently expressed each ZRK with either AtZAR1 or AtZAR1LRR as an in-frame fusion to a 5x-Myc epitope tag in N. benthamiana. We collected tissue samples and conducted co-immunoprecipitation (co-IP) experiments to test for AtZAR1-ZRK and AtZAR1LRR-ZRK interactions. As a negative control for AtZAR1 and AtZAR1LRR interaction, we cloned Botrytis-induced kinase1 (BIK1), a kinase from the PBL family which does not interact with AtZAR1 (Wang et al., 2015), with an in-frame 3x-Flag tag. We observed an interaction between AtZAR1 and all ZRKs, except for ZRK7 (Figure 2b), and between AtZAR1LRR and all ZRKs, except for ZRK7 (Figure 2c). Our findings indicate that AtZAR1 and AtZAR1LRR have identical interaction specificities and that AtZAR1LRR is sufficient for ZRK interaction. This is consistent with our previous findings that AtZAR1LRR is sufficient for ZED1 interaction (Baudin et al., 2017).
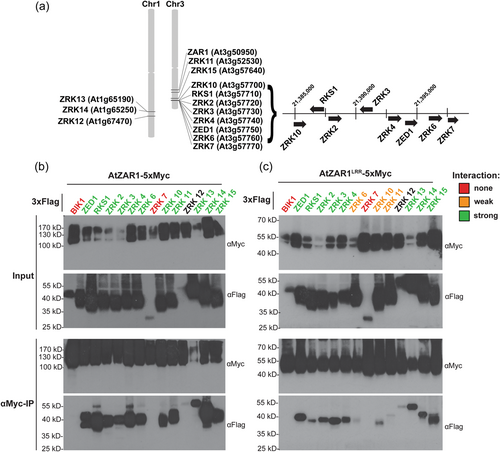
We consistently observed the strongest interactions between AtZAR1LRR and ZED1, RKS1, ZRK3, ZRK4, ZRK13 and ZRK14. Notably, these strong AtZAR1LRR interactors include all ZRKs with a previously characterised role in effector recognition through AtZAR1 (Baudin et al., 2017; Laflamme et al., 2020; Lewis et al., 2013; Martel et al., 2020; Seto et al., 2017; Wang et al., 2015), supporting our hypothesis that a strong ZAR1LRR interaction is biologically relevant for effector recognition. Moreover, the ZRKs that interact strongly with AtZAR1LRR overlap with the subset of ZRKs identified by LC-MS in A. thaliana (Figure 1b). We observed a consistently weak interaction between AtZAR1LRR and ZRK6, ZRK10 and ZRK11 (Figure 2b). While the interaction between AtZAR1 and ZRK12 was noticeably weaker when compared to other ZRKs, the ZRK12 protein was consistently harder to detect compared to other ZRKs (Figure 2b,c). Thus, we cannot make a conclusion about the strength of the AtZAR1-ZRK12 and AtZAR1LRR-ZRK12 interaction relative to the other ZRKs.
3.3 Arabidopsis ZRKs cause NbZAR1-dependent cell death in N. benthamiana
While transiently expressing ZRKs in N. benthamiana for co-IP experiments, we noticed that some ZRKs resulted in an HR in the absence of pathogen effectors. To determine if the HR is NbZAR1-dependent, we used VIGS to target NbZAR1 (hereafter NbZAR1-VIGS) or GUS (hereafter GUS-VIGS) as a negative control (Baudin et al., 2017). We expressed each ZRK in NbZAR1-VIGS and GUS-VIGS plants. We scored the HR 24 h post-dexamethasone induction as either no HR, weak HR, medium HR, or strong HR (Figure 3a). Both RKS1 and ZRK13 resulted in a strong NbZAR1-dependent autoactive HR phenotype, while the other ZRKs did not (Figure 3b). To ensure NbZAR1 was silenced, we expressed HopZ1a and ZED1 together on each leaf used across all experiments. We did not observe an HR in NbZAR1-silenced plants, indicating that NbZAR1 silencing was effective. As previously observed (Baudin et al., 2017), we consistently observed an HR when AtZAR1 was co-expressed with ZED1 and HopZ1a, since it can complement NbZAR1 silencing (Figure 3b). In addition, we did not see an HR when ZED1 was expressed alone, since ZED1 alone is not autoactive (Figure 3b). Therefore, of the 13 tested ZRKs, only RKS1 and ZRK13 caused a strong NbZAR1-dependent autoactive HR phenotype. While transient expression of ZED1 can constitute a functional effector recognition system with NbZAR1 (Baudin et al., 2017), some other ZRKs cause a HR phenotype when transiently expressed in the absence of pathogen effectors.
