Twenty-year trends of Centropages typicus (Copepoda, Calanoida) reproduction, feeding, population abundance and structure in the Gulf of Naples (Western Mediterranean Sea)
Abstract
Centropages typicus is a temperate calanoid copepod occurring in Atlantic and Mediterranean coastal waters, where its reproductive biology and population dynamics are well-known. C. typicus has also been suggested as a key species for monitoring the impact of environmental changes on copepod secondary production. The aim of this study is to investigate the seasonal and interannual reproductive (egg production and egg hatching success) and feeding (fecal pellet production) traits, population abundance and structure (sex ratio) of C. typicus over a 20-year period at the Long-Term Ecological Research site MareChiara (LTER-MC) in the Gulf of Naples (Central Tyrrhenian Sea, Western Mediterranean Sea), identifying specific trends and patterns. At a seasonal scale, egg production and hatching success were tightly linked with maxima in February–March, driving the juvenile and adult springtime population peaks but showing a poor correlation with phytoplankton abundance, pointing to an omnivorous diet. At a multiannual scale, an observed decrease in the population is backed up by a reduction in egg production, despite an increase in hatching success. The results of this study provide new elements to comprehend the links between C. typicus functional traits, population dynamics, and environmental factors and highlight the importance of running LTER series to monitor the trends in natural environments.
1 INTRODUCTION
The calanoid copepod Centropages typicus Kröyer, 1849 is one of the most abundant and best studied neritic calanoid species in temperate waters, occurring in Northern European Atlantic waters and in the Mediterranean Sea (Bonnet et al., 2007; Mazzocchi et al., 2007; Molinero et al., 2009). In the Gulf of Naples (Central Tyrrhenian Sea, Western Mediterranean Sea), C. typicus is one of the four most conspicuous copepod species (Di Capua et al., 2022; Mazzocchi & Ribera D' Alcalà, 1995), and characterizes the zooplankton associations in late spring–early summer when it reaches peak abundances (Mazzocchi et al., 2012). Considering its wide distribution and the relative ease in manipulation, a large amount of information has been collected on the behavior, reproductive biology, and ecology of C. typicus across the Mediterranean Sea and the North Atlantic Ocean (Carlotti & Harris, 2007; Di Capua & Mazzocchi, 2004; Ianora et al., 2007; Uttieri et al., 2021). In addition, other studies have investigated the interlink between seasonal cycles of reproductive traits, population abundances, and environmental parameters (Carotenuto et al., 2006; Ianora et al., 1992). These early studies conducted in the Gulf of Naples measuring in situ weekly egg production rates (EPRs) and egg hatching success, coupled with population abundances, revealed a seasonal mismatch between periods of high abundances of C. typicus and high reproduction rates (Ianora et al., 1992; Ianora & Buttino, 1990). A successive study highlighted that autotrophic biomass in terms of chlorophyll a (Chl a) did not represent a good predictor to infer hatching success and naupliar survival in the field (Carotenuto et al., 2006). Field records of population structure and abundance, coupled with laboratory results on reproductive and developmental rates, revealed that assessing survival rates of calanoid early developmental stages is of key importance to predict fluctuations in species abundances in situ (Mazzocchi et al., 2006).
The sampling station MareChiara (MC) located in the Gulf of Naples is one of the few long-term stations available in the Mediterranean Sea and is the only one along the Tyrrhenian coast. Physical, chemical, and biological data are being collected here since 1984 (with a major interruption from 1991 to 1995), and as of 2006 the site has been included in the international network of Long-Term Ecological Research stations (LTER-MC), reviewed by Zingone et al. (2019).
Simultaneous measurements of copepod reproductive traits, in terms of egg production and hatching success, feeding traits in terms of fecal pellet production, and population abundances during long-term plankton studies, are very scarce. However, they are necessary to link the temporal variability in copepod population dynamics to complex interactions with environmental parameters, and to forecast the impact of environmental changes on future plankton communities (Maud et al., 2015).
