Lumbar Intradural Disc Herniation Caused by Injury: A Case Report and Literature Review
Abstract
Background
Intradural disc herniation(IDH) caused by trauma is a rare type of disease,which is difficult to diagnose clinically and is easily misdiagnosed. We received a patient with the disease, reported the case to share the process of diagnosis and treatment and put forward our own opinions, so as to increase the probability of correct diagnosis.
Case Presentation
We report the case of a 48-year-old male who fell from a scaffold at a height of 2 m. Later, he developed low back pain, restricted movement, numbness and hyperalgesia of the lower left limb, and decreased left muscle strength. He was diagnosed with IDH. Treatment with posterior decompression and intramedullary decompression with pedicle screw internal fixation was performed. His postoperative course was uneventful, and he underwent regular follow up for 1 year. Good neurologic symptom improvement was achieved.
Conclusions
IDH is rare, and comprehensive consideration and film reading can improve the correct diagnosis rate. Accurate diagnosis and early decompression of laminae and intramedullary decompression can lead to good recovery after neurologic impingement.
Introduction
Disc herniation is a common indication for spinal surgery. When the nucleus pulposus breaks through the annulus fibrosus, posterior longitudinal ligament and dura into the dural sac, this is called IDH, which is a rare type of intervertebral disc herniation, accounting for approximately 0.27%–0.33% of cases.1, 2 In 1942, Dandy3 first found intervertebral disc tissue in the dura and a reported case of IDH, but it was later confirmed to be a rare complication of intervertebral disc herniation.4 By 2015, a total of 150 cases of IDH were reported.5 Lumbar segments account for approximately 92% of cases, mostly occurring at L4-5; thoracic segments account for 5% of cases, mostly occurring at T11-12; and cervical segments account for approximately 3% of cases.1, 6-8 Koc RK's study9 found that male patients accounted for more than 70% of IDH patients, most of whom were between 42 and 72 years old, with the oldest being 90 years old.
In the most majority of cases, IDH is due to chronic degenerative diseases, and IDH is very rarely due to trauma. In 2013, Lee10 reported a case of lumbar IDH due to a fall. The patient was initially diagnosed with a spinal subdural hematoma (SDH) at the L1 level and given conservative treatment; however, due to the symptoms persisted, the patient underwent laminectomy, during which a ruptured disc particle was found penetrating the ventral and dorsal dura.
Mutt et al.11 classified IDH into two types according to whether the nucleus pulposus compresses nerve roots in the dura: type A, in which the intervertebral disc protrudes into the dura mater without compressing the nerve root; and type B, in which the intervertebral disc protrudes into the dura mater and compresses the nerve root.
IDH is difficult to diagnose clinically and is easily misdiagnosed. On MRI, it can be misdiagnosed as a spinal cord tumor, such as a schwannoma or ependymoma, or another space-occupying lesion.12 IDH often requires a combination of surgery and postoperative pathological examination to be diagnosed.
Recently, the Second Hospital of Shanxi Medical University admitted a patient with trauma-induced IDH who developed symptoms of conus medullaris. The patient's treatment, relief and follow-up are now reported.
Case Presentation
Basic medical history: The patient was a 48-year-old male who fell from a scaffold at a height of 2 m. Later, he developed low back pain, restricted movement, numbness and hyperalgesia of the lower left limb, decreased left muscle strength, and grade III lower left limb muscle strength. At the same time, incontinence was noted as a symptom of cauda equina syndrome. Later, he was referred to our hospital.