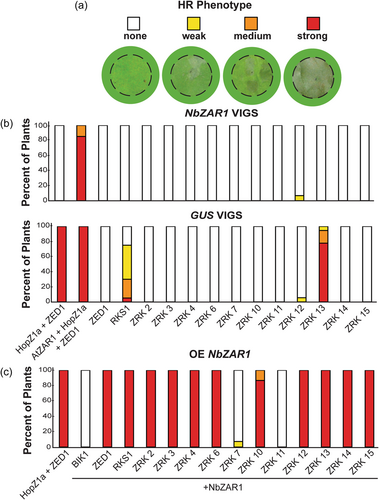
To determine if the autoactive HR is due to interspecific pairings of ZAR1 and ZRKs, we transiently expressed AtZAR1 and each ZRK in NbZAR1-VIGS plants. We did not see any strong or consistent HRs when AtZAR1 was expressed with each ZRK, suggesting that the observed autoactive HR is a result of interspecific ZAR1-ZRK pairings (Supporting Information: Figure S2). However, we did notice a weak inconsistent HR phenotype when ZRK3 and ZRK13 were coexpressed with AtZAR1 (Supporting Information: Figure S2). NbZAR1 was silenced effectively since co-expression of HopZ1a and ZED1 did not result in an HR. As previously observed (Baudin et al., 2017), the HR was complemented when we expressed AtZAR1 with HopZ1a and ZED1 (Supporting Information: Figure S2).
We hypothesised that weaker NbZAR1-dependent HR phenotypes might be missed due to low levels of natively expressed NbZAR1. To test this, we transiently expressed NbZAR1 with each ZRK in N. benthamiana plants. Since expression of NLRs can often result in HR phenotypes, we coexpressed BIK1 with NbZAR1 as a negative control for potential NbZAR1 autoactivity. Each ZRK resulted in a strong HR when expressed with NbZAR1, except for ZRK7 and ZRK11 (Figure 3c). It is likely that the lack of HR when ZRK7 and ZRK11 are respectively co-expressed with NbZAR1 is due to lack of interaction between these ZRKs and NbZAR1. In support of this hypothesis, ZRK7 cannot interact with AtZAR1 or AtZAR1LRR while ZRK11 weakly interacts with AtZAR1LRR (Figure 2c).
We therefore investigated whether the NbZAR1-dependent autoactive HR phenotype with ZRKs is correlated with NbZAR1LRR interaction strength. We cloned NbZAR1LRR (aa 496-845) as an in-frame fusion with a 5xMyc tag. We co-expressed BIK1, ZED1, RKS1, ZRK7, ZRK11 and ZRK13 with NbZAR1LRR in N. benthamiana and conducted co-IP experiments. If a strong interaction with NbZAR1LRR is the cause of the autoactive HR, RKS1 and ZRK13 should show the strongest interaction, while ZRK7 and ZRK11 should show no interaction. We observed a consistent interaction between ZED1 or ZRK13 and NbZAR1LRR (Supporting Information: Figure S3). As expected, NbZAR1LRR did not interact with ZRK7 or ZRK11, or the negative control BIK1. Surprisingly, RKS1 did not interact with NbZAR1LRR, suggesting that NbZAR1LRR interaction strength does not fully explain the autoactive HR phenotype.
3.4 Alternative variant of ZRK7 can interact with AtZAR1LRR and full-length AtZAR1
When we tested the ZRKs for interaction, the annotated ZRK7 protein was the only member of the ZRK family that did not interact with AtZAR1 or AtZAR1LRR (Figure 2). We further analysed the ZRK7 amino acid sequence compared to that of the other ZRKs and noticed that the annotated ZRK7 sequence was truncated at the C-terminus following the activation loop (Figure 4a, Supporting Information: Figure S4). This is due to an annotated intron in the coding sequence of ZRK7, which is unique among the ZRK family. We therefore searched the literature and publicly available RNA-seq data (Berardini et al., 2015; Lamesch et al., 2012), for evidence supporting the intron. The annotated spliced intron in ZRK7 is supported by 198 reads which support the junction in A. thaliana leaf RNA-seq data, and 225 reads which support the junction in A. thaliana seedling RNA-seq data (Figure 4a; Berardini et al., 2015; Lamesch et al., 2012). Interestingly, we found evidence of alternative splicing, with 73 and 79 reads that support intron retention of ZRK7 in leaf tissue and seedlings respectively (Figure 4b, Marquez et al., 2012). Intron retention results in readthrough into the annotated 3′UTR of ZRK7, which produces a protein that is similar in size and sequence to the other A. thaliana ZRKs (Figure 4b, Supporting Information: Figure S4).
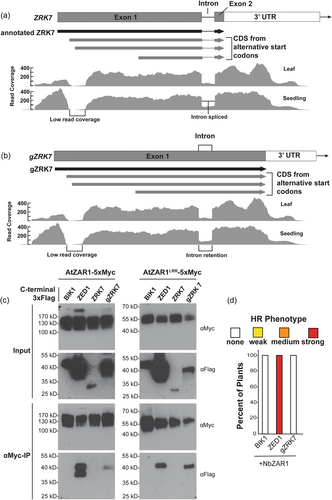
In addition, in publicly available RNA-seq data, we noticed a region of low coverage immediately following the ZRK7 start codon relative to coverage of the rest of the gene, in a pattern suggestive of an unannotated intron (Figure 4a, region labelled ‘low read coverage’). Despite high read coverage (100–400 reads) on either side of this region, no split reads spanning this region were identified that would support the presence of an intron in the data (Berardini et al., 2015; Lamesch et al., 2012). There is no clear start or end to the region of low read coverage at the 5′ end of ZRK7 (Figure 4a,b). It is possible that ZRK7 is misannotated and the transcript begins after the region of low read depth. However, the first in-frame start codon present in this transcript occurs 384 nucleotides into the annotated coding sequence (Figure 4a,b). To further investigate this region of low coverage at the 5′ end of ZRK7 as well as ZRK7 intron retention, we examined 45 datasets in the Arabidopsis RNAseq Database (Zhang, Zhang, et al., 2020). We identified evidence of intron retention in 39 of the examined datasets, while only 6 of the datasets had reads covering the region of low read coverage at the 5′ end of ZRK7, with a maximum of two reads in this region (Supporting Information: Table S1).
