SlMIR164A regulates fruit ripening and quality by controlling SlNAM2 and SlNAM3 in tomato
Summary
MiRNAs are important posttranscriptional regulators of plant development. Many miRNAs, such as the conserved miR164 species, are encoded by families of MIRNA genes, but the specific roles of individual MIRNA genes are largely undefined. Here, we characterize the functions and regulatory mechanisms of SlMIR164A, one of the primary genes of Sly-miR164, in tomato. We show that SlMIR164A is preferentially expressed at late stages of fruit development and plays a vital role in controlling fruit ripening and quality. Loss of function of SlMIR164A by CRISPR/Cas9-mediated mutagenesis results in accelerated fruit ripening and enhanced chloroplast development, which leads to altered sugar and organic acid contents and affects the nutritional quality of fruits. We also show that SlMIR164A modulates fruit ripening and quality through specific target genes, SlNAM2 and SlNAM3, which control key regulators of chloroplast function and fruit ripening processes. MIR164 genes have been shown to play conserved roles in regulating organ ageing, such as leaf senescence and fruit ripening, in a variety of plants, but whether and how their family members in tomato exert the same function remain to be elucidated. Our results reveal a previously undiscovered role of SlMIR164A in ripening control, which will further our understanding of the actions of MIR164 family, as well as the mechanisms of fruit ripening and quality control in tomato. Moreover, as loss of SlMIR164A exhibits minor impacts on organ morphology, our results can be leveraged in tomato breeding for specific manipulation of fruit ripening and quality to facilitate tomato improvement in agriculture.
Introduction
microRNAs (miRNAs) are a class of small non-coding RNAs generated from stem-loop precursors, which mediate post-transcriptional regulation of gene functions in a lot of important developmental and physiological processes (D'Ario et al., 2017; Rogers and Chen, 2013). Many plant miRNAs, especially the evolutionarily conserved ones, are encoded by multiple MICRORNA (MIRNA) genes from large families (Cui et al., 2017; Cuperus et al., 2011). These MIRNA family members may carry out partially redundant but specialized roles (Voinnet, 2009); therefore, characterizing the functions of individual MIRNA genes is critical for comprehensively deciphering the biological roles of the entire family.
miR164 is one of the conserved plant miRNAs, which plays vital roles in plant development and stress response pathways (Li et al., 2017; Liu et al., 2018). Two major functions of miR164 are the regulation of organ morphogenesis and ageing, and miR164 acts in these two processes by targeting genes in the NAM-ATAF-CUC (NAC) family (Kim et al., 2009; Sieber et al., 2007; Wang et al., 2020). Similar to other conserved miRNAs, primary miR164s in many species are transcribed from multigene families (Jasinski et al., 2010). However, the functions of individual MIR164 genes have only been investigated in a limited number of species (Kim et al., 2009; Sieber et al., 2007; Wang et al., 2020). Tomato is one of these model species in which the biological roles of specific MIR164 genes are partly characterized. The tomato miR164 (Sly-miR164) is encoded by four SlMIR164 genes, and two major genes, SlMIR164A and SlMIR164B, produce the same mature miRNA that is much more frequently identified in the published small RNA data than the other two Sly-miR164 species (Gupta et al., 2021). The loss-of-function mutants of SlMIR164A and SlMIR164B have been studied in the tomato cultivar M82, which shows that slmir164b primarily affects organ boundary specification in shoot and flower development, and slmir164a exhibits morphological defects mainly at fruit stages by influencing pericarp cell division/expansion and cuticle development (Gupta et al., 2021). These phenotypes suggest that SlMIR164A and SlMIR164B may carry out critical and specific functions in tomato organ morphogenesis. However, whether and how SlMIR164 genes regulate organ ageing, particularly fruit ripening, as their relatives in other species are still unknown. Although the slmir164 mutants in M82 do not appear to show obvious ripening phenotypes, it can be attributed to the modest expression of Sly-miR164 at ripening stages of M82 fruits (Hendelman et al., 2013). Therefore, a tomato cultivar with a strongly elevated level of Sly-miR164 in the ripening fruits, such as MicroTom ((Mohorianu et al., 2011) and our study), is probably more suitable for assessing the role of SlMIR164 genes in fruit ripening control.
To deduce whether SlMIR164 genes may act in the regulation of fruit ripening, we first examined the expression patterns of SlMIR164A and SlMIR164B during the development of MicroTom in this study. We detected specific expression of SlMIR164A, but not SlMIR164B, in the fruit ripening stages, which strongly indicates the potential role of SlMIR164A in fruit ripening control. We also generated the SlMIR164A mutant (slmir164aCR) using CRISPR/Cas9. Phenotypic examination of slmir164aCR further confirmed our hypothesis that SlMIR164A primarily controls the process of fruit ripening in MicroTom. In addition, we found that chloroplast morphology and function are affected, and nutritional metabolite contents are altered in the fruits of slmir164aCR, suggesting that SlMIR164A also modulates chloroplast development and fruit quality in MicroTom. Moreover, we also demonstrate that SlMIR164A may carry out its roles in fruit development by targeting specific NAC genes, SlNAM2 and SlNAM3, which act to control key regulators of chloroplast function and fruit ripening. We propose that the SlMIR164A-SlNAM2/SlNAM3 module represents a previously unidentified pathway to control chloroplast development, fruit ripening and quality in tomato.
