Stimulator of interferon genes (STING) expression in the enteric nervous system and contributions of glial STING in disease
Christine Dharshika and Jacques Gonzales contributed equally to the work.
Abstract
Background
Appropriate host–microbe interactions are essential for enteric glial development and subsequent gastrointestinal function, but the potential mechanisms of microbe–glial communication are unclear. Here, we tested the hypothesis that enteric glia express the pattern recognition receptor stimulator of interferon genes (STING) and communicate with the microbiome through this pathway to modulate gastrointestinal inflammation.
Methods
In situ transcriptional labeling and immunohistochemistry were used to examine STING and IFNβ expression in enteric neurons and glia. Glial-STING KO mice (Sox10CreERT2+/−;STINGfl/fl) and IFNβ ELISA were used to characterize the role of enteric glia in canonical STING activation. The role of glial STING in gastrointestinal inflammation was assessed in the 3% DSS colitis model.
Results
Enteric glia and neurons express STING, but only enteric neurons express IFNβ. While both the myenteric and submucosal plexuses produce IFNβ with STING activation, enteric glial STING plays a minor role in its production and seems more involved in autophagy processes. Furthermore, deleting enteric glial STING does not affect weight loss, colitis severity, or neuronal cell proportions in the DSS colitis model.
Conclusion
Taken together, our data support canonical roles for STING and IFNβ signaling in the enteric nervous system through enteric neurons but that enteric glia do not use these same mechanisms. We propose that enteric glial STING may utilize alternative signaling mechanisms and/or is only active in particular disease conditions. Regardless, this study provides the first glimpse of STING signaling in the enteric nervous system and highlights a potential avenue of neuroglial-microbial communication.
Key points
- Enteric neurons and glia express the pattern recognition receptor STING.
- Ablating glial STING has a minor role in IFNβ production in the myenteric and submucosal plexuses.
- Glial STING may utilize alternative signaling mechanisms that involve autophagy, but does not impact disease severity in DSS colitis.
1 INTRODUCTION
Appropriate interactions between gut commensal bacteria and host cells shape microbe–host tolerance that is essential for maintaining gut homeostasis.1-3 Disrupting this delicate balance can lead to sustained or repetitive inflammation and ultimately the development of chronic gastrointestinal (GI) diseases such as inflammatory bowel disease (IBD).1, 3, 4 The role of host–microbe communication in GI disease is complex and involves bidirectional signaling that alters both microbial factors and host responses that impact disease susceptability.5 Known mechanisms of communication focus on mucosal and immune cells as important regulators of host-dependent factors1-3 but other cells also communicate and respond to microbial factors to influence gut homeostasis and disease pathogenesis.
The enteric nervous system (ENS) is comprised of neurons and glia that regulate GI functions including motility, visceral sensation, secretion, absorption, and mucosal permeability.6, 7 The ENS is ideally situated to facilitate crosstalk between local microbe–immune interactions and to modulate systemic health through brain–gut axis signaling.8, 9 This occurs through both direct interactions between the ENS and the gut microbiome and microbial tuning of neuro-immune communication.9-14 Communication between enteric glial cells and luminal microbes is critical for ENS maintenance and host defense mechanisms. Gut microbiota regulates the formation and replenishment of enteric glia within the mucosa and lamina propria.15-17 In turn, these glial cells respond to gut dysbiosis and interact with immune cells and the mucosal barrier to produce varying proinflammatory and protective effects.18-21 Characterized mechanisms of glial-microbe signaling involve S100β and pattern recognition receptors of the toll-like receptor family; however, the mechanisms whereby glia monitor the gut microbiota and initiate functional responses remain mostly uncharacterized.
The stimulator of interferon genes (STING, also known as Tmem173) is a pattern recognition receptor that integrates signals from bacterial, viral, and host molecules to modulate inflammatory response.22-27 STING signaling has a complex role in gut homeostasis and disease as demonstrated by experiments in whole body STING knockout mice. Mice lacking STING were initially reported as having abnormal mucosal architecture and function under physiologic conditions, but these observations were not replicated in other studies.28, 29 Likewise, ablating STING has reportedly been either protective or detrimental in the dextran sodium-sulfate (DSS) colitis model.28-30 These apparently conflicting results suggest underlying complexities in STING signaling that dictate its homeostatic and pathologic roles. Most attention devoted to STING signaling focused on its role in immune cells, but STING is also present in non-canonical cell types such as neurons and glia in the central nervous system (CNS) where astrocytes activate STING signaling in response to infection and injury.31, 32 Additionally, these astrocytes enter a STING-dependent reactive state in injury as measured by glial fibrillary acidic protein (GFAP) upregulation.31 Enteric glia exhibit similar transcriptional profiles to CNS glia33, 34 and play critical roles in immune communication during colitis.35, 36 Given these similarities, it is plausible that enteric glia express STING and respond to host and microbial mediators to modify GI inflammation through the STING pathway.
The aim of this study was to assess the expression and role of STING signaling within the ENS and study its contributions to GI homeostasis and disease. We hypothesized that like CNS glia, enteric glia also express STING and that glial STING signaling is involved in colitis. We tested our hypothesis using immunolabeling and genetic mouse models including cre-lox systems to ablate STING in enteric glia (Sox10CreERT2+/−;STINGfl/fl). Our results show that STING is expressed by both enteric glia and neurons and plays a complex role in regulating downstream pathways involving IFNβ. Surprisingly, the data show that glial STING plays a minor role in canonic IFNβ responses and the pathophysiology of acute DSS colitis. These results show that while STING is a functional mechanism in the ENS, its roles are cell type and context dependent.