To determine if the intron-retained version of ZRK7 was functional, we cloned the genomic sequence for ZRK7 (hereafter gZRK7) as an in-frame fusion with a C-terminal 3x-Flag epitope tag and tested it for interaction with AtZAR1-5xMyc and AtZAR1LRR via transient expression in N. benthamiana followed by co-IP. Surprisingly, gZRK7 can interact with both AtZAR1LRR and full-length AtZAR1 (Figure 4c). As previously observed (Figure 2), BIK1 and ZRK7 did not interact with either AtZAR1LRR or full-length AtZAR1. To determine if gZRK7 can activate the NbZAR1-mediated HR, we expressed NbZAR1 and gZRK7 in N. benthamiana and scored cell death 24 h after gene induction based on a 4 category HR scale. We did not observe an HR when NbZAR1 was expressed with gZRK7 (Figure 4d). We cannot rule out the possibility that ZRK7 is a pseudogene, or that smaller reading frames within the ZRK7 gene other than the two tested in this study are of importance of the AtZAR1 recognition system. Regardless, we have shown that A. thaliana produces a ZRK7 transcript with a retained intron, resulting in a protein that can interact with both AtZAR1 and AtZAR1LRR.
We hypothesised that ZRK7 may represent an intermediate stage in ZRK evolution and conducted a selection analysis to determine if gZRK7 was under different selective pressure relative to other ZRKs. We tested for episodic diversifying selection across ZRKs using the adaptive Branch Site REL (aBSREL) model available through Hyphy. We saw evidence of gene level diversifying selection in gZRK7, as well as in ZRK12, ZRK13 and ZRK15 (Supporting Information: Figure S5). To identify residues under different selection pressure in gZRK7 compared to other ZRKs, we used the contrast-FEL model. We identified nine gZRK7 residues with significantly different dN/dS ratios relative to other ZRKs. Interestingly, 7 of the 9 residues are located before the region of low RNA-seq read coverage at the ZRK7 5′ end, suggesting that this region may be under strong selective pressure relative to the other ZRKs (Supporting Information: Figure S4; Supporting Information: Table S2).
3.5 AtZAR1 interacts with kinases outside of the ZRK family
Our LC-MS data suggested that AtZAR1-kinase interaction specificity is confined to the ZRK family. Based on trends from the LC-MS data (Figure 1b) and previously published data (Gong et al., 2022; Wang et al., 2015), we hypothesised that AtZAR1 would interact with ZRKs, but not closely related wall-associated kinase-like proteins (WAKLs) or PBLs, which form separate clades (Figure 5a). We cloned PBL2, and the kinase domain of WAKL2 (aa 346-748, hereafter WAKL2KIN) with an in-frame C-terminal 3x-Flag tag. WAKL2KIN was selected since it is the closest non-ZRK BLAST hit to ZED1 identified in A. thaliana. We carried out co-IP experiments by transiently expressing each kinase with either AtZAR1 or AtZAR1LRR in N. benthamiana. Surprisingly, we found that PBL2 and WAKL2KIN could interact with AtZAR1 (Figure 5b) and AtZAR1LRR (Figure 5c). BIK1 did not interact with AtZAR1 or AtZAR1LRR (Figures 2b,c, 5B), indicating that the interaction was specific. ZED1 interacted with both AtZAR1 and AtZAR1LRR (Figure 5b), as expected. To determine if PBL2 and WAKL2KIN interactions were specific to AtZAR1, we cloned At5g48620, a RPP8-like NLR closely related to AtZAR1, as an in-frame fusion with a 5xMyc tag, and transiently coexpressed it with PBL2 or WAKL2KIN in N. benthamiana. We have previously shown that At5g48620 does not interact with ZED1 (Baudin et al., 2021). We carried out co-IP experiments and found that neither PBL2 nor WAKL2KIN could interact with At5g48620 (Supporting Information: Figure S6), indicating that the interaction we observed for PBL2 and WAKL2KIN with AtZAR1 and AtZAR1LRR is specific.
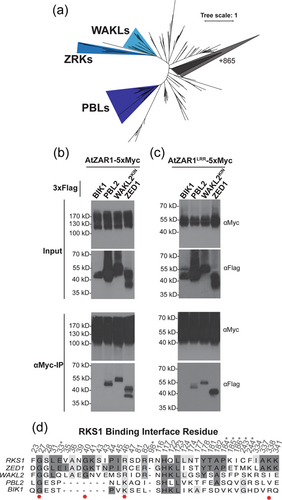
The interaction between AtZAR1 (or AtZAR1LRR), and WAKL2KIN or PBL2 is remarkable, as there is only 34% pairwise identity between ZED1 and WAKL2KIN, and 31% pairwise identity between ZED1 and PBL2 (Supporting Information: Figure S7). In addition, since AtZAR1 can interact with PBL2 and WAKL2KIN but not BIK1, this indicates that AtZAR1 can achieve interaction specificity for certain kinases, and that it can interact with kinases outside of the ZRK family. Furthermore, the similarity between AtZAR1-kinase and AtZAR1LRR-kinase interactions suggests that the LRR domain confers specificity and is sufficient for the kinase interactions. We then explored whether these interactions were sufficient to trigger an HR response in N. benthamiana. To determine if kinases outside of the ZRK family could cause an autoactive HR response, we co-expressed kinases (BIK1, ZED1, PBL2 or WAKL2KIN) with NbZAR1 since we saw NbZAR1-dependent autoactivity with most ZRKs (Figure 3c). Neither PBL2 or WAKL2KIN triggered an HR when co-expressed with NbZAR1 (Supporting Information: Figure S8). As expected, co-expression of NbZAR1 with ZED1 triggered a strong HR while the co-expression of NbZAR1 with BIK1 did not. Therefore, the NbZAR1 autoactive HR phenotype appears to be triggered specifically by the ZRK family.
