Arbuscular mycorrhizal fungi trigger danger-associated peptide signaling and inhibit carbon‒phosphorus exchange with nonhost plants
Yutao Wang and Hanwen Chen are the co-first authors.
Abstract
In soil, arbuscular mycorrhizal fungi (AMF) meet the roots of both host and presumed nonhost plants, but the interactional mechanisms of AMF with and functional relevance for nonhost plants is little known. Here we show AMF can colonize an individually grown nonhost plant, Arabidopsis thaliana, and suppress the growth of Arabidopsis and two nonhost Brassica crops. This inhibitory effect increased with increasing AMF inoculum density, and was independent of AMF species or nutrient availability. 13C isotope labeling and physiological analyses revealed no significant carbon‒phosphorus exchange between Arabidopsis and AMF, indicating a lack of nutritional function in this interaction. AMF colonization activated the danger-associated peptide Pep-PEPR signaling pathway, and caused clear defense responses in Arabidopsis. The impairment of Pep-PEPR signaling in nonhost plants greatly compromised AMF-triggered defensive responses and photosynthesis suppression, leading to higher colonization rates and reduced growth suppression upon AMF inoculation. Pretreatment with Pep peptide decreased AMF colonization, and largely substituted for AMF-induced growth suppression in nonhosts, confirming that the Pep-PEPR pathway is a key participant in resistance to AMF colonization and in mediating growth suppression of nonhost plants. This work greatly increases our knowledge about the functional relevance of AMF and their mechanisms of interactions with nonhost plants.
1 INTRODUCTION
Arbuscular mycorrhizal fungi (AMF) were present in the first land plants, and today they form arbuscular mycorrhizal (AM) symbiosis with most vascular plant species (Brundrett & Tedersoo, 2018; Brundrett, 2009). Vascular plants supply the associated fungi with sugar, and more importantly, lipids as a carbon (C) source (Bravo et al., 2017; Helber et al., 2011; Jiang et al., 2017; Keymer et al., 2017; Luginbuehl et al., 2017; Solaiman & Saito, 1997), and benefit from the fungal partner by improved mineral nutrient status, especially phosphorus (P) (Smith & Read, 2010). This C‒P exchange is the basis for the AM symbiosis, and there is evidence for a bidirectional control for ‘fair trade’ in the interaction (Grman, 2012; Hammer et al., 2011; Kiers et al., 2011). AMF develop extensive mycelia that colonize and connect roots of different plant individuals (Weremijewicz et al., 2016). This colonization may cause a continuum of effects, from positive to negative in the plant hosts (Bao et al., 2019; Johnson et al., 1997, 2015; Koch et al., 2017), and AMF diversity and community composition is a significant driver of the structure, diversity and productivity of plant communities (van der Heijden et al., 2008).
Around 29% of vascular plant species are thought to be unable to establish a functional symbiosis with AMF under natural conditions (Brundrett, 2009, 2017; Brundrett & Tedersoo, 2018). These plants, called ‘nonhost’ plants, include some economically important crops (Anthony et al., 2020; Brundrett, 2009; DeMars & Boerner, 1996; Hajiboland et al., 2020). AM symbiosis seems to be an ancestral trait in vascular plants, and the ability to form a functional symbiosis may have been lost or suppressed more than once (Delaux et al., 2014; Vigneron et al., 2018), as these nonhost species are not monophyletic (Brundrett, 2017; Delaux et al., 2014; Vigneron et al., 2018). Although there are fewer nonhost than host plants, nonhost plants can dominate a wide range of environments, such as wetland ecosystems (Brundrett, 2009; Wang et al., 2021). In nature, nonhost plants often grow together with AM hosts. Therefore, nonhost roots will often be in contact with an extensive mycorrhizal hyphal network (Francis & Read, 1994; Ocampo, 1986). Understanding the potential effects of AMF contact on the performance of host as well as nonhost plants is necessary for clarifying the ecological role of AMF in plant community assemblies. Compared to the numerous studies of the interactions between AMF and their host species, surprisingly little is known about the functional relevance of AMF for nonhost species (But see Cosme et al., 2018; Facelli et al., 2010; Glenn et al., 1985; Hajiboland et al., 2020; Ocampo et al., 1980; Veiga et al., 2013; H. Zhang et al., 2019). It has been known for some time that some nonhost plants in AM-dominated plant communities can successfully be colonized by AMF (Brundrett, 2009; Lambers & Teste, 2013; Lekberg et al., 2015; Wang et al., 2021; H. Zhang et al., 2019), even with occasional arbuscule-like structures (Cosme et al., 2018). This interaction between AMF and nonhost plants is referred to as glomeromycotan fungal colonization (Brundrett, 2009), in contrast to the interaction with host plants, which is called mutualistic mycorrhizal colonization (Brundrett, 2004). AMF colonization of the roots of individually grown nonhosts has also occasionally been observed (Cosme et al., 2021; Kruckelmann, 1975; H. Zhang et al., 2019); however, it is generally thought that a host-supported AM fungal network is required for successful colonization of nonhost roots (Cosme et al., 2018; Fernández et al., 2019; Ocampo, 1986; Veiga et al., 2013), because after initial detection of AMF, nonhost plants can exhibit strong resistance to AMF intracellular colonization (Allen et al., 1989; Cosme et al., 2021; Fernández et al., 2019; Poveda et al., 2019).
AMF are obligate biotrophs, and their growth and development are largely dependent on plant-derived C. Recent studies have elegantly demonstrated that lipids are transferred from host plants to AMF in addition to sugars, and that AMF growth and development depends on the lipid supply because AMF species lack genes for long-chain fatty acid biosynthesis (Bravo et al., 2017; Jiang et al., 2017; Keymer et al., 2017; Luginbuehl et al., 2017). C allocation from nonhost plants to AMF was reported in Atriplex gardneri and Dianthus deltoides when they were co-cultivated with mycorrhizal host plants (Allen & Allen, 1990; Lekberg et al., 2015). However, nonhost plants are generally believed to be unable to allocate C to AMF, since they have lost the orthologs of important ‘symbiotic toolkit’ genes (Delaux et al., 2013, 2014), including those required for lipid biosynthesis and exportation (Bravo et al., 2017; Jiang et al., 2017; Keymer et al., 2017; Luginbuehl et al., 2017).