The aim of this work is to analyze the 20-year temporal patterns (1995–2015) of C. typicus population abundances, structure, and reproductive and feeding traits in the Gulf of Naples, taking advantage of the long-term monitoring station LTER-MC, to (i) discern seasonal patterns and interannual variability of this species; (ii) characterize long-term dynamics in terms of stability or significant changes; (iii) relate the observed patterns to abiotic and biotic environmental conditions; (iv) understand to what extent do C. typicus life-history traits determine its population abundances at sea. Hence, this study represents the first describing these biological and ecological parameters over such a long-term period in a dominant copepod species in the Mediterranean Sea.
2 METHODS
Zooplankton samples were collected in the Gulf of Naples at LTER-MC (40° 48′ N, 14° 15′ E; ~ 75 m depth), on-board of the R/V “Vettoria”. At almost regular week frequency from January 1995 to December 2015 in morning hours (09:00–11:00 h), one vertical and one oblique tow were performed successively with a 200-μm mesh Indian Ocean net (113 cm mouth diameter) with a filtering cod-end from 50 m up to the surface. One sample was preserved in buffered formaldehyde 37%-seawater solution (final concentration of ~4%) for later enumeration of Centropages typicus juveniles (CIII-CV) and adult females and males in aliquots taken by initially using Huntsman's method (van Guelpen et al., 1982), and successively by a large-bore graduated pipette after careful mixing. The other live sample was diluted in a 5-L glass jar with surface sea water and transported to the laboratory in an insulated box within 1 h from collection.
Surface (<1 m) seawater temperature was measured with a CTD probe SBE911 mounted on a rosette sampler deployed vertically along the water column, whereas the surface concentration of Chl a was analyzed with a spectrofluorometer (see Ribera d'Alcala et al., 2004). For the analysis of phytoplankton species composition and abundances, surface water samples were collected with a Niskin bottle and fixed with buffered 37% formaldehyde solution (1.6% final concentration) in 500-mL dark glass bottles. The quantitative analysis was carried out using an inverted microscope Axiovert 135 (Carl Zeiss), following the Utermöhl method (Edler & Elbrächter, 2010). For the specific aims of this study, we will only refer to the main taxonomic groups of the phytoplankton assemblage at this sampling site, namely, phytoflagellates, diatoms, dinoflagellates, and coccolithophores (Zingone et al., 2019). Quantitative data for these groups have only been used for the multiple correlation analyses among parameters.
In the laboratory, healthy adult females of C. typicus (n = 15) were randomly sorted under a stereomicroscope (Leica) and then individually transferred to crystallizing dishes containing 100 mL of 50-μm filtered surface seawater collected at LTER-MC the same day and incubated at 20°C on a 12:12 h dark:light cycle. This temperature was chosen because it is included in the seasonal cycle of temperatures measured at LTER-MC over many years, and it is usually measured at the surface in May, when C. typicus has its maximum population abundance (Ribera d'Alcala et al., 2004). Moreover, this is also the optimal temperature for maximal reproduction of C. typicus Mediterranean populations observed in the laboratory (Halsband-Lenk et al., 2002). After 24 h, females were removed, and eggs and fecal pellets were counted under a Zeiss Axiovert inverted microscope (25× magnification) to estimate in situ rates of egg and fecal pellet production (Carotenuto et al., 2006). Since the incubations took place immediately after the catches, it is reasonable to assume that the egg production rate (EPR) might be related to the environmental state of copepods at sea (Saiz et al., 1999). Crystallizing dishes were incubated under the same conditions as above for an additional 48 h to wait for eggs to hatch into nauplii, that were then fixed by adding 15 mL of ethanol (96%). Numbers of empty egg membranes and total nauplii present on the container bottoms were counted to estimate the percentage of egg hatching success, with respect to the number of eggs produced (Carotenuto et al., 2006). As the production of fecal pellets is a quick estimate of overall copepod ingestion (Bamstedt et al., 1999), we used this biological trait as a proxy of C. typicus feeding rate.
