In the Acute Phase of Anterior Cruciate Ligament Rupture: Quantitative Assessment of Matrix Changes and Correlation between Different States of Meniscus and Adjacent Cartilage
Jiaying Zhang, Zhenyu Jia, and Yangyang Yang equally contributed to this work.
Abstract
Objective
Cartilage and meniscus are important structures that maintain the health of the knee joint. Early detection of changes in the internal components of cartilage and meniscus before morphological changes occur is essential to prevent and delay the development of osteoarthritis (OA). This study was designed to determine the changes in the matrix composition of morphologically intact cartilage and meniscus during the acute phase of an anterior cruciate ligament (ACL) rupture, as well as the effect of different states of meniscus (intact or tear) on adjacent cartilage during the acute phase.
Methods
This cross-sectional study compared and analyzed 50 patients in the acute phase of ACL rupture who underwent surgical treatment and 66 age-, weight- and height-matched healthy volunteers from May 2022 to May 2023 at our institution. Mean T2 relaxation times and effect sizes in different regions of tibiofemoral articular cartilage and meniscus were compared between the two groups using the Mann–Whitney nonparametric t-test, and correlations between different meniscal states and adjacent cartilage were analyzed.
Results
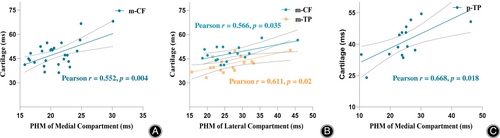
Both in the lateral and medial compartments of the knee, T2 relaxation times were significantly higher in all subregions of cartilage and meniscus in the ACL rupture group (p < 0.05), and the site of injury was predominantly centered in the medial compartment (femur, p = 0.000; tibia, p = 0.000; anterior horn, p = 0.000). In the respective compartments, the posterior horn of the lateral meniscus showed a significant positive correlation with the mid-cartilage of the femoral and tibial (r = 0.566, p = 0.035; r = 0.611, p = 0.02); and the posterior horn of the medial meniscus showed a significant positive correlation with the posterior tibial cartilage (r = 0.668, p = 0.018).
Conclusion
During the acute phase of ACL rupture, the internal composition of the cartilage and meniscus undergoes significant changes, even if the morphology is intact. More importantly, the state of the meniscus significantly affects the internal composition of the adjacent cartilage. This is an early warning sign of OA, which should be closely monitored and carefully managed in clinical practice.
Introduction
The anterior cruciate ligament (ACL) is the primary stabilizer of the knee, limiting anterior tibial translation and internal rotation,1 and the meniscus contributes to maintaining knee stability in the absence of the ACL.2-4 Rupture of the ACL is often accompanied by damage to the meniscus and other ligaments, which results in a loss of stability of the knee joint and accelerates osteoarthritis (OA).5, 6 The incidence of traumatic OA following ACL injuries has been reported to be as high as 87%.7 More than 2 million ACL ruptures are thought to occur each year worldwide.8 When an acute non-contact injury to the ACL occurs, cartilage and subchondral bone may undergo immediate lesions while causing inflammatory cytokine efflux9, 10; this could potentially be the beginning of OA. To reduce the risk of knee deterioration in the later stages of ACL injury, it is necessary to understand the changes in cartilage and meniscus components during the acute phase of ACL rupture.
Quantitative magnetic resonance imaging (qMRI) is a technique for the measurement of tissue properties via specific sequences such as T2 mapping, T1ρ, and delayed gadolinium enhancement. The T2 mapping is a method for detecting T2 relaxation times with high sensitivity and accuracy, and the latter can reflect the concentration and orientation of collagen fibers as well as the proteoglycan and water content in the cartilage matrix11, 12 and can predict the progression of OA.11 When cartilage is in a healthy state, water is tightly bound. However, as cartilage degenerates, its proteoglycan content decreases, and the collagen fibers become disordered, causing water molecules to become free. The increase in free water content leads to an increase in T2 relaxation times.13 A considerable amount of research indicates that T2 mapping provides better detection of matrix changes in the meniscus compared to T1ρ.14-16
Since ACL ruptures are often accompanied by meniscal injuries, the T2 relaxation time of the injured meniscus is significantly increased, which affects the T2 relaxation time of the adjacent cartilage.17 Apart from damaged or torn meniscus, patients with ACL ruptures still exist in which both the anterior and posterior horns of the meniscus are morphologically intact. Although there is prior literature examining changes in cartilage and meniscal matrix after ACL injury, these studies rarely exclude injured or even torn meniscus, and few of the included patients were within the truly acute period of ACL rupture (within 1 month). There is still a lack of studies on the T2 relaxation times of the medial and lateral compartment cartilage and meniscus in patients with ACL rupture in different meniscal states (intact or torn meniscus) and the correlation with different subregions of the adjacent cartilage.
Hence, there is a need to identify the matrix changes in different meniscus states (intact or tear) and adjacent cartilage during the acute phase of ACL rupture, as well as the correlation between the two. The aims of this study were (1) to accurately characterize the distribution of T2 relaxation times of knee cartilage and meniscus (intact or tear) in the acute phase (>1 week, ≤4 weeks) in patients with ACL injuries, and (2) to determine the correlation between the meniscus (intact or torn) in the acute phase of the ACL rupture and the cartilage in the adjacent region, respectively.