2 MATERIALS AND METHODS
2.1 Animal use
All experimental protocols were approved by the Michigan State University Institutional Animal Care and Use Committee (IACUC) in facilities accredited by the Association for Assessment and Accreditation for Laboratory Care (AAALAC) International. Adult male and female C57BL/6 mice 10–20 weeks of age were used for experiments unless stated otherwise. Mice were maintained in specific pathogen-free conditions and a temperature-controlled environment (Optimice cage system; Animal Care Systems) on a 12:12 hr light–dark cycle with ad libitum access to water and diet (Diet Number 2919; Envigo).
Sox10CreERT2+/−;STINGfl/fl were generated to ablate STING in enteric glia in the GI tract. Briefly, Sox10CreERT2 mice (MGI:5910373; gifted by Dr. Vassilis Pachnis, The Francis Crick Institute) were crossed with B6;SJL-Sting1tm1.1Camb STING flox mice (RRID: IMSR_JAX:031670; gifted by Dr. John Cambier, University of Colorado Denver and National Jewish Health) to generate mice hemizygous for Cre and the floxed allele (Sox10CreERT2+/−;STINGfl/wt). These mice were then backcrossed with B6;SJL-Sting1tm1.1Camb STING flox mice to generate Sox10CreERT2+/−/STINGfl/fl mice. Mice were genotyped by Transnetyx and fed tamoxifen citrate (400 mg kg−1) diet (TD.140849; Envigo) for 2 weeks to induce Cre activity.
2.2 Acute dextran sodium sulfate (DSS) induced colitis
Acute colitis was induced by adding 3% (w/v) DSS (colitis grade, M.W. 36–50 kDa; MP Biomedical) to drinking water from day 0 to day 7. DSS solution was refreshed every 2 days. Bodyweight was recorded daily, and animals were monitored for signs of disease activity such as loose stools and fecal blood. Mice were euthanized on day 8 and colon lengths were recorded.
2.3 Muscularis propria, submucosal, and myenteric plexus tissue isolation
Distal colonic samples were obtained from euthanized mice and placed in a Sylgard-coated petri dish containing ice-cold Dulbecco's modified Eagle medium/Nutrient Mixture F-12 (DMEM/F-12, 11,039,047; Gibco). Tissue was cut open along the mesenteric border and pinned flat with mucosa facing up. Live samples for IFNβ ELISA experiments were microdissected to the submucosal plexus (SMP) and muscularis propria by removing the mucosa and SMP separately. Live circular muscle-myenteric plexus (CMMP) preparations were prepared for IFNβ reporter (IFNβ-mob) experiments by removing the mucosa, serosa, and longitudinal muscle as described previously.37 For RNAscope and immunohistochemistry, tissue was subsequently fixed overnight in 4% paraformaldehyde (PFA) at 4°C and then washed with phosphate-buffered saline (PBS). Longitudinal muscle-myenteric plexus (LMMP) whole mount preparations were microdissected by removing overlying mucosa, submucosa, and circular muscle.
2.4 RNAscope in whole mount LMMP
RNAscope in whole mount myenteric plexus LMMP tissue was adapted from previous protocols13 and validated in prior published work38; using the RNAscope™ 2.5 HD Assay – RED (ACD Biosciences, Newark, CA) according to manufacturer instructions with the following adjustments for colonic LMMP tissue. Tissues were dehydrated and subsequently rehydrated by a serial ethanol gradient (25%, 50%, 75%, 100% in PBS with 0.1% Triton X-100) prior to H2O2 treatment. Tissues were then digested with Protease III for 45 min and incubated with RNAscope probes overnight at 40°C (listed in Table 1). Tissues were washed 3 × 5 min between each step with PBS (before probe incubation) or with RNAscope™ wash buffer (between amplification steps). Immunohistochemistry was performed following completion of the RNAscope protocol as described below.