Arabidopsis thaliana (L.) Heynh. (hereafter Arabidopsis) is a nonhost species that usually cannot be colonized by AMF (Brundrett, 2009; Delaux et al., 2014; Fernández et al., 2019; but see Kruckelmann, 1975) or ectomycorrhizal fungi (Vishwanathan et al., 2020). Veiga et al. (2013) and Fernández et al. (2019) found that Rhizophagus irregularis (RI) barely colonized the roots of individually grown Arabidopsis and had no effect on Arabidopsis growth. However, when Arabidopsis was grown in a host-supported AM network, hyphae and vesicles (but not arbuscules) were observed in Arabidopsis roots, and there was a decrease of Arabidopsis biomass in the presence of AMF. Further analysis suggested that this growth suppression was associated with the activation of costly defenses in colonized Arabidopsis roots, which also led to enhanced systemic immunity against a foliar necrotrophic pathogen (Fernández et al., 2019).
In plants, the sophisticated innate immune system has a diversity of plasma membrane-localized pattern recognition receptors that not only perceive conserved microbe/pathogen-associated molecular patterns from microbes or pathogens, but also recognize danger- or damage-associated molecular patterns (DAMPs) from damaged cells, and consequently activate pattern-triggered immunity (Bartels & Boller, 2015; Engelsdorf et al., 2018). In Arabidopsis, a family of plant elicitor peptides (Pep1–8) are typical DAMPs that are processed from their precursors PROPEP1–8 by the cleavage of Ca2+-dependent type-Ⅱ metacaspases upon pathogen attack or wounding (Hander et al., 2019; Shen et al., 2019). Perception of Peps by two receptors, PEPR1 and PEPR2, triggers diverse defense responses, including reactive oxygen species burst, production of secondary metabolites, hormonal regulation, and lignification, which contribute to the local and systemic immunity to various biotic stresses (Bartels & Boller, 2015; Engelsdorf et al., 2018; Huffaker et al., 2013); Meanwhile, the activation of this Pep-PEPR signaling pathway usually leads to compromised growth of the plant in a growth‒defense tradeoff (Okada et al., 2021; Ross et al., 2014). Previous works have suggested that, in the AMF and nonhost interaction, early signaling between AMF and Arabidopsis is not completely impaired and that incompatibility appears at later interaction stages, in which the unpermitted penetration of AMF led to defense responses coinciding with reduced growth (Fernández et al., 2019; Veiga et al., 2013). However, the mechanisms by which the immune pathway mediates these defense and growth outputs remain unresolved.
Our aim was to explore the direct effects of AMF on the performances of nonhost plants and the interactional mechanisms involved, without the presence of a neighbor host plant. Specifically, we investigated the following questions: (ⅰ) Do the effects of AMF on the growth of nonhost plants depend on the species of AMF, the species of nonhost plant, or the nutrient availability in the soil? (ⅱ) What is the main mechanism involved in the resistance to AMF colonization and (ⅲ) in mediating the observed growth depressions of AMF on nonhost plants? Comparative transcriptomic and physiological analyses of the wild-type Arabidopsis and its mutants (in Pep-PEPR or hormone signaling pathway) with and without AMF inoculation was used to look for the mechanisms of the interaction between AMF and nonhost plants. Isotope labeling coupled with isotope ratio mass spectrometry (IRMS) analysis was used to track the potential C delivery from Arabidopsis to AMF. Potential fungal P delivery to Arabidopsis was assessed by analyzing the expression profile of the AMF metabolism marker genes and plant P content. To the best of our knowledge, this work is the first solid evidence of the absence of C‒P exchange between AMF and colonized nonhost plant grown individually. In addition, we identify the DAMP Pep-PEPR signaling pathway as the key participant in resistance to AMF colonization and in mediating growth suppression upon AMF inoculation of nonhost plants.
2 MATERIALS AND METHODS
Detailed information about materials and methods used in this study is provided in Supporting Information (see Extended Methods).
2.1 Experimental design
We performed eight interconnected and complementary experiments, all following the randomized complete block design. Briefly, Experiment (Exp.) 1 to Exp. 4 evaluated the effects of (1) AMF inoculation potential, (2) AMF species identity, (3) N/P nutrient level and (4) nonhost species identity on the growth of nonhost plants and AMF root colonization intensity. In Exp. 1, Arabidopsis was planted in substrate with varying proportions of AMF inocula: 0% (noninoculated control), 1%, 3%, 5%, 7% and 9% of R. irregularis inoculum by volume. R. irregularis propagated in monoxenic root organ cultures (Hammer et al., 2011) was used to evaluate the accessibility of AMF to Arabidopsis roots cultivated in sterile media. Exp. 2 used a control and three different AM-inoculation treatments (R. irregularis, Funneliformis mosseae, and a mixture of these two, 5% of inoculum by volume here and in the following, except for 10% in Exp. 5 to Exp. 8). In Exp. 3, Arabidopsis plants inoculated with or without R. irregularis were planted in substrate fertilized with Hoagland solution with various levels of N and P (full N/P, half N/P or a quarter N/P). In Exp. 4, five Brassica campestris cultivars and five Brassica napus cultivars were planted in single pots containing 1.5 L substrate with or without AMF inocula (R. irregularis, F. mosseae or noninoculated control).
Exp. 5 assessed the potential C‒P exchange, that is, the basic AM functionality (Johnson, 2010), between Arabidopsis and AMF. 13C isotope labeling coupled with IRMS was used to track the potential C delivery from Arabidopsis to AMF. The potential P transport from AMF to the plant was assessed by analyzing plant P content and the fungal viability marker genes (GintPT, GintAMT2, GintMST2) in Arabidopsis root.