Methods
Study Design and Participants
This cross-sectional analysis study was approved by the Ethics Committee of the General Hospital of the Southern Theater of the Chinese People's Liberation Army [IRB 2019 No.10]. All patients with acute non-contact ACL ruptures seen in the outpatient setting and treated with inpatient surgery were considered potential candidates for this study. MRI records of all patients treated between May 2022 and May 2023 were retrospectively analyzed after informed consent was obtained from patients. Inclusion criteria: (1) age 20–40 years; (2) acute non-contact ACL injury; (3) 1 week < ACL rupture ≤4 weeks from the date of presentation to admission; (4) MR imaging showing ACL rupture; and (5) no articular injection therapy other than braking or physiotherapy. Exclusion criteria: (1) history of patellar instability; (2) history of previous knee ACL reconstruction or other knee surgery; and (3) any signs of knee osteoarthritis. One hundred and one eligible patients were included, and all of these patients underwent qMRI scans of the knee before undergoing ACL reconstruction surgery. After intraoperative arthroscopic exploration, 50 patients were selected who had no defects in the cartilage of each compartment of the knee joint and no injuries to ligaments other than ACL rupture (Figure 1). We randomly selected 66 volunteers matched for age, weight, and height who underwent health check-ups in our hospital (a total of 91 cases of healthy volunteers) as healthy controls, and informed consent was obtained. None of these controls (volunteers) had ACL injuries, discomfort such as knee pain, or a family history of OA. Finally, a total of 116 subjects (including 50 patients and 66 uninjured controls) were included (Table 1), with a total of 87 (21 patients and 66 uninjured controls) subjects with intact lateral compartment cartilage and meniscus (anterior and posterior horns intact) and 98 (32 patients and 66 uninjured controls) subjects with intact medial compartment cartilage and meniscus (anterior and posterior horns intact), 21 subjects had torn posterior horns of the lateral meniscus, and 15 subjects had torn posterior horns of the medial meniscus. The results of the arthroscopic exploration are shown in Table 2 (smooth and undamaged cartilage surfaces were observed arthroscopically in all patients with ACL ruptures).
| Groups | Male | Female | Age (years) | Weight (kg) | Height (m) |
|---|---|---|---|---|---|
| Uninjured | 60 | 6 | 25.06 ± 2.04 | 73.76 ± 12.32 | 1.73 ± 6.30 |
| Injured | 46 | 4 | 26.31 ± 6.52 | 75.69 ± 11.01 | 1.72 ± 8.05 |
| p-value | - | - | 0.15 | 0.38 | 0.53 |
| Tissue | Compartment | Lateral | Medial | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Cartilage | Femur | 0 | 0 | 0 | 0 |
| Tibial | 0 | 0 | 0 | 0 | |
| Meniscus | Anterior horn | 1 | 2 | 0 | 0 |
| abnormalities | Corpus | 7 | 14 | 3 | 6 |
| Location | Posterior horn | 21 | 42 | 15 | 28 |
| Other | 0 | 0 | 0 | 0 | |
| Meniscal tear | Horizontal | 1 | 2 | 0 | 0 |
| Radial/vertical | 19 | 39 | 15 | 30 | |
| Bucket-handle | 2 | 4 | 3 | 6 | |
| Ramp | 1 | 2 | 0 | 0 | |
| Complex | 4 | 8 | 0 | 0 | |
| Other | Popliteal tendon injury | 1 | 2 | 0 | 0 |
MRI Protocol
Each participant's knee underwent 3.0 T qMRI sequence scans with an 8-channel knee coil (Magnetom Verio; Siemens). The mean time from injury to MRI scan was 2.6 weeks (range 1–4 weeks). Imaging Protocol: T2 mapping sequence (repetition time [TR], 2060 ms; echo time [TE], 13.8, 27.6, 41.4, 55.2, 69 ms; slice thickness, 3 mm; field of view, 100 mm; flip Angle, 180°). Each patient or volunteer underwent a knee scan in the afternoon and was relaxed in a comfortable seated position for 30 min before the scan to minimize the effects of exercise on the meniscus and cartilage of the knee. Each participant had an MRI scan while lying on their back with their knee extended inside of a knee coil. The knee was stabilized with sandbags and sponges to eliminate any motion artifacts.
Cartilage and Meniscus Segmentation
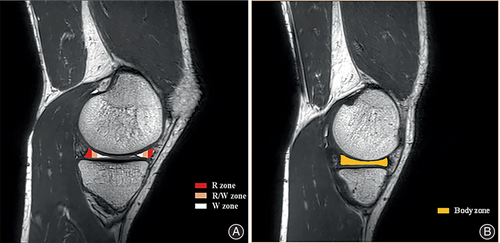
Segmentation of cartilage and meniscus was based on WORMS partitioning.18 We divide the knee articular cartilage into a weight-bearing zone and a non-weight-bearing zone. Weight-bearing zones: the cartilage connecting the tibial plateau to the central portion of the medial and lateral femoral condyles; non-weight-bearing zones: patella, trochlea, posterior-femoral condyles of the medial and lateral femoral condyle, all eight regions of interest (ROI). Then, the cartilage of weight-bearing zones was divided into three sub-regions—anterior, middle, and posterior—in which the anterior and posterior sub-regions are in contact with the meniscus (Figure 2). The meniscus was divided into the anterior, body, and posterior. Due to the orientation and type of collagen fibers and blood supply to the meniscus varying among different regions of the meniscus,19, 20 the anterior and posterior horns were further divided into red (R), red/white (R/W), and white (W) zones21 (Figure 3).