2.1 Statistical analysis
Weekly data for C. typicus egg production, fecal pellet production, egg hatching success, juveniles (CIII-CV), and male and female abundances were averaged into monthly values to have a constant number of data input for subsequent analyses. In addition, maxima, mean, and minima annual values for all biological traits (reproduction and feeding) and population abundances were also plotted for each year. Monthly data of egg production, fecal pellet production, and population abundances were log10(n + 1)-transformed, and egg hatching success values were arcsin (percentage)-transformed to calculate linear regression analyses during the 20-year period. For those datasets having statistically significant linear regressions (p < .05) but low r2, we performed an additional analysis following Bryhn and Dimberg (2011) (Bryhn & Dimberg, 2011) to confirm the Statistically Meaningful Trend (SMT). In brief, data were grouped into uniform intervals, for each of which the mean was calculated and the r2 and p obtained. The grouping was reiterated with uniform groups with increasing number of entries. If r2 ≥ .65 and p ≤ .05 in at least one reiteration, the trend could be assumed as a STM. A multiple Spearman's correlation matrix was also calculated among C. typicus monthly raw data and environmental variables.
To further investigate the possible relationship between sea surface temperature (SST) and the body size of C. typicus females, we measured females collected during spring (March) and summer (July), when SST was at its annual minimum (15°C) and maximum (25°C). Seasons were identified following the classical meteorological/climatological division, with December–February as winter, March–May as spring, June–August as summer, and September–November as autumn (Trenberth, 1983). Five years (1998, 2002, 2006, 2010, and 2014) were randomly selected, as representative of beginning, middle, and end of the sampled period. The total length of females (n = 142), from the tip of the cephalothorax to the end of the furca, was measured.
All statistical analyses were performed using GraphPad Prism version 6.
3 RESULTS
3.1 Seasonal dynamics
Monthly averages of C. typicus EPR calculated for the 20-year period 1995–2015 indicated strong seasonal fluctuations, with maximum production in March (90.9 ± 35.4 eggs fem−1 day−1), followed by a gradual decrease until a minimum production was reached in August (24.3 ± 6.4 eggs fem−1 day−1) (Figure 1a). Greatest fluctuations in the EPR occurred when reproduction was at a maximum (January–April), whereas lowest ones occurred when egg production was minimal (from May–December). By contrast, except for a few occasions, monthly values of egg hatching success showed weak seasonal fluctuations (Figure 1b). Mean hatching success was high (>78%) and stable from January to June, then diminished and was much more variable in summer and autumn, with a minimum of 68% in August–September. The average seasonal pattern of fecal pellet production showed high and variable values in spring and summer (max 20.6 ± 9.9 pellets fem−1 day−1) and low values in late autumn–winter (November–February) (min 5.6 ± 2.8 pellets fem−1 day−1) (Figure 1c).

Monthly averaged C. typicus abundances for the population and its components (late copepodites (CIII-CV) and adult females and males) showed a seasonal unimodal cycle with an increase from March to June, when highest abundances were recorded (210.2 ± 158.4 ind m−3) (Figure 2a). A sharp decline was then observed from July onward, reaching a minimum of 3.1 ± 3.2 ind m−3 in December. In the mean seasonal cycle, the contribution of C. typicus to total copepod abundance ranged from a minimum of 1.0 ± 2.8% in December to a maximum of 16.7 ± 8.8% in June. From March through September, the population was dominated by juveniles whose seasonal changes overlapped with those of adults. Females and males showed similar mean abundances throughout the year, with peaks in June of 31.8 ± 28.4 ind. m−3 and 33.1 ± 32.9 ind m−3, respectively (Figure 2b), but the sex ratio (males: females) was generally skewed toward the males during most of the year (mean 0.8–1.9, median 0.6–1.5), with very stable patterns during April–July and higher variability in autumn-winter (Figure 2c).