Exp. 6 to Exp. 8 evaluated the mechanisms involved in resistance to AMF colonization and the observed growth depressions of AMF on nonhost plants. In Exp. 6, wild-type Arabidopsis was planted with and without R. irregularis inoculation, and root and shoot tissues were harvested for transcriptome and hormones analyses. Exp. 7 evaluated the potential roles of AtPep-PEPR signaling and hormone signaling, as implied by the results from Exp. 6, in defense against AMF. The dynamic responses of the marker genes in salicylic acid (SA)-, jasmonic acid (JA)-, ethylene (ET)-, and indole acetic acid (IAA)-signaling to AMF were investigated by time-course gene expression analysis in wild-type Arabidopsis. AMF colonization intensities and growth responses upon AMF inoculation were also examined in the Pep-PEPR and the SA-, JA-, ET-, and IAA-signaling mutants. Exp. 8 was conducted to confirm the involvement of the Pep-PEPR signaling in regulation of the AMF/nonhost interaction. Profile of AMF-triggered transcriptional reprogramming was assessed by comparative analysis of the shoot (but not root, in which the Pep-PEPR pathway was not responsive to AMF colonization (23 days postinoculation) as demonstrated in Exp. 6) transcriptome between wild-type Arabidopsis and pepr1-2 pepr2-1 mutant with and without AMF-inoculation. The effects of AMF inoculation on Arabidopsis (wild-type and pepr1-2 pepr2-1 mutant) and B. campestris with or without the pretreatment of Pep1 were also investigated.
2.2 Quantification of AMF parameters
Root samples were stained with 0.05% trypan blue and the percentage of root colonization was quantified using the magnified intersection method (McGonigle et al., 1990) with some modifications (see Extended Methods). To visualize AMF structures in more detail, Arabidopsis roots were also stained with wheat germ agglutinin conjugated with AlexaFluor™ 488. The densities of extra-radical AMF hyphae and spores in the AMF-inoculated soil substrate before and after Arabidopsis cultivation were measured according to standard methods.
2.3 RNA sequencing
The RNA sequencing procedure followed standard protocols described in the Extended Methods. The root and the shoot organs of wild-type Arabidopsis with and without AMF-inoculation were sequenced using an Illumina HiSeq platform, obtaining 46.9–53.4 million clean reads (ca. 150 bp) per sample (mean 49.0 million; Supporting Information: Table S1). In a supplementary experiment, shoot organs from the AMF-inoculated and noninoculated pepr1-2 pepr2-1 mutants were also sequenced. The RNA-seq data was deposited in sequence read archive at the National Center for Biotechnology Information under BioProject ID numbers PRJNA418008 and PRJNA822488.
2.4 Quantitative real-time PCR
Relative quantification of specific mRNA levels was done using the comparative method (Livak & Schmittgen, 2001).
2.5 Measurement of plant physiological parameters
Plant physiological parameters, including biomass, photosynthesis, hormone and P contents, antioxidants levels, cell-wall composition, root architecture and lignin staining were all analyzed according to standard methods as described in the Extended Methods.
2.6 13C pulse labeling, lipid extraction and analysis of signature fatty acids
13C labeling and postlabeling processes were performed as described in our previous report (Olsson et al., 2005). AMF-derived lipids NLFA 16:1ω5 and PLFA 16:1ω5 were used as marker for AMF in roots (Graham et al., 1995; Olsson et al., 2005). NLFA 18:2ω6,9, which occurs in plants but not in AMF, was used as a signature for plant-derived lipid (Graham et al., 1995; Olsson et al., 2005).
2.7 Determination of 13C enrichment and calculation of excess 13C in fatty acids
13C enrichment in fatty acid methyl esters was measured on a Delta IV Plus IRMS coupled to a Trace GC Ultra gas chromatograph via the ConFlow IV interface (Thermo Fisher Scientific) at the Stable Isotope Facility at the Department of Biology at Lund University. 13C enrichment was calculated by subtracting background 13C levels determined from two nonlabeled controls.
2.8 Pretreatment of nonhost plants with elicitor peptides
Arabidopsis (wild-type and pepr1-2 pepr2-1 mutants) and B. campestris seedlings were pretreated with 100 nM elicitor peptides (AtPep1 or BrPep1b) and then transplanted into pots with or without AMF inoculum.
2.9 Statistical analysis
Multiple comparisons among different treatments were conducted using one-way analysis of variance (ANOVA) followed by post hoc Tukey (equal variances) or Dunnett's T3 (unequal variances) tests. A univariate two-way ANOVA followed by a post hoc Tukey test was applied to analyze the effects of AMF species, host identity (at species and cultivar level), and their interactions on AMF colonization intensity and plant root and shoot biomasses. The differences between RI and Control groups were compared using Student's t test (for normal distribution) or nonparametric Mann–Whitney U test (for nonnormal distribution). The correlation between AMF colonization intensity and AMF inoculum potential was determined using Pearson's correlation. For RNA-seq data, differentially expressed gene (DEG) analysis of RI and control plants was performed using the DESeq R package (Anders & Huber, 2012; R Development Core Team, 2014). The resulting p values were adjusted using the Benjamini–Hochberg correction for controlling false discovery rate. Genes with an adjusted p value < 0.05 found by DESeq were assigned as differentially expressed. Gene Ontology (GO) enrichment analysis of DEG was implemented by the GOseq R package (Young et al., 2010), which corrected for gene length bias. GO terms with corrected p value < 0.05 were considered significantly enriched by DEG. The KOBAS software was used to test the statistical enrichment of DEG in Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways (Xie et al., 2011).
3 RESULTS
3.1 AMF colonization and suppression of growth in nonhost plants
Arabidopsis plants grown in single pots were commonly colonized with R. irregularis to between 10% and 20% of their root length under higher AMF inoculation intensities (5%‒9%, Exp. 1; Figure 1a,b). The intensity of AMF colonization of Arabidopsis roots was positively correlated with the inoculation rates (p < 0.001; Figure 1c). Often, the AMF structures were observed in roots that seemed old, dying, or decomposing (Figure 1d–g). All typical AMF structures, including occasional stunted arbuscule-like structures, were observed in most Arabidopsis plants (Figure 1h–k), and vesicles were the most frequent structures in the roots. Arabidopsis was also colonized when inoculated with F. mosseae and a combination of both AMF species (Exp. 2), and under different levels of soil nutrient availability (Exp. 3), which did not change colonization levels (Supporting Information: Figure S1). Ten Brassica cultivars were found to be colonized by R. irregularis, but in contrast to Arabidopsis, no arbuscule-like structures were found in any of the 10 Brassica cultivars (Exp. 4). Typical vesicles and hyphae were observed in most Brassica plants but their colonization rates were generally low (<10%; Supporting Information: Figure S2).