T2 Relaxation Times Calculation
Manually selected central sections of the medial and lateral compartments (the largest weight-bearing portion of the tibiofemoral joint, showing most of the cartilage) were taken on T2 mapping images of each knee joint (8 years of experience), including two pieces in the central part of the lateral femoral condyle and the lateral tibial plateau, and two pieces in the central part of the medial femoral condyle and the medial tibial plateau. The selected image contained the cartilage of the weight-bearing area and the anterior and posterior horn of the meniscus. A total of 3248 segmented fragments were generated. T2 values were blindly measured by another two orthopaedic surgeons. T2 relaxation times for each voxel were measured through the customized codes in MATLAB software (version R2022a; MathWorks Corporation).
Reliability Analysis
T2 relaxation times were blindly measured by two orthopaedic surgeons. Inter- and intra-observer reliability was assessed by randomly selecting five subjects in each of the ACL deficient (ACLD) (which means ACL rupture) and ACL intact (ACLI) groups, respectively, before performing T2 relaxation time measurements on all subjects. The inter- and intra-observer reliability were assessed based on single-measurement intragroup correlation coefficients (ICC).
Statistical Analysis
SPSS (version 27.0; IBM Corporation) software was used for statistical analysis. The Kolmogorov–Smirnov test was used to test for the normal distribution of continuous variables; the independent samples t-test or Mann–Whitney U-test was used as determined by the normality test. The independent samples t-test results are presented as mean and standard deviation (SD), whereas the Mann–Whitney U-test results are presented as median and interquartile range (IQR). Differences were considered statistically significant at p < 0.05. Intra- and inter-observer reliability was assessed using the intraclass correlation coefficient (ICC) and ranked as slight,22 0–0.2; fair, 0.21–0.4; moderate, 0.41–0.6; good, 0.61–0.8; and excellent, >0.8. Finally, the effect size23 estimates for the Mann–Whitney test were calculated as r = Z/. The effect size was considered small for r < 0.30, medium for 0.30 < r < 0.50, and large for r > 0.50. The correlation between different states of the meniscus and adjacent region of cartilage was analyzed using the Pearson and partial correlation method with control variables (e.g., when analyzing the correlation between the posterior horn of the meniscus and the cartilage in the middle of the weight-bearing area, the effect of the anterior horn of the meniscus and the anterior and posterior cartilage of the weight-bearing area was controlled by controlling for the influence of the anterior horn and the anterior and posterior cartilage of the weight-bearing area in an attempt to obtain more accurate correlation results). During qMRI data export, the export format failed for five ACL ruptures. Missing qMRI data were replaced using missing values in order to ensure accurate results and adequate sample size (methods: serial averages).
Results
Reliability
T2 relaxation times were measured in 10 ROIs (include medial and lateral compartments) in each subject, including central femur (CF), posterior-femoral (PF) condyles, tibial plateau (TP), anterior horn of meniscus (AHM), and posterior horn of meniscus (PHM). The inter- and intra-observer reliability for each ROI is greater than 0.8 (excellent).
T2 Values in the ACL Rupture and Control Groups
Cartilage T2 Relaxation Time
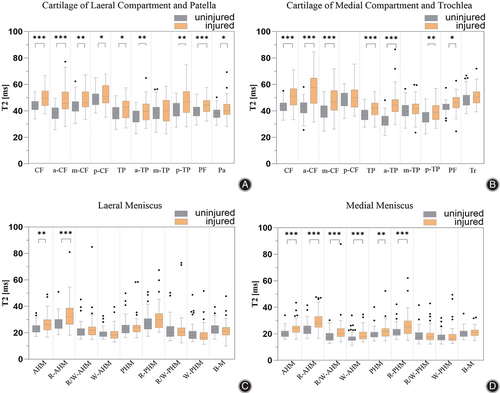
A significant increase in T2 relaxation time was observed in various subregions of the knee cartilage in patients with ACL injuries compared to controls (Figure 4A,B). In the lateral compartment, femoral weight-bearing zone cartilage, posterior femoral condyle cartilage, and tibiofemoral weight-bearing zone cartilage (except m-TP subregion) in the injured knee were statistically higher than uninjured ones. In the medial compartment, femoral weight-bearing zone cartilage (except p-CF subregion), posterior femoral condyle cartilage, and tibiofemoral weight-bearing zone cartilage (except m-TP subregion) were statistically significant in both injured and uninjured knees. In statistically significant regions of interest, most ROIs have a medium effect size. The lateral knee compartment a-CF cartilage, medial compartment a-CF, and a-TP cartilage showed large effect sizes (r = 0.52, 0.57, and 0.69). Median T2 values, interquartile ranges, and effect sizes r for each ROI for injured and uninjured knee cartilage are shown in Table 3.