3.2 Long-term variability
Long-term patterns (1995–2015) in C. typicus reproductive and feeding indicators are shown in Figure 3a. Wide interannual oscillations were observed in the EPR and fecal pellet production. Peaks in EPR were very high (90–150 eggs fem−1 day−1) in 1995–1999 and slightly lower and less variable in the successive years. Similar patterns were also observed for fecal pellet production, with interannual oscillations that tended to become smoother from 2010 onward. By contrast, fluctuations in egg hatching success were less pronounced; although some months (August 1996, November 1999, and September 2002) were characterized by very low hatching success (18%–23%), monthly values were on average above 70% (Figure 3a).
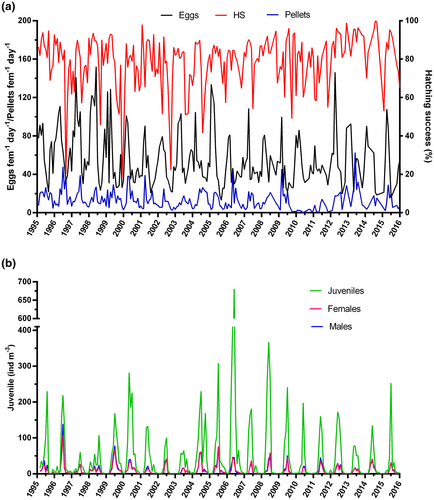
Inter-annual variability of C. typicus population structure and abundance is shown in Figure 3b. The monthly averaged abundances displayed recurrent annual patterns, characterized by a clear seasonal peak that varied in extension at the inter-annual scale, with a decreasing trend from 2008 to 2013 driven by juveniles. Maximum juvenile abundance was recorded in 2006, with nearly 700 ind m−3, followed by a progressive decrease till 2013 (~80 ind m−3). Compared to adults, juveniles had stronger inter-annual oscillations of their highest peaks, and their abundances did not always match with highest female and male abundances for those years.
In the long term, a significant negative linear trend of the EPR (Y = −0.00064x + 1.78, n = 217, r2 = .048, p < .01) and fecal pellet production (Y = −0.00136x + 1.19, n = 217, r2 = .089, p < .0001) of C. typicus appeared (Figure 4a). In contrast, hatching success had a significant long-term positive trend (Y = 0.00055x + 0.87, n = 217, r2 = .031, p < .01), thus indicating an increase in this reproductive trait over the 20-year period. Despite the low regression coefficients, the trends calculated over 30 interval divisions can, nonetheless, be considered statistically meaningful based on Bryhn and Dimberg (2011).
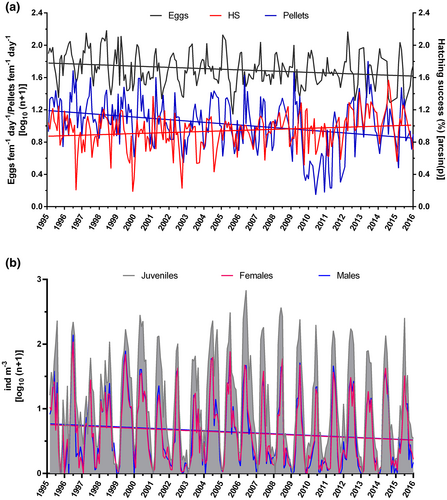
Linear regressions were not significant for the abundances of juveniles (Y = −0.001x + 1.23, n = 252, r2 = .009, p > .05), but moderately significant for those of males (Y = −0.001x + 0.77, n = 252, r2 = .018, p < .05) and females (Y = −0.00095x + 0.75, n = 252, r2 = .018, p < .05), with trends decreasing during the investigated period (Figure 4b). As before, these regressions were considered statistically meaningful trends based on Bryhn and Dimberg (2011).