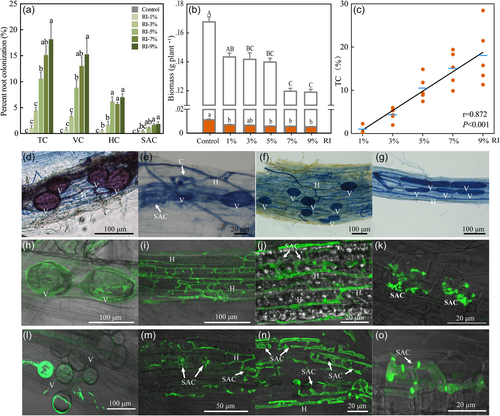
AMF inoculation reduced the biomass of Arabidopsis (Figure 1) and each of the five cultivars of B. campestris and B. napus (p < 0.05; Supporting Information: Figure S2). Growth depression increased with increasing inoculum rates (p < 0.05, Exp. 1), and the inhibitory effect of AMF on Arabidopsis was independent of AMF species identity (Exp. 2) or nutrient application (Exp. 3; Supporting Information: Figure S1). For Arabidopsis harvest at the mature stage, AMF inoculation also suppressed plant height and reduced seed production (p < 0.01; Supporting Information: Figure S3), but did not cause plant mortality. For B. napus and B. campestris, the AMF-induced inhibitory effects varied among different cultivars and AMF inocula; the identities of nonhost plant (at species or cultivar levels) and AMF species both significantly affected the inhibitory degree of AMF (two-way ANOVA, p < 0.05).
In the soil, AMF extraradical hyphal density was increased after Arabidopsis cultivation for 23 days (p < 0.05; Supporting Information: Figure S4a), while AMF spore density was significantly decreased (p < 0.05; Supporting Information: Figure S4b).
3.2 Transcriptional changes of wild-type Arabidopsis upon AMF colonization
A detailed description of the transcriptional responses of wild-type Arabidopsis to AMF inoculation is provided in the Supporting Information Results. Briefly, AMF inoculation induced an extensive and systematic transcriptional reprograming of Arabidopsis, with 2421 and 3094 DEGs assigned at the root and shoot respectively (Supporting Information: Figure S5). In the roots, KEGG pathway analysis detected four up-regulated and five down-regulated pathways with statistical enrichment of DEG, most of which are related to plant secondary metabolism (Padj < 0.05; Supporting Information: Table S4). In the shoot, the statistically up-regulated KEGG pathways were mainly related to plant defense, and the down-regulated KEGG pathways were mostly involved in photosynthesis (Padj < 0.05; Supporting Information: Table S4).
3.3 AMF-derived fatty acid contents and 13C enrichment
In AMF-inoculated plants, the contents of AMF-derived NLFA 16:1ω5 (Figure 2a) and PLFA 16:1ω5 (Figure 2b) in the roots were one to two orders of magnitude higher than the corresponding control roots. This observation confirmed successful AMF colonization in Arabidopsis roots, and enabled us to analyze potential 13C supply from the Arabidopsis host to the AMF using these signature fatty acids.
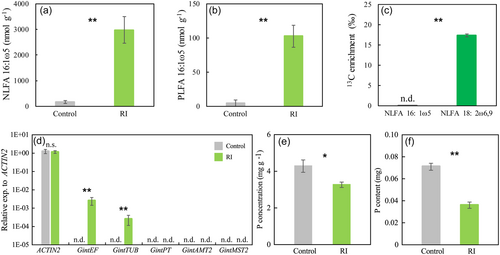
The high 13C concentration in plant-derived NLFA 18:2ω6,9 (Figure 2c) indicated that 13CO2 was successfully fixed and transported to Arabidopsis roots. There was no detectable enrichment of 13C in NLFA 16:1ω5 in the roots (Figure 2c), showing that newly assimilated C in Arabidopsis was not allocated to AMF during the labeling time. Meanwhile, there was a clear 13C enrichment in NLFA 16:0 (8.21 ± 0.76‰), a component commonly present in AMF, plants, and most other organisms (Olsson et al., 1997), and the 13C enrichment was lower than that in plant-derived NLFA 18:2ω6,9 (p < 0.01), supporting the high reliability of the results from 13C labeling experiment.
3.4 Fungal gene expression and plant P content in wild-type Arabidopsis
R. irregularis housekeeping genes GintEF and GintTUB were both detected in all the AMF-inoculated roots, while the fungal metabolism marker genes GintPT, GintAMT2 and GintMST2 were not detectable in any of the analyzed AMF-inoculated root samples (Figure 2d). These results supported successful colonization by AMF in Arabidopsis roots, but at the same time indicated that AMF in Arabidopsis root may not be functional in nutrient transportation. In accordance with this hypothesis, shoot P concentration and content in AMF-inoculated Arabidopsis were both significantly lower than those in the control plants (p < 0.01; Figure 2e,f).
RNA-seq data indicated no differences between AMF-inoculated and control plants in the expression pattern of the PHT1 gene family (Supporting Information: Table S3), which is responsible for phosphate uptake from soil (Nussaume et al., 2011). AMF-inoculated plants had higher levels of PHR1 transcript (AT1G12370, Padj < 0.01) (which controls phosphate starvation-inducible gene expression, Rubio et al., 2001), and lower expression levels of two out of the three PHO1 genes (PHO1 [AT3G23430] and PHO1; H1 [AT1G68740], Padj < 0.05) (which indicates phosphate deficiency, Rouached et al., 2011). Combined with the much lower P content in AMF-inoculated plants, these results suggest that AMF colonization leads to phosphate starvation and deficiency in Arabidopsis.