| ROI | Lateral | Medial | ||||||
|---|---|---|---|---|---|---|---|---|
| Injured | Uninjured | r | p-value | Injured | Uninjured | r | p-value | |
| Femur | ||||||||
| CF | 49 (44–55) | 44 (41–47) | −0.37 | 0.000 | 51 (44–57) | 44 (40–46) | −0.46 | 0.000 |
| a-CF | 45 (41–54) | 38 (34–42) | −0.52 | 0.000 | 58 (46–65) | 43 (38–46) | −0.57 | 0.000 |
| m-CF | 46 (43–54) | 43 (39–47) | −0.30 | 0.002 | 47 (40–55) | 39 (35–44) | −0.42 | 0.000 |
| p-CF | 51 (46–59) | 49 (44–53) | −0.19 | 0.047 | 50 (43–56) | 47 (44–53) | −0.13 | 0.158 |
| PF | 44 (39–48) | 40 (36–43) | −0.36 | 0.000 | 46 (42–50) | 43 (41–45) | −0.35 | 0.000 |
| Tibia | ||||||||
| TP | 42 (34–47) | 38 (34–42) | −0.23 | 0.014 | 42 (37–46) | 37 (33–41) | −0.39 | 0.000 |
| a-TP | 39 (33–45) | 35 (31–40) | −0.27 | 0.005 | 44 (39–48) | 33 (29–36) | −0.69 | 0.000 |
| m-TP | 40 (32–48) | 39 (34–43) | −0.08 | 0.394 | 42 (38–45) | 39 (36–44) | −0.12 | 0.220 |
| p-TP | 47 (38–54) | 41 (36–46) | −0.31 | 0.001 | 39 (33–44) | 36 (31–39) | −0.23 | 0.015 |
| Patella | 40 (37–45) | 38 (35–41) | −0.20 | 0.034 | ||||
| Trochlea | 50 (46–54) | 48 (44–52) | −0.16 | 0.096 | ||||
- Note: The p-value for the difference between the cartilage of ACLD and ACLI is shown in the table; less than 0.05 is given in boldface. The effect sizes (r) are shown in the table; greater than or equal to 0.5 is given in boldface.
Meniscus T2 Relaxation Time
Similarly, T2 relaxation times were significantly increased in all subregions of the knee meniscus in patients with ACL rupture compared to normal controls (Figure 4C,D). In the lateral meniscus, AHM and red zone of AHM (R-AHM) had statistical differences between the two groups. In the medial meniscus, the AHM (including the R, R/W, and W zones) as well as the PHM and the red zone of PHM (R-PHM) had statistically significant differences in the two groups. Small effect sizes predominate in statistically significant regions of interest of the meniscus. Only the anterior horn of the medial meniscus was observed to show a large effect size (r = 0.51). Median T2 values, interquartile ranges, and effect sizes r for each ROI for injured and uninjured knee cartilage are shown in Table 4.
| ROI | Lateral | Medial | ||||||
|---|---|---|---|---|---|---|---|---|
| Injured | Uninjured | r | p-value | Injured | Uninjured | r | p-value | |
| Meniscus | ||||||||
| AHM | 26 (22–30) | 23 (20–25) | −0.29 | 0.002 | 24 (21–26) | 20 (18–22) | −0.51 | 0.000 |
| R-AHM | 32 (26–39) | 26 (23–30) | −0.38 | 0.000 | 28 (25–33) | 23 (20–26) | −0.49 | 0.000 |
| R/W-AHM | 22 (18–24) | 20 (18–23) | −0.16 | 0.090 | 20 (18–24) | 17 (15–20) | −0.37 | 0.000 |
| W-AHM | 18 (16–21) | 19 (17–21) | −0.09 | 0.369 | 18 (16–21) | 16 (15–18) | −0.36 | 0.000 |
| PHM | 24 (21–26) | 23 (20–26) | −0.09 | 0.320 | 22 (18–24) | 18 (17–21) | −0.27 | 0.005 |
| R-PHM | 30 (24–34) | 26 (23–31) | −0.17 | 0.066 | 25 (20–30) | 21 (19–23) | −0.33 | 0.000 |
| R/W-PHM | 21 (18–24) | 20 (17–25) | −0.00 | 0.990 | 18 (15–20) | 17 (15–21) | −0.03 | 0.759 |
| W-PHM | 16 (15–20) | 18 (15–21) | −0.16 | 0.093 | 16 (15–20) | 17 (15–19) | −0.03 | 0.780 |
| B-M | 21 (18–24) | 22 (20–25) | −0.12 | 0.196 | 21 (18–23) | 20 (18–22) | −0.12 | 0.195 |
- Note: The p-value for the difference between the cartilage of ACLD and ACLI is shown in the table; less than 0.05 is given in boldface. The effect sizes (r) are shown in the table; greater than or equal to 0.5 is given in boldface.