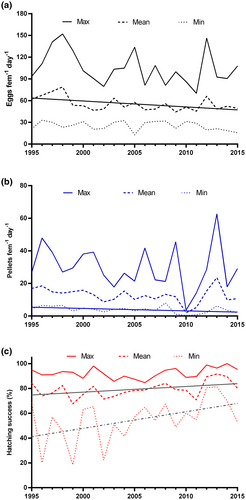
From 1995 to 2015, maximum annual values of C. typicus EPR showed a remarkable variability, decreasing from 152 eggs fem−1 day−1 in May 1998 to 70 eggs fem−1 day−1 in January 2011, whereas the annual minimum ranged from 33 eggs fem−1 day−1 in August 1996 to 13 eggs fem−1 day−1 in August 2005. Both values, however, did not show any clear long-term pattern (Figure 5a). In contrast, mean EPR values showed a significant decreasing trend, from 79 eggs fem−1 day−1 in 1998 to 49 eggs fem−1 day−1 in 2015 (Y = −0.818x + 1696, n = 21, r2 = .28, p < .05). Very ample inter-annual oscillations without a regular pattern were also observed in fecal pellet production both for maximum (4–63 pellets fem−1 day−1) and mean (2–24 pellets fem−1 day−1) annual values (Figure 5b). Conversely, minimum annual values showed a significant decreasing long-term trend, from 7 pellets fem−1 day−1 to 2 pellets fem−1 day−1 (Y = −0.150x + 304.9, n = 21, r2 = .21, p < .05). Concerning long-term trends of egg hatching success, maximum annual values were quite stable over the period (85%–100%), whereas a significant tendency of temporal increase was observed for mean (Y = 0.457x – 836.9, n = 21, r2 = .20, p < .05) and minimum (Y = 1.339x – 2631, n = 21, r2 = .22, p < .05) annual values, which increased from 70% to 90% and from 18% to 81%, respectively (Figure 5c).
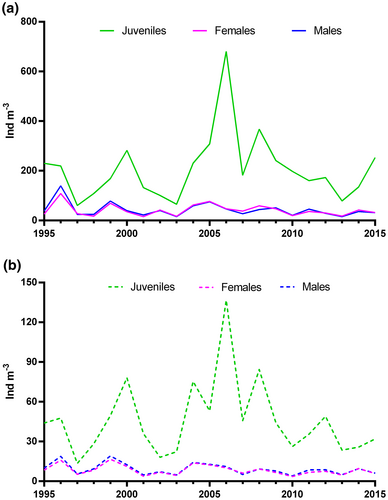
Long-term trends of maximum, mean, and minimum annual values of population abundances did not show any clear pattern, changing abruptly from year to year without any significant temporal trend. Juvenile abundances were usually more variable with up to a 10-fold difference between extremes of maximum (60 vs. 680 ind m−3) and mean (14 vs. 140 ind m−3) annual values, compared to 4–9-fold difference in adult females and males (maxima 15–140 ind m−3 for males and 15–110 ind m−3 for females, respectively) (Figure 6a,b).
3.3 Relationships among reproductive traits, abundances, and environmental parameters
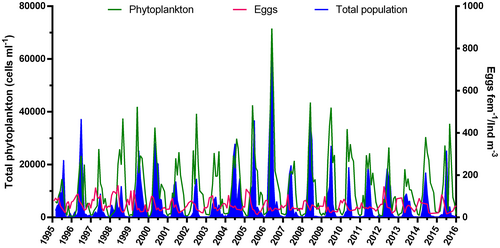
The temporal dynamics of C. typicus EPR and population abundances from 1995 to 2015 were compared with those of total phytoplankton (Figure 7). Total phytoplankton abundance, which included diatoms, phytoflagellates, dinoflagellates, and coccolithophores, showed a typical seasonal variability with one–two main annual peaks (generally in spring and autumn) and several months of low/moderate values (Figure 7). Except for the highest value measured in 2006 (72 × 103 cells mL−1), total phytoplankton density at LTER-MC ranged 20–40 × 103 cells mL−1. Phytoplankton concentration increased in the years 1995–1999 and 2003–2006, and decreased from 2008 till 2014 (Figure 7). C. typicus EPR rates were not in phase with phytoplankton abundance, with egg production peaks always occurring before peaks of phytoplankton abundances; moreover, changes in the amplitudes of these two parameters appeared not to co-vary. Total population abundance of C. typicus, on the other hand, was tuned with the EPR, with peaks in population abundance always occurring after those of the EPR. However, the highest EPR did not always correspond to highest population abundance, along the time-series. Similar EPR in 1998 and 1999, for example, corresponded to a twofold difference in population abundance during those years, whereas in 2006, which was one of the least productive years, had the highest population abundance. Interestingly, C. typicus total population often matched total phytoplankton in terms of peak amplitude and occurrence, both increasing in 2003–2006 and diminishing from 2008 to 2014 (Figure 7).