3.5 Photosynthesis and stress responses in wild-type Arabidopsis
AMF inoculation of wild-type Arabidopsis generally decreased net photosynthetic rate, transpiration rate, dark-adapted photochemical efficiency (Fv/Fm), nonphotochemical quenching and electron-transport rate (p < 0.05) regardless of inoculum type (R. irregularis, F. mosseae, or a mixture of the two; Supporting Information: Figure S6). Chlorophyll a, chlorophyll b and carotenoid contents were all significantly reduced upon AMF inoculation (p < 0.05; Supporting Information: Figure S6). At the transcriptional level, an over-representation of DEGs involved in the photosynthesis process and biosynthesis of photosynthesis-related components were detected, mostly because of lower gene expression in the shoots of AMF-inoculated plants than in control plants (Supporting Information: Table S4 and Figure S7). AMF colonization reduced the light-harvesting efficiency in Arabidopsis as revealed by the reduced amount of light-harvesting pigments and down-regulation of the photosynthetic antenna proteins pathway in AMF-inoculated plants (Supporting Information: Table S4). Down-regulation of the chlorophyll biosynthetic process (GO:0015995) can explain the decrease in photosynthetic pigments. AMF also inhibited the functional activity of the photosystem and interrupted photosynthetic electron transportation in Arabidopsis, as shown by decreased Fv/Fm and electron-transport rate, as well as a significant down-regulation of the GO terms involved in the assembly and functional role of both photosystems I and II (Supporting Information: Figure S7).
AMF inoculation led to an accumulation of peroxidases, malonaldehydes and soluble sugars in plant shoot tissue (Supporting Information: Figure S8), which are common physiological stress responses in plants. Analysis of the secondary metabolism pathway from the RNA-seq data confirmed AMF-introduced stress to Arabidopsis and activation of the defense system in nonhost plants. In the roots, a large number of up-regulated and down-regulated DEGs pertinent to metabolism and biosynthesis of secondary metabolites were detected as a response to AMF inoculation (Supporting Information: Figure S7). Genes involved in the responses to stress, chemical or organic substances, and oxygen-containing compounds were all strongly overrepresented in both down-regulated and up-regulated DEGs in the root. Specifically, in AMF-inoculated roots, the genes involved in the biosynthesis of flavonoids (GO:0009813) and phenylpropanoids (GO:0009699), two important components of secondary metabolites for defense against herbivores and pathogens, were significantly up-regulated. In the shoots, KEGG enrichment analysis also detected an overrepresentation of biosynthesis of secondary metabolites in AMF-inoculated plants compared with noninoculated control, where both flavonoid biosynthesis and phenylpropanoid biosynthesis were significantly up-regulated in the shoots of AMF-inoculated plants (Supporting Information: Table S4), indicating a systemic defense reaction.
3.6 Cell-wall composition and root architecture in wild-type Arabidopsis
The lignin content was significantly higher in roots of AMF-inoculated plants than in control plants, while cell-wall cellulose, hemicellulose and pectin contents were lower in AMF-inoculated plants (p < 0.05; Supporting Information: Figure S9). Microscopic observation of unstained Arabidopsis roots found browning in AMF-colonized segments (Supporting Information: Figure S10a,b). Lignin staining showed localized lignification in AMF-colonized root segments, and the strongest staining was observed at AMF entry point (Supporting Information: Figure S10c‒e). RNA-seq data in the roots showed a significant up-regulation of the GO terms involved in cell-wall disassembly and a significant down-regulation of the GO terms involved in biogenesis of cell-wall polysaccharides in AMF-inoculated plants (Supporting Information: Table S7). AMF-inoculated plants also showed a concerted induction of lignification-related genes (Supporting Information: Figure S10f). The lignin precursor is synthesized in the phenylpropanoid metabolism pathway, and peroxidases catalyze the final enzymatic step in the biosynthesis of lignin. The AMF-induced up-regulation of phenylpropanoid synthesis pathway and higher peroxidase activity can explain the observed lignification in AMF-inoculated roots. In the shoots, several genes encoding for the degradation of polysaccharides were specifically induced by AMF colonization (Supporting Information: Table S5), and a large number of up-regulated DEGs were related to cell-wall metabolism and synthesis (Supporting Information: Table S3).
The length, volume and average diameters of the Arabidopsis root system were all significantly lower in AMF-inoculated plants than those in control plants (p < 0.05; Supporting Information: Figure S11). The density and average length of the root hair in the four analyzed root sections (0‒1, 1‒2, 2‒3 and 3‒4 cm from the root tip) were in most cases significantly lower in AMF-inoculated roots than in control roots (p < 0.05).
3.7 Plant hormone and defense responses in wild-type Arabidopsis
In the shoots, the JA content was higher in AMF-inoculated plants (p < 0.05; Figure 3d,e), and the JA signaling processes (VSP1, VSP2, GO:0009695) were significantly up-regulated by AMF inoculation at 23 days postinoculation (Padj < 0.05; Figure 3c). In the roots, JA and SA contents were lower in the AMF-inoculated plants (p < 0.05; Figure 3d,e), and accordingly the JA- and SA-signaling genes (e.g., VSP1, VSP2, TGA1) were significantly down-regulated (Padj < 0.05; Figure 3c) at 23 days postinoculation.
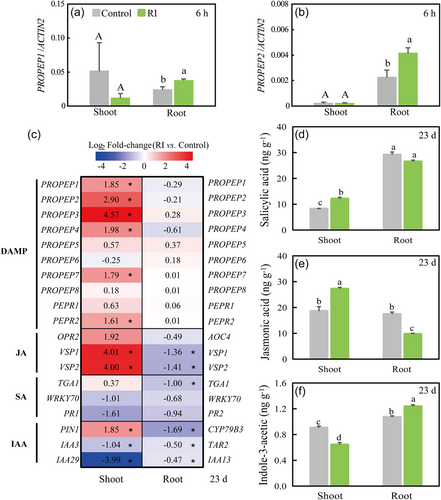
AMF-inoculated plants had lower IAA contents than noninoculated plants in shoots (p < 0.05), but higher IAA contents in roots (p < 0.05; Figure 3f). The RNA-seq analysis showed significant down-regulation of the IAA (GO:0009684) and auxin (GO:0009851) biosynthetic processes in both the shoots and roots (Figure 3c). Furthermore, there was a significant down-regulation of the KEGG pathway in tryptophan (the main IAA precursor) metabolism (ath00380) in AMF-inoculated roots. Up-regulation of PIN1 (Figure 3c), the key gene in charge of downward transportation of IAA, suggested that the increase in IAA downward transport from shoot to root may be an important reason for increased IAA content in the root.