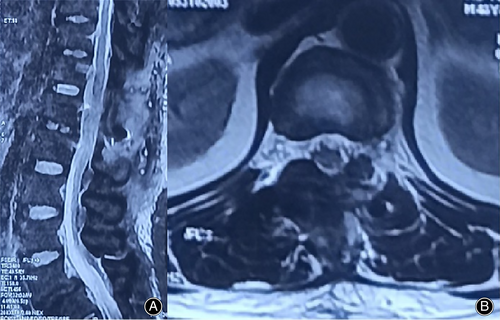
The imaging results are shown in Figure 1:
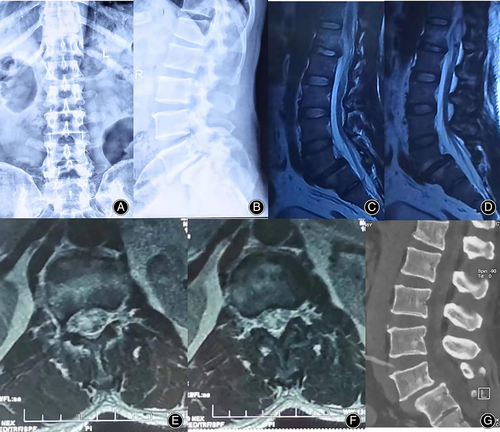
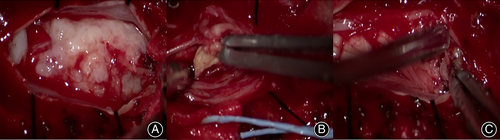
During the operation, clear cerebrospinal fluid leakage was found in the lower left lamina of L1. After laminar decompression, the dura was greatly expanded, and there was a large amount of tension (Figs. 2 and 3).
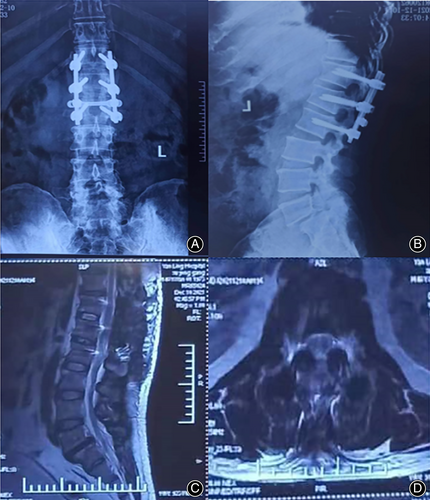
The postoperative imaging findings were as follows Figure 4:
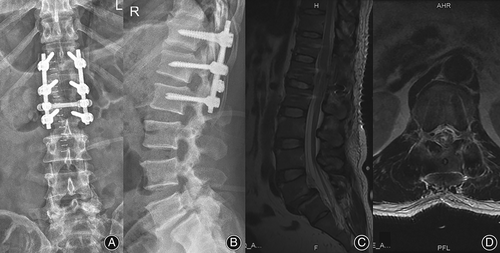
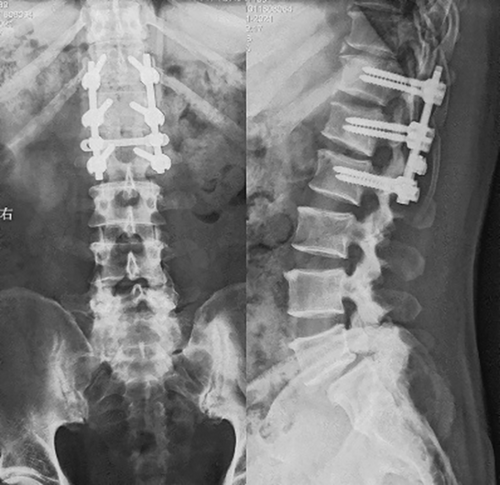
There were no serious complications, such as infection or nerve injury, after the operation. On the second day after surgery, the numbness and hyperalgesia of the lower left extremity improved, and the muscle strength of the lower left extremity recovered. On the 5th day after the operation, the patient felt urinary urges but was still unable to control them himself. On the 12th day after the operation, while the patient could urinate himself, he had not regained autonomous control of fecal excretion. Twenty days after the operation, the patient could control fecal excretion autonomously. By the 6-week follow-up, the patient had recovered from all preoperative symptoms and returned to normal life (Fig. 5). Six months and 1 year after the operation, his condition was completely normal, without recurrence(Fig. 6 and Fig. 7).