Results
Expression analysis of Sly-miR164 and SlMIR164A/SlMIR164B genes in MicroTom development
To assess the pattern of Sly-miR164 expression in MicroTom, we first conducted a northern blotting experiment on different tomato tissues at various developmental stages. Although the expression of Sly-miR164 was detected in all samples, we found that it is more highly accumulated in the shoot apical meristem, young leaf primordia and late-stage flowers (Figure 1a-b). As tomato is a model system for studying fruit development, we specifically analysed the expression of Sly-miR164 at different fruit stages. We detected that Sly-miR164 is preferentially expressed in the ripening stages (from 30 to 45 days post anthesis) during fruit development (Figure 1c), which is in agreement with the previous study in MicroTom (Mohorianu et al., 2011). Following this analysis, we also performed qRT-PCR assays on the primary SlMIR164A and SlMIR164B genes to examine their respective contributions to the overall expression of Sly-miR164. We found that SlMIR164B is more actively transcribed than SlMIR164A in the vegetative and floral stages, while the expression of SlMIR164A is primarily detected in the developing fruits, particularly at fruit ripening stages when SlMIR164B is expressed at a very low level (Figure 1d-f). When comparing this result with that of northern blotting, it can be seen that the Sly-miR164 molecules produced from SlMIR164B are probably the major contributors of the overall Sly-miR164 population in vegetative and floral organs, and those from SlMIR164A may constitute majority of the Sly-miR164 pool in fruits. Furthermore, we also generated the tomato transgenic line containing the SlMIR164Apro:GUS reporter, and detected strong SlMIR164Apro:GUS activities in the later stage fruits (Figure S1a-b), which further confirms the expression specificities of SlMIR164A shown by qRT-PCR. Together, these data demonstrate that SlMIR164A and SlMIR164B have diverse temporal and spatial expression patterns, which suggests that these two SlMIR164 genes may contribute distinctly to the overall activities of Sly-miR164 in tomato development. In particular, the specific expression of SlMIR164A and Sly-miR164 in the late fruit stages strongly implies that Sly-miR164 generated from SlMIR164A may carry out vital roles in the important processes during late fruit development, such as fruit ripening, in MicroTom.
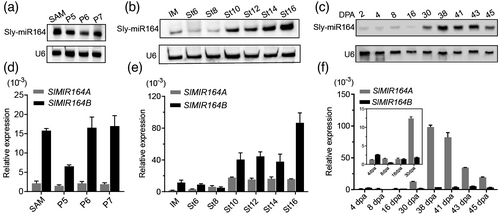
Generation and phenotypic analyses of the CRISPR/Cas9-engineered mutants of SlMIR164A
To further characterize the functions of SlMIR164A, we utilized the CRISPR/Cas9 system to create SlMIR164A mutants (slmir164aCR) in the MicroTom background. We designed a pair of guide RNAs (gRNAs) that recognize the precursor sequence of SlMIR164A and transformed them in wild-type MicroTom plants (Figure S2a). We obtained multiple independent T1 transgenic lines in which the Sly-miR164 sequence is completely deleted in the SlMIR164A gene, and selected three homozygous plants for detailed analyses (Figure S2b and c). We assessed the level of Sly-miR164 in the fruits of each transgenic line by northern blotting and detected that the abundance of Sly-miR164 was significantly decreased in all three slmir164aCR mutants compared with wild type (Figure S2d). We also found that the expression of SlMIR164B was similar in slmir164aCR and wild type (Figure S2e), suggesting that the transcription of SlMIR164B was not affected by the loss of SlMIR164A. We then performed detailed phenotypic analyses of these slmir164aCR lines. We first observed that slmir164aCR plants show almost no discernible changes in vegetative and floral organ growth as compared with wild type, except that the number of leaf serration is increased (Figure S2f-l), which is consistent with the weak expression of SlMIR164A in vegetative and floral development (Figure 1d-e). Moreover, fruits of these slmir164aCR plants also have similar perimeter, area and weight to those of wild type (Figure S2m-q), which is different from the reduced fruit growth phenotype observed in the M82 slmir164a mutant (Gupta et al., 2021). These results indicate that SlMIR164A may not play a critical role in the regulation of organ morphology in MicroTom and different SlMIR164A genes probably exert specific developmental effects in different tomato varieties.
Although loss of SlMIR164A in slmir164aCR does not result in strong alteration of fruit morphology, we notice its marked effect when the fruit undergoes the ripening process, which is in agreement with the strong expression of SlMIR164A in the fruit ripening stages (Figure 1f; Figure S1). The most important phenotype of slmir164aCR is the earlier ripening of fruits. The time from anthesis to onset of ripening is reduced by about 5 days in slmir164aCR relative to wild type (Figure 2a-b). We also examined the production of ethylene and accumulation of major pigments (lycopene, lutein and β-carotene) in the ripening fruits. We found that these key ripening-associated parameters are all at a higher level in slmir164aCR than in wild type (Figure 2c-f), which further supports that fruit ripening is accelerated by the loss of function of SlMIR164A. Moreover, we found that exogenous ethylene treatment induces the expression of SlMIR164A, leading to an increased level of Sly-miR164 in the fruit (Figure 2g-i), while application of the ethylene suppressor 1-methylcyclopropane (1-MCP) represses the transcription of SlMIR164A and accumulation of Sly-miR164 (Figure 2j-l). This suggests that the markedly elevated expression of SlMIR164A and Sly-miR164 in the ripening stages of MicroTom development is likely attributable to the induction by ethylene. As the ethylene level is increased in slmir164aCR, which shows a repressive role of SlMIR164A on ethylene biosynthesis, we propose that SlMIR164A may mediate a negative feedback regulation of ethylene production to fine-tune the process of fruit ripening in MicroTom.