In the shoots, the expression of DAMPs AtPep precursor genes (PROPEP1‒4, 7) and their receptor (PEPR2) and coreceptor (SERK1) genes were all significantly up-regulated upon AMF inoculation (Padj < 0.05; Figure 3c), suggesting a typical DAMP-triggered immunity at the transcriptional level. In the roots, the detected DAMPs marker genes PROPEP1 and PROPEP2 were significantly up-regulated as early as 6 h postinoculation (Figure 3a,b), though at 23 days postinoculation there was no difference in gene expression of the AtPep-PEPR pathway between AMF-inoculated and control roots as measured by RNA-seq data (Figure 3c).
The results of the expression dynamics of the SA-, JA-, ET-, and IAA-signaling marker genes in wild-type Arabidopsis with and without AMF inoculation are provided in the Supporting Information (Results, Figures S12 and S13).
3.8 AMF colonization and plant growth in Pep-PEPR signaling and SA-, JA-, ET- and IAA-signaling mutants of Arabidopsis
AMF colonization intensity in the single PEPR mutant pepr1-2 or pepr2-1 was similar with the wild-type Arabidopsis, as indicated by both microscopic counting and qPCR analysis (Figure 4a,b). However, the double mutant pepr1-2 pepr2-1 had a significantly higher root colonization intensity than wild-type Arabidopsis (p < 0.05; Figure 4a, more details are shown in Supporting Information Materials: Figure S14a), which was further confirmed by qPCR analysis of the AMF marker genes (p < 0.05; Figure 4b). These results suggest the participation of the Pep-PEPR signaling pathway in resistance to AMF colonization in Arabidopsis roots, and functional redundancy of PEPR1 and PEPR2 in this process.
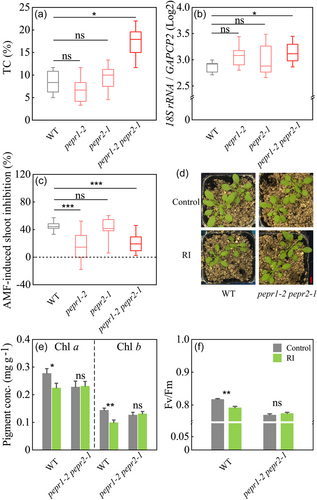
Although AMF colonization significantly reduced the shoot biomass of wild-type Arabidopsis and all the PEPR mutants (Figure 4c,d), the AMF-induced growth suppression in the pepr1-2 and pepr1-2 pepr2-1 mutants was significantly lower than that in wild-type (Figure 4c,d), suggesting the involvement of the Pep-PEPR signaling pathway in mediating growth suppression upon AMF colonization.
The results for AMF colonization and plant growth in SA-, JA-, ET-, and IAA-signaling mutants are provided in the Supporting Information (Results, Figure S15).
3.9 Transcriptional and physiological responses of pepr1-2 pepr2-1 mutants upon AMF inoculation
The global transcriptional responsiveness to AMF inoculation in pepr1-2 pepr2-1 mutant shoots was much weaker than that in wild-type Arabidopsis, with 133 up-regulated and 220 down-regulated DEGs observed in the mutant (Figure 5a). The transcriptomic responsive pattern of the pepr1-2 pepr2-1 mutant to AMF was quite different than in wild-type Arabidopsis (Figure 5b), with significantly down-regulated protein export, phagosome and DNA replication and up-regulated amino acids metabolism and degradation (Padj < 0.05; Supporting Information: Figure S16). The vast majority of AMF-induced DEGs in wild-type Arabidopsis were not changed in the pepr1-2 pepr2-1 mutant (Figure 5c). Notably, the KEGG pathways involving in photosynthesis (ath00195, ath00196, ath00860) and biosynthesis of secondary metabolites (ath01100), all of which were significantly down-regulated upon AMF inoculation of wild-type Arabidopsis, were unchanged in the pepr1-2 pepr2-1 mutant (Figure 5d).
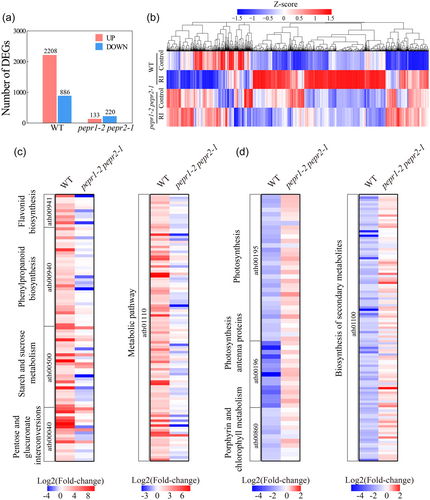
Consistent with the transcriptomic data, additional physiological measurements of the photosynthetic parameters also showed that the AMF-induced reduction of photosynthetic pigments and inhibition of Fv/Fm in wild-type Arabidopsis were abrogated in the pepr1-2 pepr2-1 mutant (Figure 4e,f).
3.10 Effects of Pep1 pretreatment on AMF colonization and growth depression in nonhost plants
Pretreatment of seedlings with AtPep1 significantly decreased AMF colonization rates in the roots of wild-type Arabidopsis (p < 0.05), but not the pepr1-2 pepr2-1 mutant (Figure 6a and Supporting Information: Figure S17a). In B. campestris (cultivar: ZC), pretreatment with Pep1 (BrPep1b) also significantly reduced root colonization by R. irregularis (p < 0.05; Figure 6c and Supporting Information: Figure S17c). These results corroborate the hypothesized participation of the Pep-PEPR signaling pathway in resistance to AMF colonization in nonhost plants.
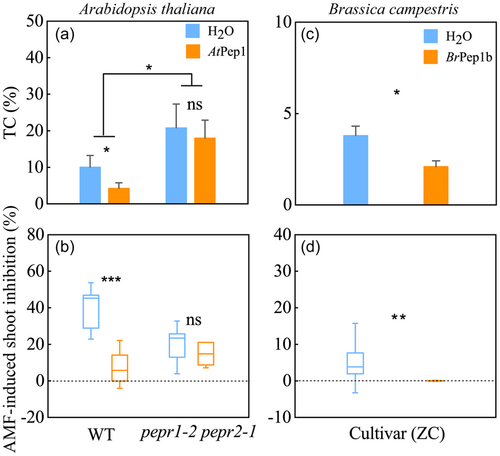
In noninoculated Arabidopsis, pretreatment with AtPep1 significantly inhibited shoot growth of the wild type, but not of the pepr1-2 pepr2-1 mutant (Supporting Information: Figure S17b). AMF-induced growth suppression was largely and significantly relieved by AtPep1 pretreatment in wild-type Arabidopsis (p < 0.05), but not in the pepr1-2 pepr2-1 mutant (Figure 6b and Supporting Information: Figure S17b). In B. campestris, pretreatment with BrPep1b also significantly reduced AMF-induced growth suppression (p < 0.01; Figure 6d and Supporting Information: Figure S17d), though this pretreatment did not significantly inhibit growth of B. campestris (p > 0.05; Supporting Information: Figure S17d). These results further corroborate the participation of Pep-PEPR signaling in mediating growth suppression upon AMF inoculation in nonhost plants.