| RNAscope Probes | |||
|---|---|---|---|
| Probe Target | Vendor | Catalog number | Channel |
| Tmem173 (Alt. name for Sting1) | ACD Bio-Techne | 413,321 | C1 |
| Immunohistochemistry (IHC) Antibodies | ||||
|---|---|---|---|---|
| Target | Vendor | Catalog number | Dilution | RRID |
| Primary antibodies | ||||
| Chicken anti-GFAP | Abcam | ab4674 | 1:1000 | AB_304558 |
| Mouse anti-HuC/D (Biotinylated) | Invitrogen | A21272 | 1:200 | AB_2535822 |
| Mouse anti-Peripherin | Santa Cruz | sc-377,093 | 1:100 | |
| Rat anti-MHCII | Novus | NBP2-21789 | 1:200 | AB_2828034 |
| Rabbit anti-Calbindin-D28K | Swant | CB38 | 1:200 | AB_10000340 |
| Rabbit anti-IFNβ | Novus | NBP1-77288 | 1:200 | AB_11039065 |
| Rabbit anti-STING | Proteintech | 19,851-1-AP | 1:500 | AB_10665370 |
| Rabbit anti-S100β | Abcam | ab52642 | 1:200 | AB_882426 |
| Sheep anti-nNOS | Millipore | AB1529 | 1:200 | AB_90743 |
| Rabbit anti-P62 | Abcam | ab109012 | 1:200 | AB_2810880 |
| Rabbit anti-LC3B | Abcam | ab192890 | 1:200 | AB_2827794 |
| Secondary antibodies | ||||
| Donkey anti-sheep Alexa Fluor 405 | Jackson Laboratories | 713–475-147 | 1:400 | AB_2340740 |
| Goat anti-chicken Dylight 405 | Jackson Laboratories | 103–475-155 | 1:400 | AB_2337389 |
| Donkey anti-chicken Alexa Fluor 488 | Jackson Laboratories | 703–545-155 | 1:400 | AB_2340375 |
| Donkey anti-mouse Alexa Fluor 488 | Jackson Laboratories | 715–545-150 | 1:400 | AB_2340846 |
| Donkey anti-rabbit Alexa Fluor 488 | Jackson Laboratories | 711–545-152 | 1:400 | AB_2313584 |
| Donkey anti-sheep Alexa Fluor 488 | Jackson Laboratories | 713–475-147 | 1:400 | AB_2340740 |
| Donkey anti-rabbit Alexa Fluor 594 | Jackson Laboratories | 711–585-152 | 1:400 | AB_2340621 |
| Goat anti-rat Alexa Fluor 594 | Jackson Laboratories | 112–585-003 | 1:400 | AB_2338372 |
| Streptavidin Alexa Fluor 594 | Jackson Laboratories | 016–580-084 | 1:400 | AB_2337250 |
| Donkey anti-rabbit Alexa Fluor 647 | Jackson Laboratories | 711–605-152 | 1:400 | AB_2492288 |
| Donkey anti-rat Alexa Fluor 647 | Jackson Laboratories | 712–605-150 | 1:400 | AB_2340693 |
2.5 Immunohistochemistry in whole mount LMMP
Immunohistochemistry (IHC) was performed as described previously.35, 36 Primary and secondary antibody details are provided in Table 1. Briefly, LMMP preparations were washed in PBS with 0.1% Triton X-100 followed by 45 min incubation in blocking solution (4% normal donkey serum, 0.4% Triton X-100, and 1% bovine serum albumin) at 20°C. Primary antibodies were diluted in blocking solution and incubated with tissue for 24–48 h at 4°C. Tissues were then washed in PBS and incubated with secondary antibodies diluted in blocking solution for 2 h at 20°C. LMMP preparations were then washed in PBS followed by a last rinse in 0.1 M phosphate buffer. Tissues were finally mounted on slides with Fluoromount-G mounting medium with or without DAPI (Southern Biotech). All antibodies are validated by prior studies.35, 39-43
2.6 Fixed frozen section immunohistochemistry
Fixed frozen slide IHC was performed as described previously.34 Distal colons were harvested, pinned at ends in Sylgard-coated dishes, and fixed overnight in 4% paraformaldehyde (PFA) at 4°C. Fixed samples were washed in 0.1 M phosphate buffer and subsequently cryoprotected in a 30% sucrose solution in 0.1 M phosphate buffer for at least 72 hours. About 10–12 μm cryosections were processed by the Michigan State University HistoPathology Laboratory. For IHC, slides equilibrated to 20°C for 2 h before use. Blocking, labeling, and washes were carried out using the same protocol as described before.
2.7 Histological staining and scoring
Cross-sections of distal colon were submerged in 10x volume of Carnoy's fixative (60% ethanol, 30% chloroform, and 10% glacial acetic acid) and fixed for 4 hours at 20°C. Tissues were transferred to 30% ethanol in distilled H2O prior to 4 μm sectioning and staining with hematoxylin & eosin (H&E) or periodic acid-schiff & hematoxylin (PASH) by the Michigan State University HistoPathology Laboratory.
Histological colitis scoring was performed using OlyVIA software (Olympus, Tokyo, Japan). Tissue damage was assessed per colonic cross section where the extent of tissue destruction was calculated based on mucosal architecture (0 = none, 1 = mild damage, 2 = moderate damage, and 3 = loss of crypt and epithelial structure), cellular infiltration (0 = normal, 1 = around crypt bases, 2 = reaching muscularis mucosa, and 3 = reaching submucosa), muscle thickening (0 = none, 1 = mild, 2 = moderate, 3 = large), and goblet cell depletion (0 = present, 1 = decreased in number/modified morphology, or 2 = depleted). A score multiplier of 1, 2, 3, or 4 was used based on the percentage of cross-section affected (25%, 50%, 75%, or 100%, respectively).