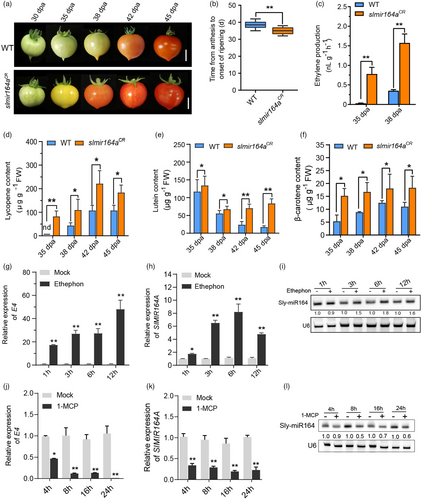
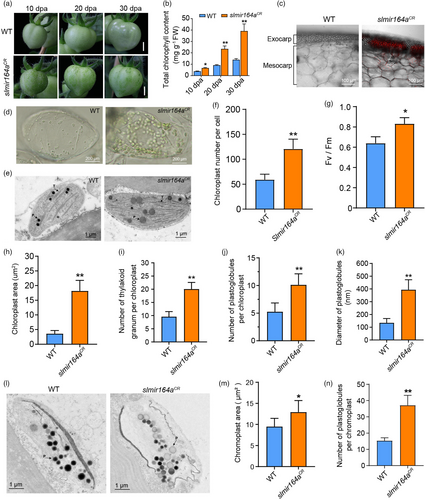
In addition to the early ripening phenotype, we also noticed that slmir164aCR fruits form numerous dark green spots at immature and mature green stages (Figure 3a), which resembles the tomato mutants with enhanced chloroplast functions (Wu et al., 2020). We then assessed the fruit chlorophyll content and chloroplast development in slmir164aCR as compared with wild type. Our results show that the chlorophyll level, chloroplast number and photosynthetic efficiency are all higher in the fruits of slmir164aCR relative to wild type (Figure 3b-d, f, g). Furthermore, we analysed the chloroplast structure in the pericarp of the dark green areas on slmir164aCR fruits and in the comparable fruit regions of wild type using transmission electron microscope (TEM). We found that the chloroplast area, the number of thylakoid grana, as well as the number and size of plastoglobules in chloroplasts are all increased in slmir164aCR compared with wild type (Figure 3e, h-k). As these dark green spots turn yellow at orange and red fruit stages, we also used TEM to examine the structure of chromoplasts in the pericarp of the yellow regions on ripening fruits. Consistently, we detected larger chromoplast areas and more plastoglobules per chromoplast in slmir164aCR compared with wild type (Figure 3l-n). Together, these results demonstrate that SlMIR164A plays a vital role to modulate the development of chloroplast and chromoplast in MicroTom.
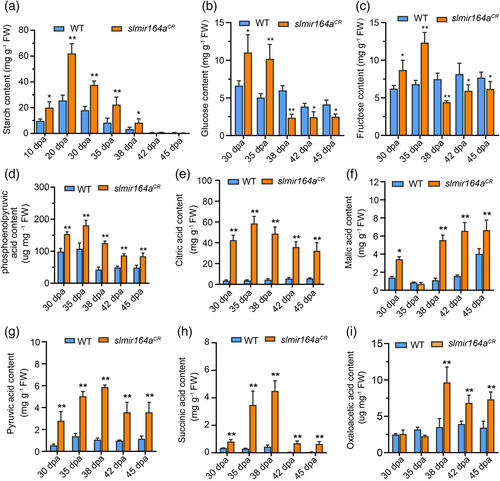
As the alteration of fruit ripening and chloroplast development both impact fruit metabolism, particularly that of sugar and organic acid compounds, which influences the nutritional quality of fruits, we also analysed the contents of starch, glucose, fructose and major types of organic acids in the ripening fruits of slmir164aCR and wild type. We found that the levels of starch and all organic acids are elevated in slmir164aCR at most of the examined fruit stages (Figure 4a, d-i), while the abundances of glucose and fructose are increased from 30 to 35dpa and declined at later stages during fruit ripening of slmir164aCR as compared with wild type (Figure 4b-c). These data show that SlMIR164A also acts in the regulation of fruit metabolism, and ultimately affects fruit quality in MicroTom.
Genome-wide analysis of the downstream genes of SlMIR164A in fruit development
To investigate the molecular mechanisms that underlie the functions of SlMIR164A in regulating fruit ripening and quality, we performed genome-wide transcriptomic studies to analyse the gene expression changes in the ripening fruits (30, 35 and 42 dpa) of slmir164aCR as compared with those of wild type (Figure S3a). We identified 8683, 11385 and 8444 differentially expressed genes between slmir164aCR and wild type at each of the above developmental stages (Figure S3b-d; Data S1). To analyse the specific patterns of expression dynamics of all DEGs during the fruit development of slmir164aCR and wild type, we performed a k-means clustering approach and classified these genes into six clusters (Figure S3e; Data S2). Specifically, cluster 1 and cluster 2 consist of genes whose expressions are in general higher in slmir164aCR than in wild type, while cluster 3, cluster 4 and cluster 5 represent genes with lower expressions in slmir164aCR than in wild type, and genes in cluster 6 show a complex pattern with decreased expression at 30dpa but increased expression at 35dpa in slmir164aCR compared with wild type (Figure S3e-f). Furthermore, the clusters with a similar pattern of gene expression changes also show specificities in the level and time of the expression differences between wild type and slmir164aCR. For example, cluster 1 and cluster 3 are composed of genes with increased and decreased expression in slmir164aCR primarily at 30 dpa, and the DEGs in cluster 2 and cluster 4/cluster 5 are specifically up- and down-regulated in the 35dpa and/or 42dpa slmir164aCR fruits respectively (Figure S3e-f). These expression patterns show diverse responses of the DEGs to the loss of SlMIR164A at different developmental stages, which implies that genes in different clusters may carry out largely distinct roles in mediating the functions of SlMIR164A. These diversified pathways constitute a complex genetic network that SlMIR164A acts through in the regulation of fruit ripening and quality in tomato.