4 DISCUSSION
4.1 AMF colonization and growth suppression of individually grown nonhost plants
This work shows that AMF can colonize individually grown Arabidopsis if high inoculum intensity is applied, providing an excellent system for further studies on the interaction between AMF and nonhost plants (see Supporting Information Discussion). The obvious inhibitory effects of AMF on the growth of Arabidopsis and Brassica species and cultivars were repeatedly observed in our microcosm experiments, without any neighboring AMF-host plants. The degree of inhibition depended on the identity of AMF and nonhost species, but not on soil nutrient levels, and inhibition also occurred when only a relatively low AMF inoculum potential was applied. The results suggest that direct inhibition by AMF is widespread in nonhost plants, at least in Brassicaceae, which includes many agronomically important crops (Brundrett, 2017; Ocampo et al., 1980). These results have important implications for our understanding of the roles AMF play in the regulation of plant community structures and in crop rotations.
4.2 The Pep-PEPR signaling pathway as a key participant in resistance against AMF colonization and in mediating AMF-induced growth suppression of nonhost plants
The gene expression profile from the Pep-PEPR signaling pathway suggested activation of DAMPs-triggered immunity upon AMF inoculation early in Arabidopsis roots and later in shoots. Further analyses captured the typical symptoms of Pep-PEPR-mediated defense responses in Arabidopsis due to AMF inoculation, including cell-wall lignification, modification of cell-wall components, photosynthesis suppression, secondary metabolism enhancement and oxidative stress. Furthermore, impairment of the Pep-PEPR pathway in Arabidopsis greatly compromised the defensive responses and photosynthesis suppression triggered by AMF, leading to higher colonization rates and reduced growth suppression upon AMF inoculation. Pretreatment with Pep peptide decreased AMF colonization and largely compensated for AMF-induced growth suppression in nonhost plants. These results show the important role of Pep-PEPR signaling in resistance to AMF colonization and in mediating growth suppression upon AMF inoculation. Previously, Pep-PEPR signaling has been reported to be an important mechanism in systemic resistance of plants to fungal pathogens, virus, nematodes and herbivores by amplifying the innate immune responses (Poretsky et al., 2020; Zeng et al., 2020; L. Zhang & Gleason, 2020). Here we identified Pep-PEPR signaling to be a key factor in the incompatible interaction between nonhost plants and AMF. The observed cell-wall modifications, induced expression of cell-wall degradation genes and extensive hormone regulations upon AMF inoculation in our experiments imply that the release of DAMPs could be triggered by certain cell-wall fragments and/or defense hormones (Engelsdorf et al., 2018; Hander et al., 2019; Huffaker & Ryan, 2007), but further research is needed to clarify this process.
Previous studies have demonstrated the involvement of SA signaling in defense against AMF colonization in nonhost plants (Fernández et al., 2019; Poveda et al., 2019). Our extensive survey of SA-, JA-, ET-, and IAA-related mutants found higher AMF colonization intensities in SA (npr1-1 and cbp60g-1) and ET (ein2-1) signaling mutants, indicating the direct or indirect participations of the SA and ET signaling pathways in resistance to AMF colonization. Meanwhile, AMF-induced growth suppression in these SA and ET mutants was generally higher than that in the wild-type plant, supporting the suggestion that single SA, ET or other hormones alone might not operate as primary mechanisms of AMF incompatibility nor of growth antagonism (Cosme et al., 2021). It has been reported that Pep-PEPR signals inhibited plant growth via crosstalk with hormone signaling (Jing et al., 2019). In this study, with the activation of Pep-PEPR signals, there were significant decreases in developmental hormones and increases in defense hormones in the shoots of AMF-inoculated Arabidopsis, resembling the symptoms of plants suffering from pathogen attack (Huot et al., 2014), in which plants mobilize immunity responses at the expense of growth inhibition via hormonal regulation (Castrillo et al., 2017). It was found that in roots, Pep-PEPR signaling undergoes crosstalk with IAA accumulation to control growth and root-hair development during immune responses (Jing et al., 2019). Here, we also observed IAA accumulation and suppressed growth and root-hair development upon AMF inoculation in Arabidopsis roots where Pep-PEPR signals had been activated at an early stage. In general, our results suggest an extensive interplay between the Pep-PEPR and hormone pathways in nonhost plants in coordination of growth and defense upon AMF inoculation, though the mechanism of their interaction is not yet clear.
Host plants also exhibit some stress and defense responses during their initial contact with AMF (Blilou et al., 1999; Gutjahr & Paszkowski, 2009) or other mutualists (Zipfel & Oldroyd, 2017), and in several host species the up-regulation of some defense-responsive genes have been reported upon AMF colonization, namely ‘defense priming’, including genes involved in lignin synthesis and phenylpropanoid metabolism (Chialva et al., 2018; Delaux & Schornack, 2021; Fiorilli et al., 2015; but see Cosme et al., 2021). However, the initial physiological defense responses are relatively mild, and they are switched off after a few days to allow for subsequent colonization in a compatible AMF host (Gutjahr & Paszkowski, 2009; Martinez-Medina et al., 2016; Pieterse et al., 2014). An important difference found in this study is that there were strong and persistent defense symptoms, with the Pep-PEPR signaling as a key participant in AMF-inoculated Arabidopsis, which suggests that the defense response activated at an early stage of AMF colonization has not been switched off in the AMF-inoculated Arabidopsis. Poveda et al. (2019) also showed AMF-induced root defenses in 21-day-old Arabidopsis plants grown alone. Fernández et al. (2019) found the activation of defenses in Arabidopsis co-cultivated with a mycorrhizal host plant, but not in the individually-grown Arabidopsis. This inconsistency may be due to the differences in cultivation systems (e.g., inoculum rates). Interestingly, activation of plant defenses has also been reported in some AM-defective mutants of host plants that were co-cultivated with mycorrhizal host plants (Blilou et al., 1999; Chen et al., 2021), and in Arabidopsis, persistent and systemic resistance also occurred upon inoculation with the ectomycorrhizal fungus Laccaria bicolor (Vishwanathan et al., 2020), implying a functional similarity of mycorrhizal or unwanted fungi in nonhost plants. More comparisons between the responses of host and nonhost plants to AMF-inoculation is provided in the Supporting Information Discussion section.