The results of a thorough evaluation of potential relationships among C. typicus biological traits (EPR, hatching success, and fecal pellet production), population abundances (juveniles and adults, CIII-CVI), and environmental parameters (temperature, Chl a, total phytoplankton, diatoms, dinoflagellates, and other phytoplankton) are shown in Table 1. The multiple correlation matrix on co-occurring monthly data indicated that the EPR was weakly correlated with pellet production (r = .18, p < .01). Moderate and strong inverse correlations, instead, were observed between egg production and total phytoplankton (r = −.33, p < .0001) and surface temperature (r = −.65, p < .0001), respectively (Table 1). Hatching success was positively but moderately correlated with EPR, fecal pellet production, and Chl a (r ≤ .22, p < .05). Fecal pellet production, on the other hand, was positively correlated with Chl a and phytoplankton (r ≥ .45, p < .05). The abundance of each population component (juveniles, males, and females) showed a positive but weak correlation with surface temperature (r ≅ .20, p < .001), and a strong correlation with all biotic parameters (r ≥ .50, p < .0001) (Table 1).
| Eggs | HS | Pellets | Juveniles | Females | Males | T | Chl a | Total phyto | Diatoms | Dinos | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eggs | |||||||||||
| HS | 0.17 | ||||||||||
| Pellets | 0.18 | 0.22 | |||||||||
| Juveniles | a | a | a | ||||||||
| Females | a | a | a | 0.90 | |||||||
| Males | a | a | a | 0.91 | 0.95 | ||||||
| T | −0.65 | −0.13 | 0.18 | 0.22 | 0.24 | 0.21 | |||||
| Chl a | −0.09 | 0.16 | 0.45 | 0.59 | 0.52 | 0.49 | 0.18 | ||||
| Total phyto | −0.33 | 0.06 | 0.49 | 0.74 | 0.69 | 0.68 | 0.51 | 0.74 | |||
| Diatoms | −0.28 | 0.08 | 0.51 | 0.68 | 0.62 | 0.61 | 0.50 | 0.75 | 0.97 | ||
| Dinos | −0.21 | 0.08 | 0.53 | 0.75 | 0.70 | 0.70 | 0.41 | 0.71 | 0.92 | 0.86 | |
| Phytoflagellates | −0.34 | 0.05 | 0.45 | 0.73 | 0.68 | 0.68 | 0.48 | 0.68 | 0.97 | 0.88 | 0.92 |
- Note: In bold, r significant at p < .05, nmin = 202, nmax = 251. Eggs (eggs fem−1), HS (hatching success, %), Pellets (pellets fem−1), juveniles, female and male abundances (ind m−3), T (temperature, °C), Chl a (mg m−3), total phytoplankton, diatom, dinoflagellate, and phytoflagellate concentration (cells mL−1).
- a Correlation not calculated.