2.8 IFNβ ELISA experiments
Muscularis propria and SMP were incubated with STING agonists in DMEM/F-12 with 100 U mL−1 Penicillin–Streptomycin (15,140,122; Gibco) for 18–24 h at 37°C (5% CO2). Media was collected at the end of the incubation period and centrifuged at 2000g for 10 min. Samples were either processed immediately for ELISA or kept at −80°C until further processing. STING agonists used included 100 μM c-di-GMP (C057; Biolog), 100 μM 2,3-cGAMP (tlrl-nacga23; Invivogen), 100 μM 3,3-cGAMP (tlrl-nacga; Invivogen), 100 μg mL−1 DMXAA (tlrl-dmx; Invivogen), and ~ 109 viral particles of Ad5-VCA095644 (a c-di-GMP producing adenovirus gifted by Dr. Christopher Waters, Michigan State University, MI). C-di-GMP and Ad5-VCA0956 ELISAs were performed after 24 h incubations using the Verikine™ Mouse IFN Beta ELISA Kit (42,400; PBL Assay Science) while the 2,3-cGAMP, 3,3-cGAMP, and DMXAA ELISAs were performed after 18 h incubations using the Mouse IFN beta SimpleStep ELISA® Kit (ab252363; Abcam). Tissue preparations were cut to the same dimensions or normalized to tissue weight. All ELISAs were conducted in duplicate following manufacturer's protocols and measured on a Synergy H1 Hybrid Multi-Mode Microplate Reader with Gen5 v3.04 software (BioTek).
2.9 Autophagy induction and detection
Live LMMP preparations from wild-type animals were incubated in DMEM/F-12 medium containing 100 μg mL−1 DMXAA (tlrl-dmx; Invivogen) for 0, 3, and 6 h at 37°C, in order to induce autophagy. Tissues were then fixed in PFA 4% and autophagy was detected by measuring immunofluorescence intensity of P62 and LC3B puncta per cellular area.35 Glial cells were identified by GFAP+ labeling and neurons were identified as GFAP− cells within the myenteric ganglia. LC3B puncta was detected by thresholding the LC3B channel via the Intermodes algorithm in the Threshold function of FIJI45 (National Institutes of Health). The quantification was assessed using the Analyze Particles function with the following settings: particle size of interest from 0 to infinity, and circularity from 0 to 1.
2.10 Imaging
RNAscope and IHC representative imaging was performed on a Zeiss LSM 880 NLO confocal system (Zeiss) or Airyscan system with 32 channel GaAsP design using Zen Black software and a 20x objective (0.8 numerical aperture, Plan ApoChromat; Zeiss). Using digital zoom 40x and 80x images were achieved within the software. Images for quantification of nNOS+ or calbindin+ neurons were captured using the 20x objective (0.75 numerical aperture, Plan Fluor; Nikon) of an upright epifluorescence microscope (Nikon Eclipse Ni) with a Retiga 2000R camera (QImaging) controlled by QCapture Pro 7.0 (QImaging) software. Neuronal counts were performed using FIJI software (NIH). Representative images for colitis scoring of histology slides were captured on an Olympus SLIDEVIEW VS200 slide scanner.
2.11 Statistical analysis
All statistical analyses were performed in GraphPad Prism v9.4.0 (GraphPad Software) and considered significant at p < 0.05. All analyses were performed using either a one- or two-way ANOVA with Dunnett's, Tukey's, or Bonferroni's multiple comparison tests where appropriate. Details on sample size and specific statistical tests are specified in respective figure legends.
3 RESULTS
3.1 STING is expressed by enteric neurons and glia
STING is expressed by neurons and glia in the CNS31, 32 but whether it is expressed in the ENS is unknown. We began assessing potential STING expression in the ENS using available RNA-sequencing datasets for enteric glia that were generated in previous publications.46, 47 Bulk sequencing of enteric glia data are represented in Figure 1A (available from Delvalle et al.47 GEO accession: GSE114780) while single cell sequencing data are represented in Figure 1B (available from Drokhlyansky et al.46 and accessed through the Single Cell Portal, Broad Institute). The canonical STING signaling cascade involves STING forming a complex with TANK-binding kinase (Tbk1) to activate transcription factors interferon regulatory factor (Irf3) and NF-κB (Nfkb1 and Rela) that stimulate the production of type I IFNs such as IFNβ.22 Type I IFNs bind to the IFNα/β receptor (Ifnar1 + Ifnar2) and activate interferon-stimulated gene factor 3 (comprised of Stat1, Stat2, and Irf9) to induce transcription of IFN-stimulated genes.22, 48 The transcriptional data show that enteric glia express STING (gene Sting1, alternate gene name Tmem173), its directly downstream signaling molecules, and genes for the proteins induced by type I IFN binding in both mice (Figure 1A,B) and humans (Figure 1B). An additional published single cell dataset was accessed and had similar human enteric glial expression patterns of these genes (data not shown).49 We confirmed these results by visualizing Sting1 expression within the myenteric plexus using RNAscope in situ hybridization. Both enteric glia (S100β+ cells) and neurons (peripherin+ cells) of the myenteric plexus express Sting1 (Figure 1C, white and yellow arrows, respectively) and labeling for Sting1 is reduced in enteric glia from glial STING KO (Sox10CreERT2+/−;STINGfl/fl) mice. Taken together, these data suggest that enteric glia express the necessary genes to utilize STING signaling through its prototypical signaling cascade and additionally respond to downstream type I IFN signaling.