T2 Values with the Meniscus in an Intact State
Lateral Compartment T2 Relaxation Time
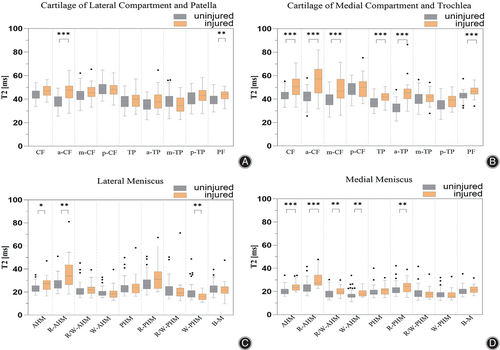
Differences in cartilage were distributed in the a-CF and PF subregions and in meniscus were distributed in the AHM, R-AHM, and white zone of PHM (W-PHM) subregions in ACL ruptured knees compared to healthy uninjured knees (Figure 5A,B). The vast majority of subregions showed small effect sizes. Complete median T2 values, interquartile ranges, and effect sizes r for both groups were shown in Table 5.
| ROI | Cartilage | Meniscus | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Injured | Uninjured | r | p-value | Injured | Uninjured | r | p-value | ||
| Femur CF | 47 (43–51) | 44 (41–47) | −0.20 | 0.069 | AHM | 27 (22–30) | 23 (20–25) | −0.27 | 0.012 |
| a-CF | 48 (41–51) | 38 (34–42) | −0.43 | 0.000 | R-AHM | 34 (26–44) | 26 (23–30) | −0.31 | 0.003 |
| m-CF | 45 (42–50) | 43 (39–47) | −0.18 | 0.093 | R/W-AHM | 22 (19–24) | 20 (18–23) | −0.12 | 0.254 |
| p-CF | 48 (44–52) | 49 (44–53) | 0.00 | 0.980 | W-AHM | 18 (15–21) | 19 (17–21) | −0.08 | 0.439 |
| PF | 44 (40–46) | 40 (36–43) | −0.30 | 0.006 | |||||
| Tibia TP | 40 (34–43) | 38 (34–42) | −0.05 | 0.663 | PHM | 24 (19–27) | 23 (20–26) | −0.03 | 0.774 |
| a-TP | 38 (32–44) | 35 (31–40) | −0.17 | 0.117 | R-PHM | 31 (23–38) | 26 (23–31) | −0.13 | 0.234 |
| m-TP | 35 (30–41) | 39 (34–43) | −0.18 | 0.092 | R/W-PHM | 19 (16–23) | 20 (17–25) | −0.09 | 0.394 |
| p-TP | 43 (39–48) | 41 (36–46) | −0.18 | 0.099 | W-PHM | 16 (13–18) | 18 (15–21) | −0.24 | 0.024 |
| B-M | 22 (19–25) | 22 (20–25) | −0.06 | 0.562 | |||||
- Note: The p-value for the difference between the cartilage of ACLD and ACLI is shown in the table; less than 0.05 is given in boldface. The effect sizes (r) are shown in the table; greater than or equal to 0.5 is given in boldface.
Medial Compartment T2 Relaxation Time
Compared with healthy knees, the differences in cartilage were concentrated in the medial compartment (except the p-CF, m-TP, and p-TP subregions) and in meniscus were concentrated in the medial compartment, including all subregions of AHM and R-PHM in ACL ruptured knees (Figure 5C,D). The vast majority of subregions showed small effect sizes. The medial knee compartment a-CF and a-TP cartilage showed large effect sizes (r = 0.53, 0.67). The AHM and R-AHM of the meniscus of the medial knee compartment showed large effect sizes (r = 0.50, 0.52). Complete median T2 values, interquartile ranges, and effect sizes r for both groups were shown in Table 6.
| ROI | Cartilage | Meniscus | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Injured | Uninjured | r | p-value | Injured | Uninjured | r | p-value | ||
| Femur CF | 51 (44–57) | 44 (40–46) | −0.40 | 0.000 | AHM | 23 (21–26) | 20 (18–22) | −0.50 | 0.000 |
| a-CF | 57 (45–65) | 43 (38–46) | −0.53 | 0.000 | R-AHM | 27 (25–34) | 23 (20–26) | −0.52 | 0.000 |
| m-CF | 47 (41–57) | 39 (35–44) | −0.37 | 0.000 | R/W-AHM | 19 (18–22) | 17 (15–20) | −0.29 | 0.005 |
| p-CF | 50 (42–55) | 47 (44–53) | −0.10 | 0.350 | W-AHM | 18 (16–21) | 16 (15–18) | −0.34 | 0.001 |
| PF | 46 (44–49) | 43 (41–45) | −0.39 | 0.000 | |||||
| Tibia TP | 42 (39–45) | 37 (33–41) | −0.38 | 0.000 | PHM | 21 (18–22) | 18 (17–21) | −0.15 | 0.139 |
| a-TP | 45 (40–49) | 33 (29–36) | −0.67 | 0.000 | R-PHM | 24 (20–27) | 21 (19–23) | −0.25 | 0.015 |
| m-TP | 42 (38–44) | 39 (36–44) | −0.11 | 0.272 | R/W-PHM | 18 (15–20) | 17 (15–21) | −0.03 | 0.735 |
| p-TP | 39 (33–42) | 36 (31–39) | −0.20 | 0.052 | W-PHM | 16 (15–19) | 17 (15–19) | −0.08 | 0.420 |
| B-M | 21 (19–24) | 20 (18–22) | −0.15 | 0.154 | |||||
- Note: The p-value for the difference between the cartilage of ACLD and ACLI is shown in the table; less than 0.05 is given in boldface. The effect sizes (r) are shown in the table; greater than or equal to 0.5 is given in boldface.