Expression and phylogenetic analyses of the Sly-miR164-targeting NAC genes in tomato
NACfamily members SlGOB, SlNAM1, SlNAM2 and SlNAM3 are major target genes of Sly-miR164 (Hendelman et al., 2013; Karlova et al., 2013). To elucidate how these four genes act in the regulation of fruit ripening and quality by SlMIR164A, we specifically examined their expressions in slmir164aCR relative to wild type in the RNA-seq data. We found that the expressions of SlNAM2 and SlNAM3 are more dramatically increased than those of SlGOB and SlNAM1 in slmir164aCR as compared with wild type (Figure S4b). We further confirmed the RNA-seq result using qRT-PCR, which shows a specific induction of SlNAM2/SlNAM3 transcription in the slmir164aCR fruits at 30, 35 and 42 dpa (Figure S4c-e). We also assessed the expressions of these NAC genes in the fruit developmental stages of wild type (Figure S4f) and compared their patterns with that of SlMIR164A (Figure 1f). We observed that SlNAM2 and SlNAM3 are highly expressed at early fruit stages, which shows a pattern complementary with that of SlMIR164A expression, while SlGOB and SlNAM1 both have low transcript levels during fruit development (Figure S4f; Figure 1f). These results suggest that SlNAM2 and SlNAM3 may operate as primarily targets to mediate the function of SlMIR164A in fruit ripening and quality control. This hypothesis is also supported by our phylogenetic analysis of the miR164-targeting NAC genes in Arabidopsis, tomato and another climacteric fruit model species kiwifruit (Actinidia deliciosa), which shows that SlNAM2/SlNAM3 are more closely related to senescence and ripening-associated NACs, such as ORE1, AdNAC6 and AdNAC7 (Kim et al., 2009; Wang et al., 2020) (Figure S4a).
SlNAM2and SlNAM3 promote the transcription of chloroplast/photosynthesis-related genes in cluster 1 and fruit-ripening-associated genes in cluster 2
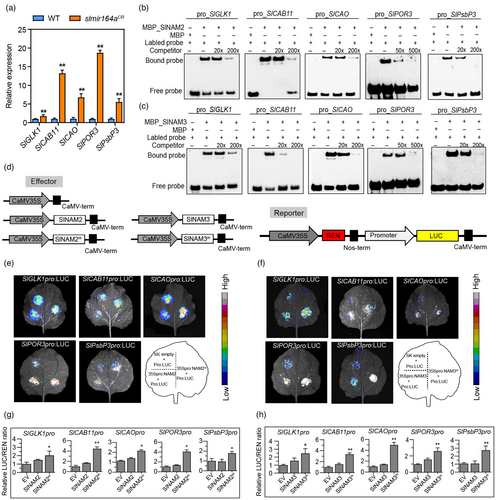
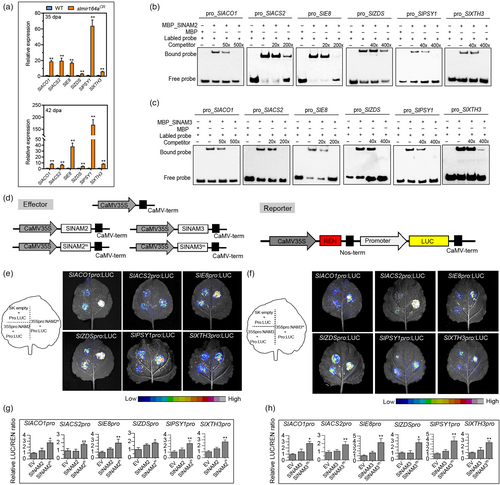
To further investigate how SlNAM2 and SlNAM3 function through their targets to mediate the regulation of SlMIR164A on fruit ripening and quality, we assessed the distributions of putative target genes of NAC transcription factors in the downstream pathways of SlMIR164A revealed by the RNA-seq result. We first analysed the presence of the NAC binding sites (NACBS (Lindemose et al., 2014)) on the promoters of DEGs in the six clusters in (Data S3), and found that NACBSs are enriched in cluster 1 and cluster 2 (Figure S5a). This indicates that a significant number of genes in these two clusters may be directly regulated by SlNAM2 and SlNAM3. Interestingly, GO analyses suggest enrichments of regulators of photosynthesis and chloroplast development in cluster 1, and those of fruit ripening processes in cluster 2 (Figure S5b,c; Data S4), which are in congruence with the two key functions of SlMIR164A shown by the phenotypic assays of slmir164aCR. Specifically, cluster 1 contains important chloroplast development-associated genes GOLDEN2-LIKE1 (SlGLK1) (Nguyen et al., 2014) and CHLOROPHYLL A/B BINDING PROTEIN11 (SlCAB11) (Liu et al., 2020; Piechulla et al., 1991), chlorophyll biosynthetic genes CHLOROPHYLLIDE A OXYGENASE (SlCAO) (Meng et al., 2018) and PROTOCHLOROPHYLLIDE OXIDOREDUCTASE3 (SlPOR3) (Bassa et al., 2012), and photosystem II protein-encoding gene PsbP DOMAIN-CONTAINING PROTEIN 3 (SlPsbP3) (Liu et al., 2020), while cluster 2 includes 1-AMINOCYCLOPROPANE-1-CARBOXYLIC ACID (ACC) OXIDASE1 (SlACO1), ACC SYNTHASE2 (SlACS2) and SlE8 that act in the regulation of ethylene biosynthesis and signalling (Li et al., 2019), ZETA-CAROTENE DESATURASE (SlZDS) and PHYTOENE SYNTHASE1 (SlPSY1) that are involved in carotenoid biosynthetic processes (McQuinn et al., 2020), and XYLOGLUCAN ENDOTRANSGLUCOSYLASE/HYDROLASE3 (SlXTH3) that may modify cell wall properties during fruit ripening (Miedes and Lorences, 2009). Expressions of these genes were also assessed by qRT-PCR, which confirmed the increased levels of SlGLK1, SlCAB11, SlCAO, SlPOR3 and SlPsbP3 in the 30dpa fruits (Figure 5a), and SlACO1, SlACS2, SlE8, SlZDS, SlPSY1 and SlXTH3 in the 35dpa and 42dpa fruits of slmir164aCR relative to wild type (Figure 6a). Furthermore, all the eleven genes contain the putative NACBS on their promoters (Figure S6b; Figure S7b), which suggests that SlNAM2/SlNAM3 may directly bind these genes and regulate their transcription. To test this hypothesis, we carried out EMSA experiments to assess the direct interactions of SlNAM2 and SlNAM3 with the promoter fragments that encompass the putative NACBS on these genes. The result showed that SlNAM2 and SlNAM3 were able to bind all eleven promoter regions and the association of SlNAM2 or SlNAM3 with each biotin-labelled fragment could be attenuated by excess amount of unlabelled competitor DNA with the same sequence (Figure 5b-c; Figure 6b-c). We also conducted dual luciferase assays in the leaves of Nicotiana benthamiana and demonstrated that overexpression of the Sly-miR164-resistant form of SlNAM2 or SlNAM3 (SlNAM2m and SlNAM3m) could strongly induce the promoter activities of all eleven genes, although overexpression of the native SlNAM2 or SlNAM3 failed to do so, which was possibly attributable to the suppression by the N. benthamiana miR164 (Figure 5d-h; Figure 6d-h). These results indicate that SlNAM2 and SlNAM3 may function as direct positive regulators of these important chloroplast development and fruit ripening-related genes in the downstream pathways of SlMIR164A.