To further explore the interaction between AtZAR1 and these kinases, we used Swiss-Model to generate structural homology models of AtZAR1 in complex with either BIK1, PBL2 or WAKL2KIN based on the AtZAR1-RKS1 structure (Supplemental Data S1; PDB:6j5w) (Wang, Wang, et al., 2019; Waterhouse et al., 2018). Homology models of all tested kinases showed that they are predicted to interact with AtZAR1 through the same surface as RKS1 (Supporting Information: Data S1). It is possible that this similarity is due to conserved kinase architecture and explains the diversity of AtZAR1-kinase interactions. Next, we used PRODIGY to predict binding affinities (ΔG) and dissociation constants (Kd) of our homology models as well as the AtZAR1-RKS1 structure (Xue et al., 2016). Interestingly, the predictions from PRODIGY based on homology models for direct interactions between AtZAR1 and kinases are consistent with our experimental findings through co-IP. AtZAR1-BIK1 binding is predicted to be weakest, AtZAR1-PBL2 binding strength is predicted to be intermediate, and AtZAR1-WAKL2KIN and AtZAR1-RKS1 binding are predicted to be strongest and relatively similar (Supporting Information: Table S3). Thus, AtZAR1 appears to achieve remarkable plasticity in its interactions with kinases, which may have facilitated its evolution in the recognition of so many distinct effector proteins. While our experiments and analyses suggest that AtZAR1 directly interacts with PBL2 or WAKL2KIN, we cannot rule out the possibility that AtZAR1 and these kinases interact indirectly in N. benthamiana.
3.6 Selection analyses identify residues important for AtZAR1 interaction specificity
We hypothesised that residues important for AtZAR1 interaction would be under significantly stronger negative selection pressure in ZRKs when compared to PBLs and WAKLs, and that ZRK family members evolved to have a role in pathogen recognition based on our current understanding of AtZAR1-mediated immunity. While PBL2 and WAKL2KIN can interact with AtZAR1, we hypothesised that this interaction may be due to similarities in kinase architecture, and expected that selective pressure to gain and maintain AtZAR1 interactions would be more pervasive in the ZRK family. Using the contrast-FEL model through hyphy, we identified 8 residues in the ZRKs which were under significantly stronger negative selection compared to WAKLs and PBLs (Supporting Information: Figure S9, Supporting Information: Table S4). To place this analysis in the context of AtZAR1-kinase interactions, we used Chimera and the inactive RKS1-AtZAR1 structure (PDB: 6j5w) to identify RKS1 residues in the AtZAR1 binding interface (Figure 5d). Out of the 8 residues identified in the selection analysis, G27, G40, R46 and K338 are in the RKS1-AtZAR1 binding interface (Figure 5d). As a validation of our findings, the G27A mutation in RKS1 has been previously shown to hinder ETI, while the G29E mutation in ZED1 (corresponding to G40 in RKS1) has been shown to both hinder ETI and weaken the AtZAR1 interaction (Hu et al., 2020; Wang, Wang, et al., 2019). In addition, three positions adjacent to binding interface residues (C38, S115, E339) and one activation loop residue (K220) are under significantly stronger negative selection in the ZRKs compared to PBLs and WAKLs (Supporting Information: Table S4), suggesting that these residues may be functionally relevant. While PBL2 and WAKL2KIN can interact with AtZAR1, evidence from our selection analysis suggests that clear differences in selective pressure are present in the ZRK family. Moreover, the specific residues under significantly different selective pressure in the ZRK family suggest that the ZRKs are likely under selective pressure to interact with AtZAR1.