T2 Values in the ACL Rupture Group with Different Meniscal States
T2 Relaxation Time for Intact Menisci and Torn Meniscus
The torn meniscus was mainly concentrated in the posterior horn in our included subjects with ACL rupture. Therefore, only the posterior horn of the meniscus was analyzed. T2 values were overall higher in torn meniscus compared to morphologically intact meniscus. A statistically significant difference was found only in the R/W and W zones of the posterior horn of the lateral meniscus and had a moderate effect size (R/W, p = 0.016, r = 0.36; W, p = 0.026, r = 0.33) (Table 7) (Figure 6).
| ROI | Lateral | Medial | ||||||
|---|---|---|---|---|---|---|---|---|
| Intact | Tear | r | p-value | Intact | Tear | r | p-value | |
| Meniscus | ||||||||
| PHM | 23 (19–25) | 24 (23–28) | −0.25 | 0.092 | 21 (18–23 | 23 (19–27) | −0.22 | 0.135 |
| R-PHM | 28 (23–35) | 30 (27–35) | −0.10 | 0.520 | 25 (20–39) | 28 (20–30) | −0.15 | 0.312 |
| R/W-PHM | 19 (16–22) | 21 (20–27) | −0.36 | 0.016 | 18 (15–20) | 17 (16–25) | −0.13 | 0.399 |
| W-PHM | 16 (14–18) | 18 (15–25) | −0.33 | 0.026 | 16 (15–19) | 16 (16–24) | −0.21 | 0.163 |
- Note: The p-value for the difference between the cartilage of ACLD and ACLI is shown in the table; less than 0.05 is given in boldface. The effect sizes (r) are shown in the table; greater than or equal to 0.5 is given in boldface.
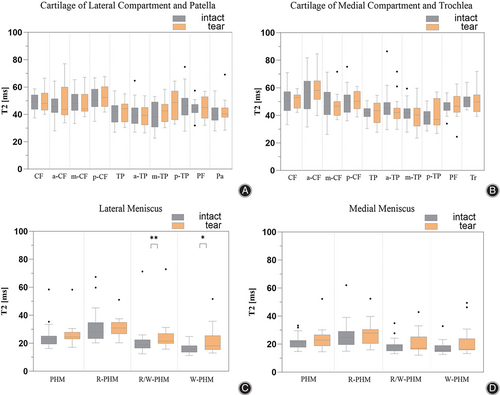
T2 Relaxation Time for Cartilage Adjacent to Intact Meniscus and Torn Meniscus
Statistically significant differences were not found in all cartilage subregions, and all subregions showed small effect sizes. Complete median T2 values, interquartile ranges, and effect sizes r for both groups were shown in Table 8 (Figure 6).
| ROI | Lateral | Medial | ||||||
|---|---|---|---|---|---|---|---|---|
| Intact | Tear | r | p-value | Intact | Tear | r | p-value | |
| Femur | ||||||||
| CF | 49 (44–54) | 48 (43–56) | −0.08 | 0.590 | 51 (43–57) | 53 (45–55) | −0.06 | 0.682 |
| a-CF | 46 (41–51) | 44 (40–60) | −0.01 | 0.944 | 57 (45–65) | 58 (52–65) | −0.10 | 0.500 |
| m-CF | 48 (43–55) | 44 (42–55) | −0.08 | 0.574 | 47 (40–57) | 47 (40–50) | −0.03 | 0.838 |
| p-CF | 50 (46–59) | 52 (46–60) | −0.09 | 0.615 | 50 (42–55) | 50 (45–57) | −0.11 | 0.455 |
| PF | 44 (41–47) | 45 (37–53) | −0.01 | 0.944 | 46 (44–49) | 47 (42–54) | −0.05 | 0.754 |
| Tibia | ||||||||
| TP | 42 (34–47) | 44 (35–48) | −0.07 | 0.640 | 42 (39–45) | 43 (35–49) | −0.01 | 0.923 |
| a-TP | 39 (33–45) | 39 (32–45) | −0.03 | 0.815 | 45 (41–49) | 42 (38–45) | −0.27 | 0.139 |
| m-TP | 38 (31–49) | 43 (35–48) | −0.16 | 0.271 | 42 (38–45) | 40 (32–45) | −0.06 | 0.665 |
| p-TP | 46 (39–52) | 49 (36–57) | −0.06 | 0.691 | 39 (33–43) | 37 (33–52) | −0.08 | 0.613 |
| Patella | 39 (36–45) | 40 (38–45) | −0.09 | 0.527 | ||||
| Trochlea | 50 (46–54) | 50 (43–55) | −0.01 | 0.923 | ||||
- Note: The p-value for the difference between the cartilage of ACLD and ACLI is shown in the table; less than 0.05 is given in boldface. The effect sizes (r) are shown in the table; greater than or equal to 0.5 is given in boldface.