SlNAM2/SlNAM3 act as main effectors of SlMIR164A in controlling fruit ripening and quality
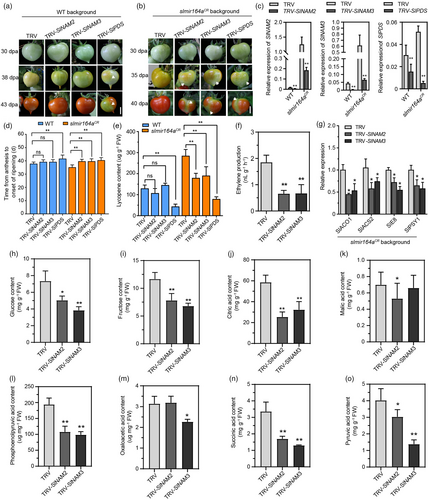
Direct activation of the key regulators of chloroplast development and fruit ripening by SlNAM2/SlNAM3 supports a model that SlMIR164A may act through SlNAM2/SlNAM3 to control fruit ripening and quality in tomato development. To further test this model, we utilized the VIGS system with the tobacco rattle virus (TRV) vector to specifically suppress the expression of SlNAM2/SlNAM3 at fruit ripening stages in wild type and slmir164aCR. We also applied VIGS on the PHYTOENE DESATURASE (SlPDS) gene as a positive control, and transformed the empty TRV vector in fruits as a negative control. We detected decreased expression of SlNAM2, SlNAM3 or SlPDS in the wild-type and slmir164aCR fruits infected with the corresponding TRV construct, suggesting success in target gene suppression by VIGS (Figure 7c). We also observed large sectors of photo-bleached pericarp tissue in both wild-type and slmir164aCR fruits inoculated with TRV-SlPDS (Figure 7a, b). In accordance with this visual phenotype, the TRV-SlPDS- infected fruits also experience a longer interval from anthesis to onset of ripening and have a decreased lycopene content as compared with the negative control (Figure 7d, e), which shows the effectiveness of VIGS system in our analysis. Different from the similar strong impact of SlPDS suppression on both wild-type and slmir164aCR fruits, silencing of SlNAM2 and SlNAM3 appears to exhibit distinct phenotypic effects in these two genetic backgrounds. Specifically, slmir164aCR fruits infected with TRV-SlNAM2 and TRV-SlNAM3 ripe later than those with the empty TRV vector, which is shown by an extended time from anthesis to onset of ripening and a lower fruit lycopene level in the former than in the latter (Figure 7d, e). Consistently, in the fruits of TRV-SlNAM2 slmir164aCR and TRV-SlNAM3 slmir164aCR, we also detected reduced production of ethylene and expressions of the ethylene biosynthesis and response genes SlACO1, SlACS2 and SlE8, as well as the decreased transcription of the carotenoid biosynthesis regulator SlPSY1 in comparison with the negative control (Figure 7f, g). These results suggest that suppression of SlNAM2 or SlNAM3 can reverse the early ripening phenotype caused by the loss of function of SlMIR164A, which strongly supports our model that SlMIR164A impacts tomato fruit ripening through SlNAM2 and SlNAM3. However, we did not notice any discernible change of these ripening-associated parameters in wild-type fruits after inoculation with TRV-SlNAM2 or TRV-SlNAM3 (Figure 7a, d, e), which is probably attributable to the low expression of SlNAM2 and SlNAM3 in the ripening stages of wild-type fruit development (Figure S4f). Furthermore, we also measured the contents of glucose, fructose and major organic acids in the TRV-SlNAM2 slmir164aCR, TRV-SlNAM3 slmir164aCR and negative control fruits. We found that most of these compounds have decreased abundances in the SlNAM2- or SlNAM3-silenced fruits as compared with control at 35dpa (Figure 7h-o). This indicates that suppression of SlNAM2/SlNAM3 also reverses the increase of sugar and organic acid metabolites in slmir164aCR at early ripening stages, thus affecting the quality of slmir164aCR fruits. Together, these results show a previously unidentified role of SlNAM2 and SlNAM3 in the regulation of fruit ripening and quality, and demonstrate that these two NAC genes are major effectors of SlMIR164A in the ripening and quality control of tomato fruit development.