3.7 Binding interface analyses identify residues important for AtZAR1 interaction specificity
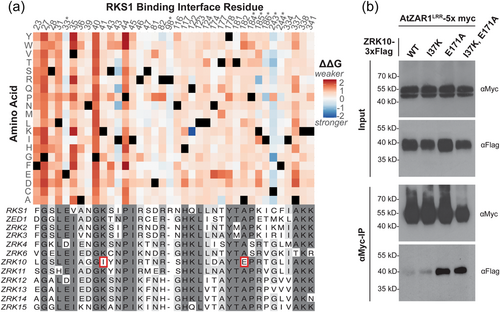
To identify additional residues that are important in the AtZAR1-kinase binding landscape, we used MutaBind2 to predict the change in binding affinity (ΔΔG) resulting from every possible single amino acid substitution in the RKS1-AtZAR1 binding interface based on the previously published inactive structure (Wang, Hu, et al., 2019; Wang, Wang, et al., 2019). Mutations in the N-terminal region of RKS1 (aa 23–44) are predicted to reduce binding affinity more than those in other regions of RKS1 (Figure 6a). Notably, binding interface residues in the N-terminal region of RKS1 are almost exclusively in the AtZAR1LRR interface. The only AtZAR1NBD contact in the N-terminal region of RKS1, E33, is not predicted to have a large effect on binding affinity, regardless of the amino acid substitution. Residues G27, G40 and P44 are predicted to strongly reduce binding affinity regardless of the alternative amino acid and are in the AtZAR1LRR interface (Figure 6a). These residues stand out as binding ‘hotspots’ and are conserved across all interacting ZRKs (Figure 6a). Both G27 and G40 were identified in our selection analysis as being under significantly stronger negative selection pressure in the ZRK family when compared to WAKLs and PBLs (Figure 5d). Furthermore, both G27 and G40 have been shown to weaken the AtZAR1 interaction and abolish RKS1 and ZED1-mediated ETI (Hu et al., 2020; Wang, Wang, et al., 2019). While R46 and K338 were also identified in our selection analysis, neither of these residues stand out as binding hotspots, and it is possible that selection pressure on these regions is for reasons other than AtZAR1LRR interaction. Interestingly, the RKS1I43N and RKS1Q122K mutations were predicted to strengthen RKS1 interaction, and both alternative residues are found in other interacting ZRKs (Figure 6a). We also noted that mutations in the seven identified RKS1-AtZAR1NBD contacts (E33, R98, P184, K185, I186, C243 and F244) are predicted to result in more neutral changes to binding affinity overall, further supporting the importance of AtZAR1LRR in kinase binding (Figure 6a).
3.8 A single amino acid substitution in ZRK10 strengthens the interaction with AtZAR1LRR
Our previous data strongly support the importance of ZAR1-ZED1 interaction for pathogen recognition, as we previously identified single nucleotide polymorphisms in AtZAR1 that affected the interaction with ZED1 and that resulted in a loss of HopZ1a recognition (Baudin et al., 2017, 2021). As a proof of concept for rationally engineering interactions with AtZAR1, we used our findings from the AtZAR1-kinase co-IPs and the binding interface analysis to determine if we could strengthen the interaction between AtZAR1LRR and ZRK10. We specifically focused on the ZRK10-AtZAR1LRR interaction as it was consistently observed to be weak relative to other ZRKs (Figure 2c). We identified two residues in the RKS1 binding interface alignment that were unique to ZRK10 (Figure 6a). All AtZAR1-interacting ZRKs have a lysine that aligns with RKS1K41, while ZRK10 has an isoleucine in the corresponding position (ZRK10I37; Figure 6a, Supporting Information: Figure S4). Similarly, AtZAR1-interacting ZRKs have an alanine that aligns with RKS1A182, while ZRK10 has a glutamic acid in the corresponding position (ZRK10E171; Figure 6a, Supporting Information: Figure S4). In addition, the ΔΔG values from our binding interface analysis suggest that the two differences unique to ZRK10 would result in a decrease in binding affinity if they were introduced in RKS1. Specifically, RKS1K41I, which aligns with ZRK10I37, is predicted to weakly decrease RKS1-AtZAR1 interaction strength (ΔΔG = 0.52) while RKS1A182E (which aligns with ZRK10E171) is predicted to result in a greater decrease in RKS1-AtZAR1 interaction (ΔΔG = 1.13; Supporting Information: Figure S10). Since these differences in ZRK10 are predicted to negatively affect binding affinity, we investigated if introducing the consensus residue in ZRK10 would increase ZRK10-AtZAR1LRR interaction strength.
We generated ZRK10I37K, ZRK10E171A and ZRK10 I37K/E171A mutants and transiently co-expressed AtZAR1LRR as an in-frame fusion to a 5x-Myc tag, with each ZRK10 mutant as an in-frame fusion with 3x-Flag, in N. benthamiana. We then tested for differences in interaction strength with AtZAR1LRR by co-IP. Remarkably, we observed a consistently stronger interaction between ZRK10E171A and AtZAR1LRR when compared to ZRK10 (Figure 6b). In agreement with our predictions, ZRK10I37K had no consistent detectable effect on the AtZAR1LRR interaction. Similarly, we did not observe a stronger interaction between ZRK10I37K/E171A and AtZAR1LRR when compared to ZRK10E171A, suggesting that the I37K mutation had little effect on AtZAR1LRR interaction when combined with the E171A mutation. Therefore, the binding interface analysis identified a key residue that strengthened the ZRK10-AtZAR1LRR interaction in co-IP experiments. Moreover, we have shown that AtZAR1LRR interaction specificity is sensitive to single amino acid substitutions in a ZRK.