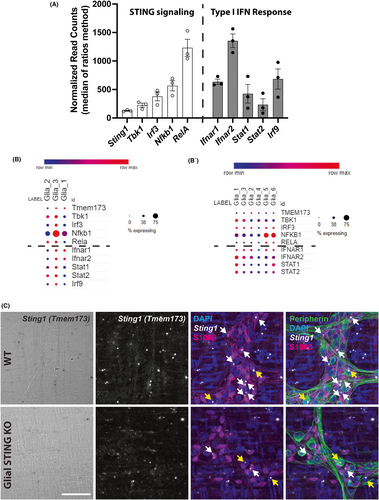
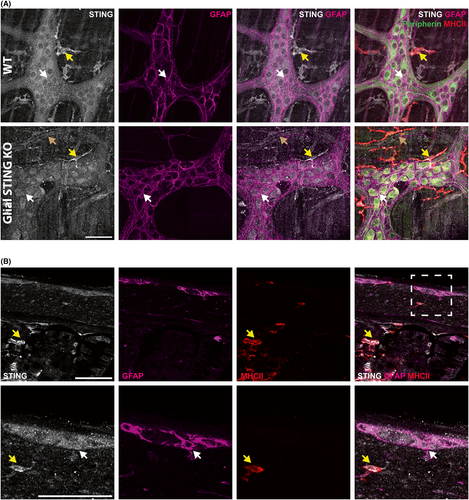
We next determined whether STING protein is expressed by enteric neurons and/or glia using immunohistochemistry. STING immunoreactivity was observed in both neurons and glia throughout the myenteric plexus in WT mice (Figure 2A). Immunoreactivity for STING was also observed in neighboring MHCII+ immune cells, which are likely resident muscularis macrophages (yellow arrows). Interestingly, STING is also expressed in endothelial cells22 and positive immunolabeling of blood vessels adjacent to ganglia was observed (brown arrow). Despite the highly overlapping nature of enteric neurons and glia that express STING, the loss of STING expression of enteric glia in STING KO mice was evident in regions surrounding enteric neurons (white arrows denote glial cell bodies surrounded by GFAP+ processes in WT and glial STING KO panels) which led to increased definition of neuronal cell bodies (Figure 2A). Immunolabeling in cross-sections of colon showed clear labeling in the myenteric plexus including neurons and enteric glia (white arrow denotes a glial cell body) (Figure 2B) and stronger STING labeling of MHCII+ immune cells in the muscularis propria and mucosa/lamina propria (yellow arrows) in WT animals. These data suggest that enteric neurons and glia express STING, albeit at lower levels than immune cells.
3.2 Myenteric plexus IFNβ is primarily localized to enteric neuron cell bodies and neuronal and/or glial processes, and fiber tracts
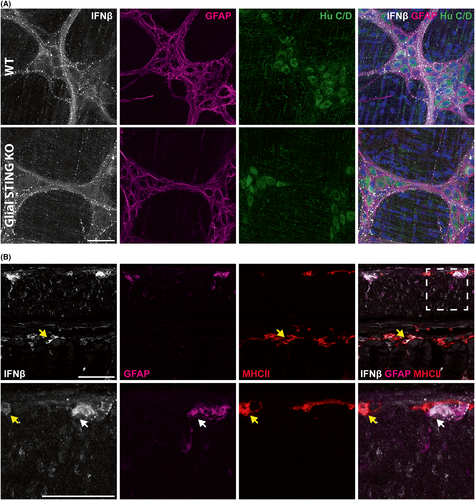
Given that enteric neurons and glia express STING RNA and protein, we assessed whether type I IFNs, the primary mediator of STING activity,22 are also expressed by these cells. We began by examining expression of the gene encoding IFNβ (Ifnb1) in enteric glial transcriptional datasets. Interestingly, Ifnb1 was not expressed at detectable levels in bulk enteric glial RNA-sequencing datasets47 and was negligible in single cell transcriptional sequencing datasets for enteric glia (data not shown),46, 49 suggesting that enteric glia do not express IFNβ. This was confirmed using immunolabeling which revealed that IFNβ is primarily localized to neuronal cell bodies and neuronal fiber tracts (Figure 3A). These data suggest that enteric neurons are responsible for IFNβ in the ENS and that enteric neurons traffic IFNβ along their processes. We conducted labeling in cross-sections of mouse colon to visualize the extent of IFNβ expression in the gut wall and relative expression in other cell types compared to the ENS. Similar to STING, IFNβ is primarily expressed in the myenteric plexus and mucosa/lamina propria (Figure 3B). However, unlike STING the relative expression of IFNβ in the ENS (denoted by white arrows) is comparable to MHCII+ immune cells (denoted by yellow arrows). This supports IFNβ as a functional immune mediator in the ENS and enteric neurons whether through STING-dependent or -independent pathways.