Discussion
MIR164genes have been shown to have important functions in organ ageing processes, such as fruit ripening and leaf senescence in a variety of plants (Kim et al., 2009; Li et al., 2013; Wang et al., 2020); however, whether the tomato MIR164 genes carry out a similar role still remains to be clarified. In this study, we demonstrate that loss of function of SlMIR164A in the tomato cultivar MicroTom results in early ripening of fruits, which uncovers a previously unidentified action of tomato MIR164 in fruit ripening control. Consistent with this result, we found that SlMIR164A is preferentially expressed in the ripening stages of fruit development, which supports the mechanism shown by former studies that the functional specificities of MIRNA genes are tightly associated with their diversified expression patterns (Lian et al., 2021; O'Maoileidigh et al., 2021; Sieber et al., 2007). This mechanism may also help explain why the loss of SlMIR164 genes does not result in a prominent impact on fruit ripening in M82 (Gupta et al., 2021), as the expression of Sly-miR164 is only modestly increased at ripening stages of M82 fruit development (Hendelman et al., 2013).
In agreement with the phenotypic assays, we also show by genomic and molecular studies that SlMIR164A regulates important genes and pathways related to fruit ripening. Most importantly, we demonstrate that SlMIR164A acts through specific NAC family genes, SlNAM2 and SlNAM3, to exert its function in ripening control. SlNAM2 and SlNAM3 are key target genes of Sly-miR164, which have been shown to play vital roles in mediating the action of Sly-miR164 in the morphogenesis of leaves, flowers and fruits (Gupta et al., 2021; Hendelman et al., 2013). In this report, we present a newly characterized function of SlNAM2 and SlNAM3 in the SlMIR164A-directed regulation of fruit ripening. We show that SlNAM2 and SlNAM3 are induced by the loss of SlMIR164A specifically at fruit ripening stages (Figure S4) and their suppression can reverse the delayed ripening of fruits in slmir164aCR (Figure 7a-g). We also show that both SlNAM2 and SlNAM3 may directly regulate the transcription of important ripening-associated genes, including those involved in ethylene function and fruit pigment biosynthesis (Figure 6). These genetic circuits support that the SlMIR164A- SlNAM2/SlNAM3 module may operate as a key hub to control the ripening processes of tomato fruits.
The functions of MIR164 genes in controlling plant ageing are often related to the actions of the ageing-related phytohormone ethylene (Kim et al., 2009; Li et al., 2013; Wang et al., 2020). For example, ethylene represses the expression of Ade-MIR164b during kiwifruit development, which in turn increases the level of its targets AdNAC6 and AdNAC7 to modulate fruit ripening. Interestingly, AdNAC6 and AdNAC7 also function to activate the ethylene biosynthetic genes AdACS1 and AdACO1, thus mediating a positive feedback loop to enhance the production of ethylene in this process (Wang et al., 2020). In contrast to these results, we found in our study that SlMIR164A and SlNAM2/SlNAM3 may interact with ethylene in a different manner. Specifically, although SlNAM2 and SlNAM3 upregulate the ethylene biosynthetic genes SlACS2 and SlACO1, and SlMIR164A suppresses SlNAM2/SlNAM3 expression and ethylene production (Figure 2c; Figure 6; Figure S4), the transcription of SlMIR164A is induced by ethylene in tomato fruits (Figure 2g-i). These findings support a model that SlMIR164A and SlNAM2/SlNAM3 may operate in a negative feedback circuit, in which ethylene auto-inhibits its own biosynthesis during tomato fruit ripening. Tomato is a typical kind of climacteric fruits that experience two phases of ethylene regulation, referring to as ‘system I’ and ‘system II’, during fruit development (Forlani et al., 2019; Liu et al., 2015). System I is responsible for producing basal ethylene levels in immature fruits and is ethylene auto-inhibitory, while system II acts at ripening stages when ethylene production is auto-catalytic. These two systems are regulated by specific sets of ethylene biosynthetic genes. In particular, system II relies on the functions of SlACS2, SlACS4, SlACO1 and SlACO4, whose expressions increase with the climacteric rise of ethylene production during fruit ripening. Some of these key ethylene biosynthetic genes, such as SlACS2 and SlACO1, have been shown to be directly activated by the MADS-box transcription factors RIPENING INHIBITOR (RIN) and TOMATO AGAMOUS-LIKE1 (TAGL1), while the ethylene signalling gene ETHYLENE INSENSITIVE3 (EIN3) acts upstream of these two MADS factors to promote their transcription (Lu et al., 2018). These pathways suggest a positive feedback circuit between ethylene with RIN and TAGL1, which operates as an important mechanism for the autocatalytic regulation of ethylene production in system II. As our results show that SlNAM2/SlNAM3 also positively control SlACS2 and SlACO1, we propose that the SlMIR164A-SlNAM2/SlNAM3-mediated negative feedback pathway may interact with the MADS-regulated positive feedback loop to fine-tune the system II ethylene production in the ripening process of tomato development.
In addition to the accelerated ripening of fruits, we also found that the contents of sugar and organic acids that determine the nutritional quality of fruits are altered in slmir164aCR relative to wild type (Figure 4). In particular, the starch levels are substantially elevated from immature to ripening fruits, which reflects a potential increase of fruit photosynthesis in slmir164aCR. In support of this hypothesis, we detected enhanced chloroplast development, which leads to a higher photosynthetic efficiency in the fruits of slmir164aCR compared with wild type (Figure 3). Consistently, we also identified key regulators of photosynthesis and chloroplast development in the top-ranked GO categories of the downstream genes of SlMIR164A (Figure S5b; Figure S6; Data S4). Furthermore, we show that SlNAM2 and SlNAM3, the major target genes of SlMIR164A, may directly control the activities of some of these chloroplast regulators (Figure 5). These results together demonstrate the critical role of the SlMIR164A-SlNAM2/SlNAM3 pathway in promoting chloroplast function. However, the abundances of glucose and fructose, two major products of starch catabolism, are increased in slmir164aCR fruits only at 30dpa and 35dpa, and declined afterwards during fruit ripening (Figure 4b-c). This is probably because the early ripening of slmir164aCR fruits induces precocious demand of high energy consumption, which triggers strong activation of glycolysis and results in the reduction of glucose/fructose levels at late ripening stages (Batista-Silva et al., 2018; Beauvoit et al., 2018). This speculation is supported by our RNA-seq analysis that a number of GO terms related to glycolysis and its downstream processes in cellular respiration, such as ‘glycolytic process’, ‘tricarboxylic acid cycle’ and ‘pyruvate kinase activities’, are enriched in the downstream pathways of SlMIR164A in the ripening fruits (Figure S5c; Data S4). In addition, we also detected markedly increased levels of major organic acids that are intermediates of the TCA cycle in slmir164aCR fruits (Figure 4d-i), which further indicates the activation of fruit glycolysis and respiration by the loss of SlMIR164A. Together, these results demonstrate that the alteration of sugar and organic acid contents that influence the quality of slmir164aCR fruits may be attributable to the combinatory effect of enhancement of chloroplast function and acceleration of the ripening process during the fruit development of slmir164aCR.