Discussion
Etiology and Pathogenesis
At present, there are three common hypotheses regarding the etiology of IDH: (1) The acute external force injury hypothesis: Sharma et al.13 believe that when the spine is suddenly subjected to external force, the pressure in the intervertebral disc will increase sharply. If the fibrous ring is broken at the same time, the nucleus pulposus may break through the fibrous ring, PLL and dura mater to form IDH.13 At present, this view is recognized by most clinicians. (2) Chronic injury hypothesis: Lee and Fairholm et al.14 believe that long-term mechanical compression and wear of intervertebral discs and osteophytes cause thinning of the dura mater, which makes it possible to penetrate the nucleus pulposus. (3) The adhesion hypothesis: It is generally believed that there is a gap and loose connection between the PLL and the dura mater, but some factors, such as iatrogenic injury and chronic inflammation, can make them adhere closely, resulting in thinning or even rupture of the dura mater under long-term stimulation.10, 15, 16 Lyons17 found that 15% of IDH patients underwent related-segment surgery and proposed that a history of previous surgery is one of the reasons for the disease. Shizan also made a similar report18,indicating that approximately 1/3 of the patients had previously undergone lumbar disc herniation (LDH) surgery. Floeth et al.6 found that in the case of acute trauma, intervertebral disc fragments can penetrate the dura mater or arachnoid space. In the case of chronic dural degeneration, adhesion, thinning and damage, intervertebral disc fragments can penetrate the dural sac or arachnoid space.6
The case of IDH we report here is consistent with hypothesis 1. We believe that the intradural disc tissue was derived from the L1-2 disc for the following reasons: preoperative MRI showed degenerative changes in the L1-2 intervertebral disc, and local bulging was observed during the operation, combined with dura mater rupture, cerebrospinal fluid leakage, and local hematoma formation at the lower level of the lumbar vertebral body. Therefore, we suspect that this acute trauma led to partial injury of the L1-2 intervertebral disc. The acute pressure caused substantial damage to the annulus fibrosus and herniation of the nucleus pulposus, which broke through the damaged PLL and dura mater and entered the dural sac. According to the intraoperative findings, the intervertebral disc tissue was continuously scattered in the dura mater and between the nerve bundles, causing compression injury to the conus medullaris. IDH caused by trauma is very rare. Considering these findings in combination with Lee's previous report.10 we suspect that IDH caused by trauma is more likely to occur in thoracolumbar segments under greater stress.
Clinical Manifestations
The manifestations of IDH vary according to whether the pathogenesis is acute or chronic and according to the location of the affected disc. Kataoka et al.2 found that 79% of patients had a history of more than 1 year, while few patients had acute attacks. The common symptoms of IDH are low back pain, radicular pain of the lower limbs, and acute cauda equina syndrome in some patients.18 In the present case, the patient experienced an acute onset, which has a relatively low incidence rate among upper lumbar segments. In acute cases, patients often have no relevant clinical manifestations before the injury and then an acute onset after the injury; in chronic cases, patients often have long-term low back pain and sudden neurological symptoms, such as cauda equina syndrome and lower extremity root pain. The clinical symptoms of IDH are often severe, and sometimes the findings of imaging examinations are difficult to explain.19
Diagnosis of IDH
The imaging manifestations of IDH are similar to those of spinal cord tumors or other space-occupying lesions, such as neurofibromas, lipomas, meningiomas, epidermoid tumors, arachnoid cysts, arachnoiditis, metastases and hematomas. Therefore, it is difficult to make a clear diagnosis, and IDH is easily misdiagnosed.20, 21 D'Andrea et al.22 reviewed 122 cases of IDH described in the literature and found that only 8 cases could be diagnosed before surgery. They believe that only through surgery in combination with medical examinations can a final positive diagnosis be made.
With the progression of technology and the improvement of imaging modalities, more exploration of ways to diagnose IDH is being performed. At present, some special signs and related examinations have been used for its diagnosis. While X-ray examination has no obvious value in the diagnosis of IDH, CT, discography, myelography and MRI can be used to diagnose IDH.23
Regarding CT, in some cases, the density of intradural space-occupying objects can be found in the soft tissue window to be consistent with the density of intervertebral discs. Hidalgo-Ovejero et al.24 found gas signs in the spinal canal on CT, and the risk of IDH in patients with gas signs was 6 times that of patients without gas signs. Therefore, when a patient's CT shows the phenomenon of air accumulation in the spinal canal, IDH should be considered.