3.3 Enteric glial STING does not affect ENS IFNβ production but plays a role in glial autophagy
Since STING and IFNβ are both expressed in the ENS, we next sought to determine if ENS STING could activate production of IFNβ and whether enteric glial STING contributed to this response. Canonical STING signaling responds to cyclic dinucleotide (CDN) molecules produced by microbes and in response to self-leaked DNA during host damage to upregulate type I IFNs.22
ENS IFNβ production with STING activation was measured by ELISA from supernatants of ENS preparations incubated in vitro with STING ligands for 18–24 h (Figure 4A–C). Muscularis propria tissue were incubated with either the bacterial CDN c-di-GMP or an adenovirus construct designed to promote host c-di-GMP production (AdVCA or Ad-VCA0956).44 An adenovirus containing no transgenic material (AdNull) was used as an additional negative control. The myenteric plexus produced IFNβ in response to both c-di-GMP and AdVCA (p < 0.01; Figure 4A) indicating that ENS STING can be canonically activated by bacterial c-di-GMP. However, there are a variety of other STING ligands including the microbial-produced CDN 3,3-cGAMP, the host-produced CDN 2,3-cGAMP, and the murine pharmacologic agonist DMXAA.22, 24, 25, 50, 51 Differential responses to these ligands by the ENS may suggest specified function of ENS STING and therefore, we tested IFNβ in response to these ligands as well. Both the myenteric (p < 0.01; Figure 4B, left) and submucosal (p < 0.001; Figure 4B, right) plexuses produce IFNβ in response to STING ligands. However, IFNβ production between ligands was not significantly different suggesting that ENS STING responds similarly to these STING ligands. Interestingly IFNβ production in response to all ligands was higher in the submucosal than myenteric plexus (p < 0.0001; Figure 4C). This is likely due to the additional STING+ cell types that reside within and adjacent to this layer (Figure 2B), therefore it is unclear to what degree enteric neurons and/or glia contribute to total IFNβ production in the submucosal plexus.
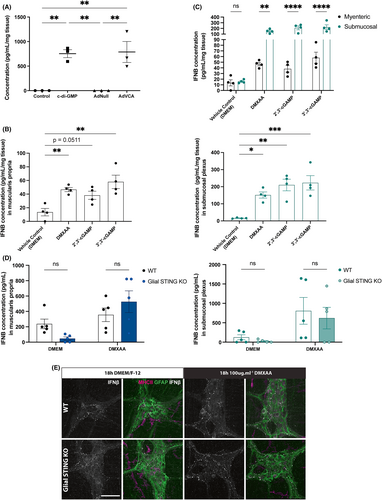
Even though ENS IFNβ is primarily produced in enteric neurons, glial STING may still indirectly contribute to IFNβ production. STING transcription is induced by type I IFNs and STING in turn potentiates CDN-triggered type I IFN production.52 Neuronal IFNβ could potentiate STING in enteric glia and in turn, enteric glial STING could potentiate neighboring neurons. CNS neuroglia complete STING signaling with the help of other cell types,53 and enteric neurons and glia also divide and share other signaling cascades like that of the antioxidant glutathione.41 Thus, we performed ELISA on supernatants from WT and glial STING KO mice after 18 h incubation with 100 μg mL−1 DMXAA. Genotype did not significantly affect IFNβ production in either the myenteric (Figure 4D, left) or submucosal (Figure 4D, right) plexus. These results were confirmed by immunolabeling of myenteric plexus after 18 h incubation with 100 μg mL−1 DMXAA. There are no appreciable differences in IFNβ staining within the myenteric ganglia of WT and glial STING KO mice (Figure 4E) suggesting that enteric glial STING does not play a significant role in ENS IFNβ production.
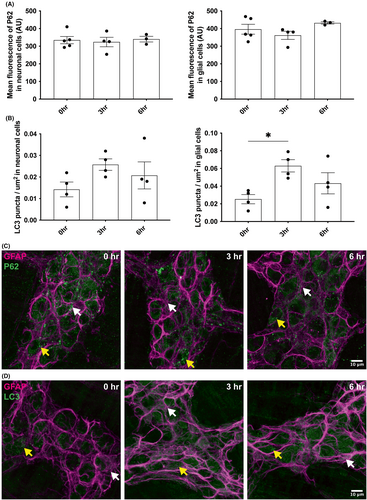
A previous study reports that STING could activate autophagy pathways in an IFNβ-independent manner.54 We measured the level of the autophagy markers p62 and LC3 at different time points after incubation of the myenteric plexus with 100 μg mL−1 of DMXAA (Figure 5A–D). The neuronal and glial expression of p62 is not modified by the presence of DMXAA (Figure 5A,C). However, we observed an increased expression of LC3 in enteric glia (GFAP+ cells) after 3 hours of incubation with the STING agonist (Figure 5B,D), while it had no effect on the neuronal expression of LC3. These results suggest that glial STING plays a role in enteric glial autophagy.
3.4 Enteric glial STING does not play a major role in GI health or ENS neuronal populations in acute DSS colitis
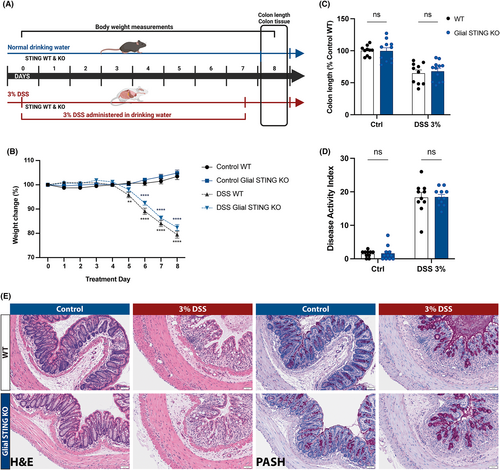
The pathogenesis of DSS colitis involves compromised mucosal barrier integrity and exposure of deeper gut layers to luminal contents including bacterial metabolites.55, 56 Prior work assessing the susceptibility of whole-body STING KO mice to acute 3% DSS colitis reported opposing results,28, 29 suggesting that more complex and specific factors may influence STING's role in acute DSS colitis. In our study, WT and glial STING KO mice were given 3% DSS in their drinking water for 7 days and euthanized on day 8 (Figure 6A). Significant weight loss (p < 0.0001; Figure 6B) and colonic shortening (p < 0.0001; Figure 6C) occurred in DSS-treated mice compared to controls with no significant difference between genotypes. DSS significantly increased the histological disease index in both WT and glial STING KO mice (p < 0.0001; Figure 6D) to a comparable degree with no observed sex differences.