4 DISCUSSION
Here, we demonstrate that AtZAR1 can interact with diverse A. thaliana kinases while simultaneously being sensitive to single amino acid substitutions in kinase interactors such as ZRK10. Under native conditions in A. thaliana, we show that AtZAR1 can interact with 6 out of 13 ZRKs (Figure 1). Using a N. benthamiana transient expression system, we showed that AtZAR1 can interact with all ZRKs except for ZRK7 (Figure 2). We found that some ZRKs result in a strong effector-independent HR phenotype when coexpressed with native and/or overexpressed NbZAR1 (Figure 3). Although AtZAR1 can interact with an unannotated splice variant of ZRK7, subsequent analysis suggested ZRK7 is likely a pseudogene (Figure 4). While all known ZAR1-mediated effector recognition requires a kinase in the ZRK family (Laflamme et al., 2020; Lewis et al., 2013; Martel et al., 2020; Seto et al., 2017; Wang et al., 2015), we showed that AtZAR1LRR can interact with kinases outside of the ZRK family with greater strength compared to some ZRKs (Figures 2 and 5). The overlap between AtZAR1 and AtZAR1LRR interaction profiles demonstrates that AtZAR1LRR is sufficient for interaction with kinases and suggests that AtZAR1LRR confers specificity for kinases (Figures 2 and 5). The specificity of AtZAR1LRR interactions can be manipulated by single amino acid substitutions in the kinase interactor (Figure 6).
Interaction profiles of AtZAR1 in A. thaliana during HopZ1a recognition suggest that AtZAR1 interacts with multiple ZRKs during effector recognition. The strongest AtZAR1LRR interactors observed in co-IP experiments in N. benthamiana overlap with the ZRKs detected through LC-MS in A. thaliana (Figures 1 and 2). This suggests that the interactions observed in N. benthamiana between AtZAR1 and ZRK2, ZRK4, ZRK6, ZRK10, ZRK11 and ZRK12 were too weak to detect in A. thaliana, possibly due to the sensitivity of LC-MS, experimental conditions, native expression levels or subcellular or tissue localisation of the proteins. It is also possible that some AtZAR1-ZRK interactions are strengthened in the presence of a corresponding effector, although ZED1 and AtZAR1, RKS1 and AtZAR1 or ZRK3 and AtZAR1 interact in the absence of the effector (Baudin et al., 2017; Lewis et al., 2013; Seto et al., 2017; Wang et al., 2015). While we cannot exclude the possibility that AtZAR1-ZRK interactions are facilitated by a protein complex with ZRK homologues in N. benthamiana, we have previously shown that the genetic requirements for HopZ1a recognition are analogous in N. benthamiana and A. thaliana; recognition requires ZAR1 and ZED1 as well as the catalytic activity of HopZ1a (Baudin et al., 2017). We feel that the sensitivity of our LC-MS experiment and/or the collection of tissue samples during HopZ1a recognition is the most likely explanation for the observed differences in AtZAR1-ZRK interactions between our LC-MS data in A. thaliana and our co-IP data in N. benthamiana. We cannot rule out the possibility that these differences may also be due to native expression in A. thaliana compared to overexpression in N. benthamiana, although transient expression assays in N. benthamiana have been extensively used to characterise protein-protein interactions of NLRs from different plant species (Huh et al., 2017; Ortiz et al., 2017; Saile et al., 2021; Xi et al., 2022; Yin et al., 2021). Regardless, since AtZAR1 is necessary for the recognition of multiple effector proteins (Laflamme et al., 2020), the additional ZRK interactors identified here (Supporting Information: Figure S11) suggest that additional effectors may be recognised through AtZAR1-ZRK complexes. Identifying these additional unknown effectors may require isolation, sequencing and analysis of diverse P. syringae strains and other bacteria to identify new effector families, as well as functional tests to look for ZAR1-dependent recognition. Future research can prioritise ZRKs that are strong AtZAR1LRR interactors to examine for a role in effector recognition. Our data suggest that ZAR1 has remarkable plasticity in its interactions with kinases, which likely enables ZAR1 to recognise so many distinct effector proteins.
A remaining question in the ZAR1 system is whether the resistosome pentamer can contain different ZRKs upon effector recognition, or if it is comprised of only the ZRK responsible for mediating recognition to a specific effector. Given the difference in AtZAR1LRR-ZRK interaction strengths, it is unclear whether all interactions are represented under native conditions. If ZAR1 is present at a low level under native expression and ZRKs competitively bind to ZAR1, this would result in only identifying a subset of the strongest interacting ZRKs. We have previously shown that AtZAR1 alleles from A. thaliana ecotypes may have stronger or weaker interaction strength with ZED1 relative to Col-0 (Baudin et al., 2021). It is possible that selection pressure imposed by pathogens can influence broader AtZAR1-ZRK affinities across A. thaliana ecotypes. While clear differences in interaction strength were visible in AtZAR1LRR-ZRK co-IP experiments, all ZRKs appeared to interact strongly with full-length AtZAR1, except for ZRK7 (Figure 2). The tomato NLR Prf forms hetero-oligomeric complexes that associate with Pto kinase and multiple Pto kinase family members in its inactive state, and these additional members contribute to P. syringae resistance (Gutierrez et al., 2010). It is tempting to speculate that AtZAR1-ZRK complexes are similar to Prf-Pto kinase complexes, and that additional ZRKs other than the one required for effector recognition are present in oligomeric resistosomes. These additional ZRKs may have a role in conferring broader resistance to P. syringae, although further work is needed to test this hypothesis.