In summary, our study uncovers important functions of SlMIR164A and its target genes SlNAM2/SlNAM3 in the regulation of fruit ripening and quality in tomato. Our results not only provide new insights in understanding the regulatory mechanisms of the conserved MIR164 gene family in plant development but also are of potential significance for application in tomato breeding. Particularly because loss of SlMIR164A has negligible effects on organ morphogenesis, manipulation of SlMIR164A activities can help to create new tomato mutants that are altered in their fruit ripening and quality characteristics, but normal in overall growth. These mutant varieties, especially the ones in the commercially important cultivars, can be leveraged in breeding schemes that specifically target fruit ripening and quality attributes, thus greatly facilitating tomato improvement in the field.
Materials and methods
Plant materials and growth conditions
Tomato (Solanum lycopersicum L. cv MicroTom) plants were grown in the greenhouse at a constant temperature of 25 °C under natural daylight with a 16-h light/8-h dark regime. Floral developmental stages were classified as described by Brukhin et al. (2003). For the measurement of time to ripening, flowers were tagged at anthesis, and fruit developmental and ripening stages were recorded as days post-anthesis (dpa). Fruits of wild-type and slmir164aCR were harvested at the same dpas for phenotypic and molecular analyses.
Plasmid construction and tomato transformation
For gene editing in tomato, gene-targeting vectors were constructed based on the pYLCRISPR/Cas9Pubi-H system provided by the study cited herein (Ma et al., 2015). The target sequences were selected using the website (http://skl.scau.edu.cn/targetdesign/), and inserted into the gRNA through BsaI site and then cloned into the pYLCRISPR/Cas9Pubi-H binary vector. For constructing promoter:GUS reporter, ~2000 bp promoter fragment upstream of SlMIR164A was amplified using primers in Table S3, cloned into PLP100_GUS (Chaabouni et al., 2009). These constructs were introduced into Agro-bacterium strain GV3101 and transferred to tomato (Wang et al., 2005). The resulted homozygous slmir164aCR mutants were first confirmed with PCR genotyping using primers in Table S3, and further verified by sequencing. As three independent slmir164aCR lines (Figure S2) show similar phenotypes, all of them were examined in the subsequent morphological, physiological and molecular analyses and each quantitative result was calculated from the values of all three slmir164aCR mutants. However, images of slmir164aCR-a3 were used as the representatives to show the mutant phenotypes in each figure.
RNA extraction, quantitative real-time PCR and RNA-seq experiment
Total RNA from various tissues of wild-type and slmir164aCR were extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and reverse transcribed. The resulted cDNA was used in quantitative real-time PCR with TransStart Tip Green qPCR SuperMix kit (TransGen, China). Gene expression levels were calculated using the 2−ΔΔCt method with SlACTIN (Solyc11g005330) as the internal control. All primers used for qRT-PCR are listed in Table S3. Three biological replicates were analysed for each experiment.
Total RNA from fruits at different stages of wild-type and slmir164aCR (Figure S3) were analysed in the RNA-seq experiment. Detailed steps of library construction and sequencing are described in Methods S1.
Northern blotting
Northern blotting for Sly-miR164 was performed as previously described (Cai et al., 2018). Briefly, approximately 10 µg of denatured RNAs was resolved on a 15% (W/V) TBE-urea polyacrylamide gel, transferred onto a nylon membrane and hybridized with biotin-labelled probes (Table S3). The northern blotting signals were detected using a Chemiluminescent Nucleic Acid Detection Module Kit(Thermo Fisher Scientific, Rockford, Illinois, USA) and relative expression levels of Sly-miR164 were calculated against the internal control U6 using Image J (https://imagej.en.softonic.com/). Three biological replicates were analysed for each experiment.
Protein–DNA interaction assays
Interactions of SlNAM2 and SlNAM3 with the promoter sequences of target genes were analysed in vitro using electrophoretic mobility shift assay (EMSA), and in Nicotiana benthamiana leaves with the dual-luciferase reporter system. Detailed procedures of these two experiments are provided in Methods S1.
Ethylene emission detection and ethylene/1-MCP treatments
Detection of ethylene emission was performed as described in (Pranamornkith et al., 2012), with modifications. Tomato fruits were weighed and sealed in 50-mL airtight jars at room temperature for 6 h to reduce harvest stress. Ethylene in the headspace was measured with an ETD-300 laser-based photoacoustic detector (Sensor Sense, http://www.sense.com). Experiment for each transgenic line was carried out with three biological replicates.
Ethephon treatment was performed as previously described (Wang et al., 2017). Briefly, harvested fruits at 30 dpa were immersed in 50 mmof ethephon solution or water for 15 min and placed at room temperature for the durations indicated in Figure 2. For the treatment of ethylene inhibitor, fruits at 38 dpa were treated with 1-MCP (1.0 mg/L) or air, and incubated at room temperature for durations indicated in Figure 2. After each treatment, RNA was extracted from fruit pericarps for subsequent qRT-PCR and northern blotting experiments according to the procedures described above.