MRI is currently the best imaging method for diagnosing IDH.23 Choi et al.25 believe that when MRI shows interrupted PLL continuity and intervertebral disc herniation with a “hawk-beak sign” in the T2 phase, IDH may be suspected. Wasserstrom et al.26 found that intradural intervertebral disc tissue showed circular enhancement on gadolinium-enhanced MRI, namely, the “ring sign”, and diagnosed IDH through enhanced MRI for the first time. Subsequently, it was confirmed that vascular inflammatory granulation tissue showed circular enhancement. Since then, the “ring sign” has been considered a typical MRI sign. Snow confirmed the diagnosis of IDH in 2 cases by the “ring sign” before surgery,27 but not all IDH patients show “the ring sign”. Since it is the result of the long-term penetration of granulation tissue such that it surrounds the disc fragments, edge enhancement is not observed in cases of acute disc herniation.26, 28
In addition, with the rapid development of imaging technology, Crivelli29 could clearly observe extensive penetration of the PLL, ventral dura mater and dural sac by the L2-3 intervertebral disc through 3D constructive interference in steady-state (CISS) MRI, resulting in compression of the cauda equina nerve. This technology also provides a new method for IDH diagnosis.
Tomiya30 reported confirmation of a suspected case of IDH by discography and X-ray tomography (disco CT) before surgery and proposed that discography and disco CT are both useful preoperative tools for the diagnosis of IDH.
Through a review of all the literature, we found that the accuracy of the preoperative diagnosis of IDH can be improved by the following methods: 1. Accompanied by trauma, most of the acute neurological symptoms appear in the thoracic and lumbar segments, and MRI can show intervertebral space narrowing, intervertebral disc degeneration and the olecranon sign. Confounding signals can be seen in the spinal cord, as well as spinal cord compression, causing tortuous and dispersed signals, which can be used to diagnose IDH. 2. Noting previous long-term low back pain, the sudden appearance of acute lower extremity radiating pain or cauda equina syndrome. The diagnosis of IDH should be considered if there is a history of surgery or a long-term history of chronic lumbar disc degeneration, PLL rupture at the corresponding segment on MRI, confounding signals in the spinal cord, spinal cord compression causing tortuous and dispersed signals, the olecranon sign on axial imaging or the “ring sign” on enhanced MRI. If an intervertebral disc compresses the spinal cord for a long time, it will cause irreversible nerve damage; thus, special attention should be given to the morphology of the spinal cord and the possibility of IDH should not be overlooked.
If the following key points are confirmed during the operation, a dural incision and exploration for IDH need to be considered: (1) the dural sac is full, with high tension or local abnormal bulging; (2) the dura mater adheres closely to surrounding tissues, such as the PLL9; and (3) the patient's clinical symptoms are severe, but imaging shows mild compression, and only a small amount of intervertebral disc tissue is observed in the epidural region during the operation.
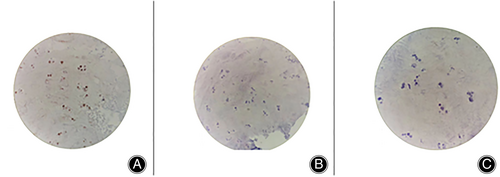
Postoperative pathology may reveal degenerative intervertebral disc cartilage. Nucleus pulposus cells are chondroid cells derived from mesenchymal cells, and their differentiation can be induced by notochord cells. The main substances of the nucleus pulposus are a large number of proteoglycan complexes, collagen fibers and fibrocartilage. With increasing age, the proteoglycan in the nucleus pulposus becomes depolymerized, the water content gradually decreases, and the collagen thickens and is gradually replaced by fibrocartilage; thus, the nucleus pulposus becomes mostly cartilage tissue.
In conclusion, the diagnosis of IDH is difficult, and it is necessary to make a comprehensive judgment based on the medical history, symptoms and imaging data of a patient. The key findings during the operation will also be of great help, and finally, the medical examination will be used to collect evidence. When the above situations occur, remember the possibility of IDH.