Our findings provide additional evidence that ZAR1LRR is responsible for determining guardee specificity. We did not identify any kinases that could interact with full length AtZAR1 but not AtZAR1LRR and vice-versa (Figure 2). Three lines of evidence support the assertion that ZAR1LRR determines guardee specificity across all kinase interactors: the overlap between interaction trends with AtZAR1LRR and AtZAR1 presented here, the cryo-EM structure of AtZAR1 in complex with RKS1, and previous findings on AtZAR1 domains in the context of ZED1 interactions (Baudin et al., 2017; Lewis et al., 2013; Wang, Hu, et al., 2019; Wang, Wang, et al., 2019). These findings further support our previous conclusion that ZAR1LRR interaction is required for ZAR1-mediated HR (Baudin et al., 2017, 2021).
Using a binding interface analysis, we were able to rationally increase the interaction strength between AtZAR1LRR and kinase interactors (Figure 6). We have previously shown that the S584R mutation in AtZAR1LRR, from A. thaliana ecotype Cvi-0, can strengthen the interaction between AtZAR1LRR and ZED1 (Baudin et al., 2021). We have also shown that mutations resulting in weakened AtZAR1LRR-ZED1 interaction lead to a loss of HR triggered by HopZ1a (Baudin et al., 2021). Although mutations in ZED1 can cause a loss of the AtZAR1LRR interaction (Baudin et al., 2017), mutations in a AtZAR1 guardee that increase affinity for AtZAR1LRR have not been previously described. Here, we show that a single amino acid substitution in ZRK10 is sufficient to increase interaction strength with AtZAR1LRR (Figure 6). Our selection of ZRK10E171A was based on the consensus residue of ZRKs that interact with AtZAR1 at that position, as well as the predicted effect of mutating that site based on the RKS1-AtZAR1 binding interface analysis (Figure 6a, Supporting Information: Figure S4, Supporting Information: Figure S10). It is possible that our rationale for targeting this specific residue can be extended to improve ZAR1 interaction strength with other kinases. Engineering NLRs is a complex problem since it requires balancing protein-protein interactions with appropriate activation of the NLR. The NLR RPS5 has been engineered to enable recognition of different effectors. RPS5 is normally activated when its kinase guardee PBS1 is cleaved by P. syringae effector AvrPphB (Ade et al., 2007). Kim et al. (2016) were able to introduce new cleavage sites into PBS1, allowing recognition of proteases from other pathogens. More recently, the recognition specificity of the rice NLR RGA5 was expanded through modifications of the RGA5 integrated-domain effector-binding interface, leading to recognition of an additional effector (Cesari et al., 2022). The functional conservation of ZAR1 across angiosperms (Gong et al., 2022; Lewis et al., 2010) provides a unique opportunity to engineer ZAR1 guardees for use across species with native ZAR1 orthologs (Baudin et al., 2017). Since we have previously demonstrated that an interaction with AtZAR1LRR is required for effector recognition (Baudin et al., 2021), the ability to fine-tune AtZAR1-ZRK10 kinase interaction strength is a major advancement toward the rational design of a ZAR1 guardee. It is possible that advances in our understanding of ZAR1 interaction specificity may allow for the rational design of ZAR1 guardees from existing pathogen-targeted kinases that are not guarded by ZAR1.
The A. thaliana Col-0 genome encodes a version of ZRK7 which can interact with AtZAR1 and AtZAR1LRR, while the annotated spliced version of ZRK7 is not capable of AtZAR1 or AtZAR1LRR interaction (Figures 2 and 4). We were unable to find concrete evidence that gZRK7 is transcribed in its entirety or is functionally relevant for AtZAR1 immunity. However, we did find evidence of intron retention in the annotated ZRK7 gene based on publicly available RNA-seq data. Furthermore, evidence of ZRK7 intron retention was previously identified in transcriptomic analysis investigating alternative splicing in A. thaliana (Marquez et al., 2012). Several ZRKs, including ZRK7, are present in a genomic cluster in the A. thaliana genome (Figure 2a) (Lewis et al., 2013), suggesting tandem duplication as a mechanism for generating ZRK diversity. Pseudogenes are frequently derived from tandem gene duplication events in A. thaliana, and may represent an intermediate step in gene duplication when found in this context (Mascagni et al., 2021). A previous genome-wide analysis of A. thaliana pseudogenes identified ZRK7 as a pseudogene based on two separate prediction pipelines (Xiao et al., 2016). ZRK7 also has high sequence similarity to a known A. thaliana pseudogene At1g43895 (Xiao et al., 2016). The data suggest that ZRK7 is a pseudogene and might have the potential to functionalise under the right selective pressure or was functional at some point in the distant past. It is also possible that genomic ZRK7 may have a detrimental effect (i.e., cell death) when expressed in A. thaliana, and became a pseudogene to avoid this. Tandemly arrayed genes involved in biotic stress are often under strong selection pressure, which can result in pseudogenes (Zou et al., 2009). Our selection analysis identified selective pressure unique to amino acids at the N-terminus of gZRK7, as well as gene-level positive selection on gZRK7, ZRK12, ZRK13 and ZRK15 (Supporting Information: Figure S5, Supporting Information: Table S2). While ZRK7 appears to be present in all A. thaliana genomes, it is difficult to identify specific ZRK7 homologues across other species due to the high sequence similarity of the ZRK family. Further investigation of ZRK7 may yield insights into the evolution of A. thaliana ZRKs.