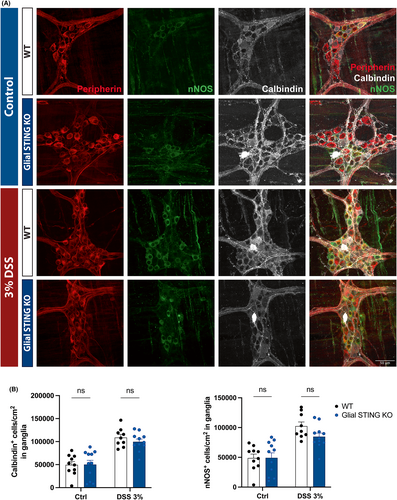
Acute DSS colitis affects enteric neuroplasticity and can alter proportions of cholinergic and nitrergic neurons in the colonic myenteric plexus.57-60 We assessed neurochemical coding in WT and glial STING KO mice using immunolabeling for calbindin and nNOS as broad markers of excitatory and inhibitory neuron populations, respectively (Figure 7A). Positive cells were counted per ganglion and normalized to ganglionic area. DSS-treated mice exhibited increased proportions of calbindin+ neurons (p < 0.0001; Figure 7B, left) which is consistent with prior reports,57, 58 however we observed an increase in the proportion of nNOS+ nitrergic neurons (p < 0.01; Figure 7B, right) where previous studies reported no difference or loss of nitrergic neurons.58-60 Genotype did not have a significant effect on the proportions of cholinergic or nitrergic neurons and sex differences were not observed (data not shown). Taken together, these data support the conclusion that enteric glial STING does not play a major role in acute DSS colitis.
4 DISCUSSION
Appropriate host-microbial communication is essential for both tolerance of commensal microbes and anti-pathogen defense where perturbations in signaling result in inflammatory damage and disease.1-5 The STING responds to microbial cues to modulate inflammation22, 23, 25, 26 and plays a complex role in acute colitis by either exacerbating or ameliorating colonic damage.28-30 We hypothesized that cell-specific STING activation contributes to STING's detrimental or protective role and enteric glial STING plays a part in this balance. We provide the first known examination of STING and IFNβ expression in the ENS and determined that both neurons and glia of the myenteric plexus express STING, while primarily enteric neurons express IFNβ. We also showed that ENS STING can be activated to produce IFNβ in enteric neurons or to induce autophagy in enteric glia. Finally, we demonstrated that glial STING does not play a major role during acute DSS colitis. Our data provide the first investigation of STING and IFNβ expression and activation in the ENS and potential roles of ENS STING signaling in disease.
Neurons and glia in the central nervous system express STING in culture and in situ albeit at lower levels than professional immune cells such as microglia.31, 32, 61 Our in situ transcriptional labeling and immunohistochemistry similarly support that ENS cells express STING but less than neighboring MHCII+ immune cells (Figures 1C and 2). Furthermore, transcriptional datasets support that enteric glia express genes necessary for STING signaling (Figure 1A,B). Enteric glia express genes for NF-κB (Nfkb1 and RelA) at relatively higher levels than other STING components. While relative gene expression does not necessarily correlate to protein expression or activity, this likely reflects the less specific role of NF-κB than other STING signaling components as it is involved in many other immune signaling pathways including other pattern recognition receptors.62 This could suggest a role for other STING-like cytosolic DNA sensors in enteric glia as they are expressed in CNS glia and induced in neurodegenerative disease.61 Future research could examine the role of these other cytosolic DNA sensors in the ENS and enteric glia.