Profiling of tomato fruit metabolites
To profile metabolites from tomato fruits, pericarps were harvested from fruit ripening stages shown in Figures 2, 4 or Figure 7, and ground into a fine powder in liquid nitrogen. Carotenoid quantification was conducted according to the study cited herein (Li et al., 2021). In brief, 100 mg of frozen ground pericarp samples was homogenized with 1 mL hexane/acetone/ethanol (1:1:1, v/v/v) solution and centrifuged. The supernatant was collected, vacuum-dried and dissolved with 200 µL of hexane/acetonitrile/methanol (15:30:55, v/v/v). The resulted solution was analysed for the levels of lycopene, β-carotene and lutein using the Shimadzu HPLC system (LC-16, Shimadzu, Kyoto, Japan). For quantification of the starch content, a starch assay kit (Solarbio, China) was used by following the manufacturer’s protocol. To assess the contents of soluble sugars, analyses were carried out according to (Li et al., 2021). Briefly, 200 mg frozen pericarp powder was mixed with 1 mL 80% ethanol solution for 30 min at 35 °C. After centrifugation for 30 min at 20 000g, the supernatant was collected, vacuum-dried and dissolved with 200 µL of ultrapure water. The contents of glucose and fructose in the solution were measured by the Shimadzu HPLC system (LC-16, Shimadzu, Kyoto, Japan). To quantify organic acids, the method described in (Flores et al., 2012) was utilized. Organic acids were first extracted from 200 mg frozen pericarp powder with 1 mL of ultrapure water at 80 °C for 30 min. After being centrifuged for 30 min at 20 000g, the supernatant was collected, vacuum-dried, and dissolved with 200 µL of ultrapure water. The levels of various organic acids (Figures 4 and 7) were measured in this solution with the Shimadzu HPLC system (LC-16, Shimadzu, Kyoto, Japan).
Chlorophyll analysis and plastid observations
To measure the chlorophyll content in each fruit sample in Figure 3, 1 mL of 80% acetone was used to extract chlorophyll from 200 mg fruit tissues. Absorbance of the resulted solution was detected using a microplate fluorometer at 663 and 646 nm and the level of chlorophyll in each sample was calculated with the established equations (Powell et al., 2012). To examine the chlorophyll autofluorescence in pericarp, equatorial pericarp pieces were harvested from 30 dpa fruits of wild-type or slmir164aCR and cut into thin slices, which were observed under a Zeiss780 laser scanning confocal microscope as described by the study cited herein (Barry et al., 2012). For the quantification of fruit photosynthetic efficiency, the whole plant was dark-adapted for 30 min immediately before fruits were detached for each measurement. Fruit chlorophyll fluorescence was measured using pulse amplitude-modulated (PAM) fluorimeter (IMAG-MAXI; Heinz Walz, Effeltrich, Germany), and the data were used to calculate the Fv/Fm ratio that provides an estimation of the maximum photochemical efficiency of photosystem II in dark-adapted materials (Xia et al., 2009). To examine the ultrastructure of chloroplast and chromoplast in fruits according to the method in (Nguyen et al., 2014), pericarp tissues were fixed in 2.5% glutaraldehyde with 0.2 M sodium phosphate buffer (pH 7.2) at 4 °C overnight. After being dehydrated in an ethanol series, all samples were embedded in Spurr’s low-viscosity resin, and polymerized at 70 °C for 24 h. The ultrathin sections of samples were obtained on an ultramicrotome, and observed under a Hitachi-7650 transmission electron microscope (Tokyo, Japan).
Virus-induced Gene Silencing (VIGS)
VIGS of MicroTom fruits was performed as previously described (Fu et al., 2005). cDNA fragments of SlNAM2, SlNAM3 and SlPDS were amplified using the primers listed in Table S3, cloned in pTRV2 to construct TRV- SlNAM2, TRV- SlNAM3 and TRV- SlPDS, and introduced in A. tumefaciens strain GV3101. Bacteria containing TRV- SlNAM2, TRV- SlNAM3 or TRV- SlPDS were mixed in equal volume with those containing the pTRV1 plasmid, and injected into immature green stage fruits of wild-type and slmir164aCR plants. Agro-injected fruits were photographed at the stages shown in Figure 7. qRT-PCR was used to detect the efficiency of gene silencing in fruits, with primers listed in Table S3. Tomato fruits infiltrated with empty VIGS vectors were used as control. Each inoculation was carried out three times, and six different plants were infiltrated each time.
Accession numbers
The RNA-Seq raw data in this study can be found under NCBI BioProject accession ID PRJNA760997. All the gene IDs used in this study are given in Table S2.
Acknowledgements
We thank Prof. Yaoguang Liu (South China Agricultural University, China) for kindly providing the pYLCRISPR/Cas9Pubi-H binary vector, and Prof. Hongwei Guo and Dr. Wei Huang (Southern University of Science and Technology, China) for technical assistance on ethylene measurement. We also thank the Instrumental Analysis Center of Shenzhen University (Lihu Campus) and Central Research Facilities of College of Life Sciences and Oceanography for helping us with the microscopy experiments. This work was supported by Guangdong Innovation Research Team Fund (2014ZT05S078), National Natural Science Foundation of China (NSFC) Grant (31772322), Guangdong Special Support Program for Young Talents in Innovation Research of Science and Technology (2019TQ05N940) and China Postdoctoral Science Foundation (2018M633137).
Conflict of interest statement
The authors declare no conflict of interest.
Author contributions
DL and TH designed the research. DL, XZ, BQ, ZG, PT, ZL, ZL and YZ performed the research. DL and TH analysed the data. DL and TH wrote the manuscript.
Open Research
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