Treatment
The treatment of IDH is relatively routine. Due to the potential severity of the clinical symptoms, most scholars prefer early surgical treatment.2, 5, 9, 10, 18, 31 Ljubisa B15 reported spontaneous intradural fragment absorption in a case of intervertebral disc herniation, which presents the possibility of the conservative treatment of IDH. When there are no neurological symptoms, conservative treatment can be attempted.
For lumbar IDH, posterior laminectomy and decompression are usually recommended. It is recommended to use a microscope to more clearly separate the intervertebral disc tissue and cauda equina under the safe conditions of a good operating field to reduce the surgical risk.8 Sung KH32 reported the first use of an intervertebral foraminoscope to complete resection of the L2-3 segment in IDH and repair of the dura mater; they found that a good effect was maintained at the follow-up 8 months after the operation, demonstrating a minimally invasive approach and the feasibility of endoscopic surgery for IDH. For thoracic IDH, it is generally believed that an anterolateral or anterior approach is relatively safe.9 For IDH in the cervical spine, Borm W et al.33 draw a conclusion: patients with cervical IDH treated via a posterior approach have varying degrees of postoperative neurological damage, and posterior surgery may aggravate the neck and increase the risk of spinal cord injury. But, Gunasekaran A34 advocates the use of posterior surgery, because a large part of the patients who receive the posterior approach have improved their recovery. These procedures are safer when performed with the assistance of a microscope. As these procedures involves operations in the spinal canal, they can be completed with neurosurgical assistance when necessary.
Prognosis
If the operation is performed in a timely manner and the intraoperative decompression is thorough, low back pain and lower extremity pain can be significantly relieved, and symptoms affecting lower extremity sensation and muscle strength can be partially or completely resolved, resulting in a relatively satisfactory surgical effect. However, 62% of patients who also have symptoms of cauda equina nerve injury can fully recover within 3 weeks to 32 months,22 and the remaining patients will have some residual dysfunctions, such as sensory disturbances in the perineal area.10, 25 Over time, neurological symptoms may become more severe,2 which lessens the chance of full neurological function recovery. A history of previous surgery can cause scar adhesion and increase the difficulty of the operation, which is also not conducive to the recovery of nerve function.
From the second day after the operation in this case, the numbness and hyperalgesia of the lower left extremity improved, and the muscle strength of the lower left extremity recovered. On the 5th day after the operation, the patient felt urinary urges but was still unable to control them himself. On the 12th day after the operation, while he could autonomously control urination, he could not fully control fecal excretion. At 6 weeks after the operation, the sensation and muscle strength of the lower left limb were completely restored, fecal excretion was completely controlled, and normal life was restored. Six months and 1 year after the operation, his condition was completely normal, without recurrence.
IDH is rare, and comprehensive consideration and film reading can improve the correct diagnosis rate. Intradural intervertebral disc protrusion should not be overlooked as a differential diagnosis of unknown intramedullary foreign bodies. Accurate diagnosis and early laminar decompression and intramedullary decompression can improve the nerve shock.
Author's contribution
BZ, YZB, XDL, XFZ and YZJ treated the patient and completed the operation. YZB and XDL designed the whole diagnosis and treatment process. YZB and BZ designed the study, developed the retrieval strategy, and made the final revision to the article. DTQ, XNW, and RTZ searched and screened the summaries and titles of manuscripts and completed follow-up visits. YZJ completed the entire initial manuscript. All authors read and approved the final draft.
Ethics approval and consent to participate
The current clinical research program was performed according to the Helsinki Declaration and approved by the Ethics Review Committee of the Second Hospital of Shanxi Medical University. The number is 2019YX NO.244. During the treatment, the patient provided consent for us to share his case and continue the study.
Consent for publication
All authors provide their consent for publication.
Conflict of interest statement
The authors have no conflicts of interest or relevant financial activities outside the submitted work.
Funding information
This work was supported by a project from Fund Program for the Scientific Activities of Selected Returned Overseas Professionals in Shanxi Province (project number: 2020040) and a project from Key Research and Discovery Program of Shanxi Province (project number: 201903D321151).