We have shown that interspecific pairings of ZAR1 orthologs and ZRKs, but not BIK1, PBL2 or WAKL2KIN, can lead to an autoactive HR, suggesting that these ZRKs might be able to trigger cell death by ZAR1 when activated by pathogen effectors (Figure 3, Supporting Information: Figure S8). Some ZRKs result in a strong effector-independent HR phenotype when co-expressed with native and/or overexpressed NbZAR1 (Figure 3). While others have observed an HR resulting from expressing RKS1 with several different ZAR1 orthologs including NbZAR1 (Gong et al., 2022), our data highlights the differential levels of autoactivity resulting from one gene family paired with one ZAR1 ortholog. In addition, expression of RKS1 or ZRK13 induced an HR (Figure 3), which was not observed when these ZRKs were co-expressed with AtZAR1 (Supporting Information: Figure S2), suggesting that co-evolution between ZAR1 and ZRK proteins is critical for maintaining an inactive state. Heterologous expression of certain NLRs has been previously shown to result in cell death in the absence of pathogen effectors due to the lack of negative regulation from a ‘paired’ NLR or guardee (Césari et al., 2014; Day et al., 2005; Huh et al., 2017). Here we show that heterologous expression of a guardee results in autoactive cell death mediated by NbZAR1. Our data suggest that the autoactive cell death phenotype is not dependent on the interaction with NbZAR1LRR (Supporting Information: Figure S3) and is specific to kinases in the ZRK family (Supporting Information: Figure S8). There are several possible explanations for the interspecific ZAR1-ZRK autoactive HR. First, NbZAR1 may be easier to activate than AtZAR1. In support of this hypothesis, NbZAR1 can be autoactivated by introducing the HRD mutation, while the same mutations do not result in an autoactive AtZAR1 protein (Baudin et al., 2019; Harant et al., 2022). Second, NbZAR1 interspecific ZRK pairings, but notably not those with BIK1, PBL2 or WAKL2KIN, may result in autoactivation of NbZAR1 immunity, due to a lack of co-evolution between NbZAR1 and ZRKs. Third, the interaction between NbZAR1 and ZRKs may result in a clash with the NbZAR1NBD. Based on the cryo-EM structure, AtZAR1 activation occurs after the RKS1 activation loop is stabilised by PBL2 interaction (Wang, Hu, et al., 2019; Wang, Wang, et al., 2019). In the inactive state, the activation loop of RKS1 is not well resolved. However, several other regions of RKS1 are near the AtZAR1NBD. Slight differences in binding orientation between the cryo-EM structure and the ZRK-NbZAR1 interaction could result in a clash between ZRKs and NbZAR1NBD, leading to activation in the absence of effector perturbation (Wang, Hu, et al., 2019; Wang, Wang, et al., 2019). Identifying the cause of interspecific autoactive HR phenotypes is a crucial next step toward interspecific pairings of A. thaliana ZRK recognition pathways with ZAR1 orthologs in non-model species.
The current mechanistic model of ZAR1 activation based on the cryo-EM structure is unable to connect some experimental findings within the ZAR1 system, and many unanswered questions about ZAR1 function under native conditions remain. Previous studies have demonstrated that ZRKs may interact with diverse PBLs in a dynamic fashion in the presence and absence of pathogen effectors (Bastedo et al., 2019; Seto et al., 2021). We observed an AtZAR1-PBL2 interaction under transient expression in a heterologous system in the absence of a pathogen effector (Figure 5) and demonstrated that this interaction is specific to AtZAR1 (Supporting Information: Figure S6). A direct AtZAR1-PBL2 interaction was not observed in the AtZAR1-RKS1-PBL2 structure or previous work on AvrAC recognition in A. thaliana (Wang et al., 2015; Wang, Hu, et al., 2019; Wang, Wang, et al., 2019). However, we previously demonstrated that the N. benthamiana system is remarkably sensitive and can distinguish weak interactions that may result in loss-of-function in A. thaliana (Baudin et al., 2017; Baudin et al., 2021). While it is possible that PBL2 interacts with AtZAR1 through an endogenous ZRK in N. benthamiana, we find this unlikely since previous studies have shown that PBL2 does not interact with RKS1 in the absence of the pathogen effector (Wang et al., 2015). ZAR1 interaction specificity under native conditions may be limited to the strongest ZRK interactors due to competitive binding, preventing ZAR1-kinase interactions outside of the ZRK family. In addition, ZRKs may have different tissue or developmental expression compared to PBL2. We also showed that AtZAR1 also displays strong interactions with WAKL2KIN, which is the closest non-ZRK kinase to ZED1. The ZRKs are believed to have evolved from WAKLs through the loss of their extracellular domain (Gong et al., 2022), which would affect their subcellular localisation. The subsequent expansion of ZRKs coupled with this change in localisation would give ZAR1 the opportunity for selective and versatile interactions with diverse kinases, resulting in the current immunodiversity conveyed by this key plant immunity hub.
ACKNOWLEDGEMENTS
The authors would like to thank Jack Kim for assistance with cloning, Dina Nazarchuk for assistance screening Arabidopsis thaliana transgenic lines, and Dr. Sheng Luan for providing the BIK1 template used in cloning. Research presented here was supported by USDA ARS 2030-21000-046-00D and 2030-21000-050-00D, and NSF Directorate for Biological Sciences IOS-1557661 and the NSF Graduate Research Fellowship Programme.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