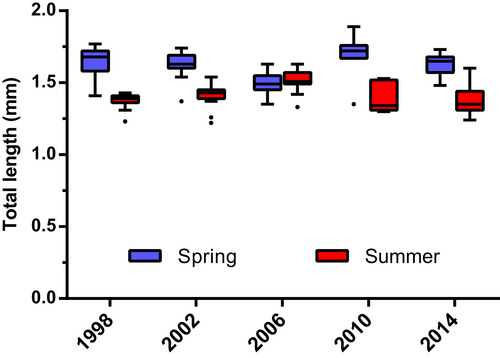
In terms of temperature–body size relationship in C. typicus, the total length of adult females was significantly higher in spring than in summer during 1998, 2002, 2010, and 2014, when females measured 1.65 ± 0.04 mm (mean ± standard deviation) in March and 1.39 ± 0.02 mm (mean ± standard deviation) in July (Figure 8) (Multiple unpaired t test with Šídák–Bonferroni correction, adj-p value < .0001). In 2006, female sizes were similar in both months (1.50 mm) (Multiple unpaired t test with Šídák–Bonferroni correction, t = 0.13 df = 28.0, adj-p value > .05). The female length affected, in turn, the individual reproductive rate, as body size and the corresponding EPR were strongly correlated (Pearson, r = 0.84, p < .01). We also calculated a significant linear regression between EPR (Y) and body size (x) of C. typicus in the Gulf of Naples (Y = 248.1x − 314.8, n = 10, r2 = .71, p < .01).
4 DISCUSSION
The present study is the first long-term time series focused on the copepod C. typicus, investigating the temporal variability of reproductive traits and fecal pellet production, together with population abundance and structure, over a two-decade period in the Gulf of Naples. Our results indicate that C. typicus reproduces during the entire year but less and more intensively in summer (August–September) and in late winter–spring, respectively, with a peak of EPR in March and hatching success in April. EPR and hatching success were tightly coupled and preeceded increases in population abundances that occurred about 1–2 months later in spring–early summer, with juveniles peaking in May and adults in June. Similar patterns were previously observed in the late 1980s by Ianora and Buttino (1990) and by Ianora et al. (1992) in the same study area. This lag-phase between fecundity and population abundance likely results from the nearly 2-months generation time reported for C. typicus at 10°C (Smith & Lane, 1985), which is close to the minimum temperature of 15°C registered at LTER-MC during January–March (Ribera d'Alcala et al., 2004).
The seasonal dynamics of the C. typicus population in the Gulf of Naples confirms that this species has a wide tolerance for temperature (Bonnet et al., 2007; Molinero et al., 2009) since it occurs year-round at LTER-MC over the 15–26°C temperature range (Ribera d'Alcala et al., 2004), and with a peak abundance in May–June corresponding to a surface temperature range of 20–23°C. However, interannual variations in abundances and SST values were only weakly correlated (r ≅ .20), thus suggesting that surface temperature might not be the right indicator for seasonal and interannual variations of C. typicus populations in the Gulf of Naples. This is supported by other studies of C. typicus in the North Sea (Helgoland Roads), the English Channels, and the Bay of Biscay, on different time-spans over the period 1975–2004 (Bonnet et al., 2007). In these regions, maximum abundances of juveniles and adults were generally lower and delayed compared to the Gulf of Naples, with peaks recorded in June–September (Mazzocchi et al., 2007). Timing and intensities of C. typicus reproductive traits in these coastal sites are also markedly different from those in the Gulf of Naples. For example, maximal reproduction occurred in summer–autumn at the Helgoland Road and during May–June in the English Channel (Bonnet et al., 2007). In both sites, moreover, EPRs were higher than in the Gulf of Naples (Halsband-Lenk et al., 2004; Ianora et al., 2007; Ianora & Buttino, 1990), probably due to higher food availability in those areas.
In the adult C. typicus population at LTER-MC, the sex ratio, oscillating around 1 during most of the year, was slightly skewed toward males in winter months. Higher male abundance could be related to the different survival rate of the two sexes, although, to our knowledge, copepod females usually endure harsh conditions better than males (e.g., Pseudodiaptomus marinus (Uttieri et al., 2020) and Oithona davisae (Svetlichny et al., 2016)). Another reason could be the diet: Bonnet and Carlotti (2001) found male skewed sex ratio in C. typicus depending on the diet offered in the laboratory, although Kouwenberg (1993) reported that in mixed feeders, such as Temora stylifera and C. typicus, sexes were about equally represented throughout the year in the northwest Mediterannean Sea. Not much is known on whether female C. typicus require remating for the continued production of viable eggs, which would explain why males are present all year round. Overall, the positive correlation between hatching success and male and female abundances suggests that this reproductive trait is likely associated with a balanced population structure and successful mating.