4.3 Lack of significant C‒P exchange during the Arabidopsis-AMF interaction
Using 13C isotope labeling supplemented with the profiling of AMF metabolism marker gene expression, we clearly showed that Arabidopsis allocated little C, if any, to the lipid synthesis in AMF. This observation may be explained by the absence of symbiotic toolkit genes in Arabidopsis (Delaux et al., 2014), and might be closely related to the near absence of arbuscules in Arabidopsis roots, including the pepr1-2 pepr2-1 mutant. The lack of C allocation to the lipid synthesis of AMF should be an important strategy for nonhost plants in restricting AMF proliferation in their roots. AMF are obligate symbionts that depend on host lipids to complete their life cycle (Jiang et al., 2017; Luginbuehl et al., 2017), though their extraradical mycelium may be able to take up certain sugars (Helber et al., 2011). Therefore, an unanswered question is, what was the main C source (particularly lipids) for the AMF structures in Arabidopsis roots? Previous studies found that R. irregularis colonization in Arabidopsis activated the biosynthesis of strigolactones and exudation of scopoletin (a coumarin), both of which can stimulate extensive hyphal branching and fungal metabolism (Akiyama et al., 2005; Cosme et al., 2021; Fernández et al., 2019). Those observations may well explain the reduction in AMF spores and increase of extra-radical hyphal density in soil after Arabidopsis cultivation in this study. Our results imply that the amount of lipids stored in AMF spores from the inoculum could have sustained to a limited extent nonsymbiotic fungal growth and colonization of Arabidopsis roots. This view would also be supported by the positive relationship between colonization level of Arabidopsis roots and the application rates of AMF inoculum. Further research is needed to fully reveal the C source for AMF growth in nonhost plants.
We provided several lines of evidence that the ‘AM pathway’ might only contribute to a minor part, if any, of P acquisition in Arabidopsis: (ⅰ) the mycorrhizal metabolism marker genes GintPT, GintAMT2 and GintMST2, which are usually abundantly expressed in AMF from host roots (Bao et al., 2019; Fiorilli et al., 2013; Helber et al., 2011), were not detectable in AMF-inoculated Arabidopsis roots, as observed here and by Fernández et al. (2019); (ⅱ) the shoot P content in the AMF-inoculated plants were lower than that in control Arabidopsis, which can be additionally explained by suppressed root development and/or a potential competition for soil P between AMF and the plants; (ⅲ) the arbuscular structure, where P delivery occurs, was underdeveloped and nearly absent in nonhost roots; (ⅳ) the level of P applied to the nonhost plants showed neither an effect on AMF colonization intensities nor on the inhibitory effects of AMF to Arabidopsis growth. Although more research on the potential movement of P between AMF and nonhost plants is still needed to reveal the mechanisms, our results suggest the absence of significant mycorrhizal P delivery to Arabidopsis. This effect may be related to the lack of plant C allocation to AMF, since in AM symbiosis, the C‒P exchange is functionally linked (Hammer et al., 2011; Kiers et al., 2011). Therefore, this study suggests that there is no significant C‒P exchange during the interaction between Arabidopsis and AMF, even when nonhost plants were P limited and suffered from impaired acquisition capacity for P (Figure 2 and Supporting Information: Figure S11). AM symbiosis is clearly more complex than simply C costs and P benefits (Hoeksema et al., 2010); for example, the AM-induced increase of defense hormones (SA and JA) in Arabidopsis shoots, as observed here, probably enhances plant resistance to biotic stress, as was seen by Fernández et al. (2019). However, the C‒P exchange is the cornerstone of AM symbiosis and a key factor in predicting the outcome of this relationship (Johnson, 2010). The loss of this basic symbiotic function should be closely linked to symbiotic incompatibility during interactions between AMF and nonhosts, and may be a critical diagnosis criterion for nonhost species.
5 CONCLUSION
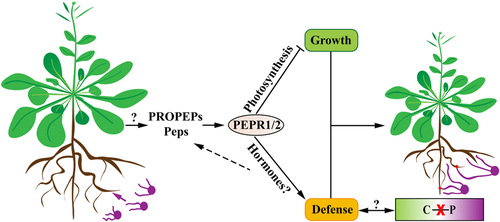
We observed AMF colonization in individually grown nonhost plants, which caused inhibitory effects, and identified the Pep-PEPR pathway as a key participant in resistance to AMF colonization and in mediating growth suppression of nonhost plants upon AMF inoculation (Figure 7). 13C isotope labeling and physiological analyses detected no significant C‒P exchange between Arabidopsis and AMF, indicating loss of the nutritional function in this interaction. This work has increased our knowledge of the functional relevance of AMF and their interactional mechanisms with nonhost plants.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Lars Olof Björn (Lund University) for helpful suggestions and Dr. Jürgen Kuhn (Lund University) and Dr. Jing Zhang (Chinese Academy of Sciences) for technical assistance in IRMS and PLFA analyses. We thank Dr. Lai Jianbin (South China Normal University) for providing Arabidopsis pepr1-2, pepr2-1 and pepr1-2 pepr2-1 seeds. This work was financially supported by grants from the National Natural Science Foundation of China (32272203, 31772397, 31400365), National Key Research & Development Program of China (2022YFC3103700), the Guangdong Pearl River Scholar Funded Scheme (2012), the Leading Scientists Project in Guangdong Province (2010-475) and the Pearl River S&T Nova Program of Guangzhou (201806010186).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