Correlation of T2 Relaxation Times in Different States of Meniscus and Adjacent Weight-Bearing Zone Cartilage in ACL Ruptures
The correlation between meniscal T2 relaxation time and adjacent cartilage T2 relaxation time in different knee compartments was counted according to whether the meniscus morphology was intact or not in patients with ACL rupture. In patients with ACL ruptures in which both the anterior and posterior meniscus horns were intact, we found that only the posterior horn of the medial meniscus showed a significant positive correlation with the m-CF cartilage of the medial compartment (Pearson r = 0.552, p = 0.004). In patients with ACL ruptures in which the posterior meniscal horn was torn, the posterior horn of the lateral meniscus showed a significant positive correlation with m-CF and m-TP cartilage of the lateral compartment (Pearson r = 0.566, p = 0.035 and Pearson = 0.611, p = 0.02; respectively); the posterior horn of the medial meniscus showed a significant positive correlation with p-TP cartilage of the medial compartment (Pearson r = 0.668, p = 0.018) (Figure 7).
Discussion
Remarkable changes in the internal composition of even morphologically intact cartilage and meniscus during the acute phase of ACL injury are the main findings of this study. The area of injury was dominated by the medial compartment of the knee. In addition, the condition of the meniscus significantly affects the changes in the internal composition of the adjacent cartilage and is associated with damage in more cartilage subregions. These findings deserve more attention, and early and careful treatment may delay or prevent the onset of osteoarthritis.
T2 Results of Cartilage and Meniscus in the ACL Rupture Group and Controls
Previous studies have demonstrated13, 24, 25 that increased T2 values are associated with free water content and collagen fiber damage in cartilage and meniscus, and are an early indication of the development of OA24 of the knee. In general (irrespective of whether the meniscus was damaged or not [Figure 4]), we found that the T2 relaxation times in all subregions of the knee cartilage and meniscus were significantly increased in patients with ACL ruptures compared to normal controls (Figure 4). This result suggests that the collagen fiber structure within the cartilage and meniscus has been altered with increased free water content. It is a precursor of morphological damage to the cartilage and meniscus. Medium effect sizes were observed in most subregions on the cartilage side, small effect sizes predominated on the meniscus side, and large effect sizes were observed only in the anterior horn of the medial meniscus (r = 0.51). The effect size indicates the magnitude of the difference between the two groups, and from the results it can be seen that the difference between the cartilage is larger, indicating that the internal composition of the cartilage is more altered in patients with ACL injury. The current findings are consistent with previous studies26-29 which used quantitative MRI techniques to assess cartilage and meniscus after ACL injury or after reconstruction of ACL injury.
T2 Results between ACL Rupture and Control Groups with Intact Meniscus Morphology
By contrast, the current study further quantitatively assessed ACL ruptures and healthy knees with intact cartilage and meniscus (Figure 5). The T2 relaxation time of an injured meniscus is not only significantly higher than that of a morphologically unaltered meniscus but also affects the T2 relaxation time of the cartilage in the neighboring region.17 With the aim of reducing this effect and obtaining a more accurate and realistic cartilage T2 relaxation time, we analyzed injured and uninjured knees with intact medial and lateral compartment cartilage and meniscus, respectively. In the lateral compartment, the T2 difference between the cartilage and meniscus were observed in the a-CF and PF subregions and the AHM, R-AHM, and W-PHM subregions in injured and non-injured knees, respectively. The vast majority of these subregions showed small effect sizes (Table 5). In the medial compartment, the T2 difference between the cartilage and meniscus were observed in all subregions of femur and tibial (except the p-CF, m-TP, and p-TP subregions) and AHM, R-PHM subregions in injured and non-injured knees, respectively. The vast majority of these subregions showed small effect sizes (Table 6).
ACL Injury Group: T2 Relaxation Time between Meniscus and Cartilage and Correlation between the Two
In addition, we analyzed the T2 relaxation time between meniscus in different states and adjacent cartilage and the correlation between the two. In patients with ACL rupture, torn meniscus had higher T2 relaxation times compared to intact meniscus, but statistically significant differences were found only in the subregions of the lateral posterior horn R/W and W zones (Figure 6). Although no statistically significant differences were found in all subregions between cartilage adjacent to different states of meniscus, we found a stronger correlation between torn meniscus and neighboring cartilage, with more areas of correlation (Figure 7). This phenomenon indicates that when the meniscus is damaged, without any intervening therapeutic measures, as the meniscus deteriorates, the cartilage composition of more neighboring areas changes and deteriorates with it.
Regardless of whether the integrity of the meniscus was taken into account or not, the highest T2 values were observed in medial compartment cartilage in ACL ruptured knees, especially in the weight-bearing zone of the femoral cartilage (CF) and its anterior aspect (a-CF) (Tables 3, 5, and 6). This result is consistent with the findings of Chavez et al.30 and Cox et al.31 regarding the arthroscopic study of patients with ACL injuries, which found that cartilage lesions in the medial and lateral compartments occurred predominantly medially and also suggests that the medial compartment is most affected by primary knee OA.32 In addition, the study by Slauterbeck et al.33 also obtained the result that the cartilage of the medial compartment of the tibiofemoral joint was the most susceptible to injury.