Interestingly while STING is expressed by both ENS neurons and glia, IFNβ is only expressed by enteric neurons. This is supported by both gene expression (Figures 1A,B) and protein localization in situ (Figure 3A,B). IFNβ has not been previously investigated in the ENS and our results support enteric neurons IFNβ production and trafficking, suggesting that this may play a functional role in GI health and/or disease. In the brain IFNβ plays a neuroprotective role during HIV infection but it is unclear whether this IFNβ is produced within neurons themselves or other cell types.63 Additionally, IFNβ is detectable but relatively lowly expressed in the brain during basal conditions64, 65 and increased during viral infection,63 suggesting it more likely plays a pathologic and not homeostatic role. This is contrasted in the ENS as our data demonstrated relatively high expression of IFNβ in enteric neurons comparable to resident MHCII+ immune cells (Figure 3B). Taken together these data suggest enteric neuronal IFNβ may play a role in physiologic and infectious conditions but further investigation is required to elucidate this. Our data show ENS STING activation and IFNβ production in both the myenteric and submucosal plexus (Figure 4B,C), and IFNβ induction in the muscularis propria by Ad5-VCA0956 (Figure 4B), a virus which infect neurons.66, 67 However, our in vitro tissue preparations to assess IFNβ production likely contain STING+ immunes cells which could participate in IFNβ production. Our data suggest that enteric neurons and not enteric glia that utilize STING-dependent IFNβ signaling in the ENS. This would be interesting to investigate in future studies and additionally compare to the CNS. To date, central neuroglia works contrast our ENS findings and support higher STING expression in glia than neurons and glial STING activation in disease states.31, 32, 53
Even though enteric glia do not affect canonic STING signaling to produce type I IFNs in response to CDNs (Figure 4D,E), there are reports of specialized STING signaling mechanisms. For instance, STING can respond to etoposide-induced DNA damage to produce IFNβ response in keratinocytes and fibroblasts.68 STING can also activate autophagy in an IFN-independent mechanism in fibroblasts, such demonstrated on a sea anemone Nematostella vectensis.54 In parallel, ENS is known to be a highly immune organ and regulate host–microbe interaction in primordial creatures such as Hydra.69 Recent work in mice supports a role for enteric glia in mammalian immunity35, 36 and even specifically highlight glial autophagy mechanisms during inflammation to communicate with lymphocytes.35 Our data show that mainly enteric glia autophagy is activated after STING activation. The differential autophagy activation between neurons and glia (Figure 5A,B) is a phenomenon already described in the CNS where metabolic stress activates robustly autophagy only in astrocytes.70 Enteric glia autophagy, following STING activation, lead to an increase in LC3 expression (Figure 5B) and no change in P62 expression, suggesting that STING activates non-canonical autophagy signaling independent of P62.71 Autophagy mechanisms in enteric nervous system cells are not well described; however, based on what is known about the roles of autophagy in related types of neuroglia such as astrocytes and Schwann cells, it is possible that autophagy in enteric glia could function to improve neuroprotection during inflammation by decreasing the inflammatory environment with phagocytosis of neurotoxic protein or cellular debris72, 73 and by increasing the recruitment of T and B lymphocytes close to the myenteric plexus.35 Our findings suggest that enteric glial STING retains a primordial autophagy-related function independent of type I IFNs.
It is also possible that the role of enteric glial STING in ENS and GI function is more specialized and explains why we do not see any significant effects in acute DSS colitis between WT and glial STING KO mice (Figures 6 and 7). Traumatic brain injury induces STING-dependent reactivity in central nervous system glia31 and perhaps enteric glia are important in GI disease states similar to this. The pathophysiology of traumatic brain injury is highly dependent on dysfunctional autophagy31, 74-77 and thus further supports both these specialized signaling mechanisms and functional responses in enteric glia. Another possibility is that enteric glia do play a role in inflammatory disease but only in chronic conditions and not during the acute state we studied. Type I IFNs play opposing roles in acute and chronic DSS colitis through myeloid cells where IFN signaling is protective during the acute phase of injury but prevented resolution of inflammation in chronic settings.78 While our data suggest enteric glia do not express IFNβ or use STING-dependent type I signaling in homeostasis or acute colitis, it is possible these genes could be upregulated only during chronic inflammatory states. Another possibility is that glia respond to IFNβ produced in other cell types via the IFNβ receptor and downstream signaling molecules (Figure 1A,B) to potentiate STING expression and function as is seen in other reports.52 This is possible as enteric glia express the IFNβ receptor and its downstream signaling molecules (Figure 1A,B).
One potential limitation of our findings is the efficacy of our genetic STING KO as it is difficult to appreciate certain loss of labeling in enteric glia in situ by either RNAscope or immunolabeling due to the highly overlapping nature of enteric glia with enteric neurons and the diffuse expression of STING across the myenteric ganglia (Figures 1C and 2A). This could mean our lack of glial STING contribution to IFNβ production and acute DSS colitis are due to ineffective glial STING reduction. However both our Sox10CreERT2 line and the floxed STING line (B6;SJL-Sting1tm1.1Camb) are independently validated.79-82 Another limitation is because of murine colonic shortening in acute DSS, the exact distal or proximal location of tissue used to determine neuronal proportions may vary. To correct this, we normalized to the number of neurons per ganglionic size instead of total neuronal number as the proximal colon has different proportions of neuronal types and total neuronal numbers.6, 83, 84 Regardless, this may still explain our increased number of nNOS+ neurons in acute DSS compared to previous studies that reported no changes or decreased proportions.58-60 However, other studies utilized 5% DSS instead and therefore nNOS neuronal alterations in DSS colitis may be dependent on colitis severity.
Overall, this research represents the first known investigation of STING signaling and IFNβ production in the ENS. Our data support novel important findings including the potential of enteric neurons to utilize STING and IFNβ signaling while enteric glial STING is likely important in more specialized roles such as autophagy. Furthermore, we discuss other possible functions of enteric glial STING that future research could uncover. Understanding the circumstances where enteric glial STING is active and how it is activated could help further characterize both the role of enteric glia in micro-immune communication and additional functions for STING in GI health and disease.
AUTHOR CONTRIBUTIONS
BDG and CD were responsible for the overall project conception, design, and supervision. Experiments were performed by CD, JG, AC, and WMS. Manuscript written by BDG, CD, and JG, with all authors contributing to revisions and editing.
ACKNOWLEDGMENTS
This project was supported by grants R01DK103723 and R01DK120862 to B.D.G. from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. The content is solely the responsibility of the Authors and does not necessarily represent the official views of the National Institutes of Health.
CONFLIC OF INTEREST STATEMENT
The Authors have no financial, professional, or personal conflicts that are relevant to the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