Analysis of Possible Causes of Decreased T2 Relaxation Time in Some Subregions of the ACL Injury Group
In patients with knee injuries, besides the region of interest with increased and statistically significant T2 relaxation times, there are also cartilage and meniscus subregions with reduced cartilage and meniscus T2 relaxation times. Almost all of these subregions are not statistically significant and have small values of change. This phenomenon was also noted in the study on cartilage quantification by Casula et al.23 and was interpreted as possibly an initial physiological response of the tissue as it attempts to repair or adapt to abnormal mechanical demands. Since our study was cross-sectional research and the mechanism of repair in terms of histology is not clear, we have only described such phenomena. Further research found that in the case of including the meniscus regardless of its integrity, T2 values in all subregions of cartilage of the medial and lateral compartments in patients with ACL rupture were greater than in healthy knees, and four subregions of the meniscus had smaller T2 values than in healthy knees (three in the lateral, one in the medial). When comparing only injured and uninjured knees with intact meniscus morphology, two cartilage subregions of the lateral compartment and a total of four meniscus subregions (three in the lateral, one in the medial) of the medial and lateral compartments had smaller T2 values than healthy knees. Meniscal lesions often occur in knees with acute ACL tears34 and are associated with an increased likelihood of articular cartilage injury in the same compartment.17 So, the above phenomenon can be explained by the fact that when the injured meniscus was not excluded, the neighboring cartilage with a greater likelihood of injury (although morphologically intact) was also included in the statistical analysis, resulting in all subregions of the injured knee cartilage being larger than that of the healthy knee. Differences in cartilage T2 relaxation times in different subregions before ACL reconstruction have been studied in the literature. T1ρ and T2 were shown to be significantly higher in the injured knee compared to the uninjured knee quickly after ACL injury, particularly in the posterior lateral tibia, according to a multicenter study by Russell et al.35 Differences in T2 distribution in the meniscus of patients with ACL rupture have been less well studied. Wang et al.36 found that acute ACL injuries are associated with significantly increased meniscal T1ρ and T2 values in both patients with and without meniscal lesions or tears and Paproki et al.37 found that T2 values were significantly higher in tear than in non-tear medial meniscus for the full meniscus and its subregions. Similar to our current study is the study on meniscus by Wang et al. However, they used up to 6 months after ACL injury as an inclusion criterion, which does not really reflect the changes in the meniscus during the acute phase of ACL injury.
Strengths and Limitations of this Study
Reactive synovitis, joint congestion bone marrow oedema can occur in the injured knee during the first few days of an ACL rupture. It is not clear whether inflammatory factors within the knee joint at the beginning of an ACL rupture have an effect on the T2 relaxation time of cartilage and meniscus. Therefore, in order to avoid, as much as possible, the influence of inflammatory factors at the beginning of the injury and to ensure that the patients were in the acute phase of ACL rupture, the current study selected patients who were more than 1 week and less than 4 weeks after the acute rupture of the ACL. Simultaneously, this study is the first to quantify morphologically intact cartilage and meniscus in both injured and uninjured knees, excluding the effect of the injured meniscus on the adjacent cartilage. It also subdivides the cartilage and meniscus into different subregions with the aim of obtaining a more accurate soft tissue state of the knee. However, this study still has some limitations. First, it is a cross-sectional study limited to the acute phase, and long-term follow-up is still needed to see whether the T2 relaxation time of the soft tissues of the knee varies with changes in the acute phase. Second, although the vast majority of subjects in the current study underwent MRI scanning 2–3 weeks post-injury, whether bone marrow oedema, for example, affects T2 relaxation time in the acute phase also requires further study. Third, the next step needs to be to continue to expand the sample size with a view to obtaining more data.
Conclusion
During the acute phase of ACL rupture, the internal composition of the cartilage and meniscus undergoes significant changes, even if the morphology is intact. More importantly, the state of the meniscus significantly affects the internal composition of the adjacent cartilage. This is an early warning sign of OA, which should be closely monitored and carefully managed in clinical practice.
Author Contributions
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, J. Zhang, Z. Jia, and Y. Yang. Methodology, J. Zhang, L. Zhang, and T. Huang. Validation, J. Zhang. Formal analysis, J. Zhang. Data Curation, J. Zhang, Z. Jia, L. Zhang, and T. Huang. Writing—Original Draft, J. Zhang and Z. Jia. Visualization, J. Zhang and Z. Jia. Software, Y. Yang. Writing—Review & Editing, T. Tsai and P. Li. Supervision, P. Li. Funding acquisition, P. Li.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81871808), Military Project of Application Fundamentals (CLB21J021, 20QNPY084, and 21FYFH06).
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Authorship Declaration
All authors listed meet the authorship criteria according to the latest guidelines of the Internet Committee of Medical Journal Editors, and all authors are in agreement with the manuscript. The manuscript has been read and approved by all the authors, and all authors listed meet the authorship criteria according to the latest guidelines of the International Committee of Medical Journal Editors.
Ethics Statement
This cross-sectional analysis study was approved by the Ethics Committee of the General Hospital of the Southern Theatre of the Chinese People's Liberation Army [IRB 2019 No.10] and informed consent was obtained from all subjects.