Concerning C. typicus egg hatching success, this trait remained relatively high and stable (>78%) on an inter-seasonal scale, though it slightly declined in summer when females were producing less eggs. Similar annual dynamics and mean annual values in the area were also reported in 1989 (73%) (Ianora et al., 1992), in 1990 (84%) (Ianora & Buttino, 1990), and in 2000 (80%) (Carotenuto et al., 2006), thus indicating high seasonal stability of this reproductive trait in the study area. Similarly high values of hatching success were reported for C. typicus at Helgoland Roads and in the Bay of Villfrance in the period 1995–1999 (Halsband-Lenk et al., 2004). Unfortunately, interannual comparison with other time series on C. typicus could not be made as this reproductive trait has not been investigated in these areas (Bonnet et al., 2007).
Factors that likely affect hatching success in copepods have been widely debated in the last two decades, since the discovery that diatoms produce lipid-derived cytotoxic molecules (oxylipins) impairing copepod embryo development (Russo et al., 2019). Several field studies showed strong fluctuations in hatching succeess in some copepod species during intense diatom blooms (Halsband-Lenk et al., 2005; Ianora et al., 2004; Pierson et al., 2005). A recent study examined weekly production of diatom oxylipins in the natural phytoplankton assemblage at the LTER-MC site, during a whole year in 2017 (Russo, d'Ippolito, et al., 2020). The work confirmed the previously reported occurrence of oxylipin-producing diatom species in the Gulf of Naples (Barreiro et al., 2011) and demonstrated that oxylipin production was not related to changes in diatom community composition, but was diatom-density dependent, with higher concentrations in late spring–summer months, when diatoms dominated the phytoplankton community (Russo, d'Ippolito, et al., 2020). We could speculate, hence, that the observed minimum hatching success of C. typicus in summer (<60%) could be related to higher concentrations of oxylipins in the diatom community. However, as hatching success was usually very high, it is possible that C. typicus may be able to partly detoxify these compounds, as proposed in certain copepod species feeding on oxylipin-producing diatoms (Asai et al., 2020; Lauritano et al., 2012). Alternatively, other maternally related traits not investigated in the present study may have been affected by diatom occurrence. Low survival of non-feeding naupliar stage I of C. typicus (67%) and T. stylifera (12%–25%) has been associated to high diatom abundance and oxylipin production in this same area (Carotenuto et al., 2006; Russo, Lauritano, et al., 2020). More studies will be needed to further confirm whether diatom oxylipins affect early larval development of C. typicus population in the Gulf of Naples.
Production rate of fecal pellets, used as a proxy of grazing rate in copepods (Bamstedt et al., 1999), was highest in C. typicus during March–August, when the phytoplankton biomass at LTER-MC reaches its high values triggered by nutrient availability and water column stratification (Ribera d'Alcala et al., 2004; Zingone et al., 2019). During these months, oligotrophic and eutrophic conditions alternate, driving phytoflagellates and diatoms, the most abundant groups characterizing the area, to dominate (D'Alelio et al., 2015). This suggests that although animals were well-fed during these spring–summer months, food type was not always adequate to sustain high fecundity and/or hatching. In autumn, nutrients of terrestrial origin are dispersed by a wide circulation pattern (Cianelli et al., 2015) and summer diatoms peak again, whereas in winter, though phytoplankton reaches its minimum, episodic blooms of few diatom species may occur (Zingone et al., 2010). In these months, C. typicus may have been feeding on alternative prey such as microzooplankton or other phytoflagellates that are very abundant in this period (Zingone et al., 2019). Scanty information is available about the feeding preferences of this copepod, but C. typicus is generally described as an omnivorous species with mixed feeding strategy privileging herbivory (Benedetti et al., 2016, 2018). The fact that in our 20-year study egg production was inversely correlated to phytoplankton concentration (total, diatoms, and phtyoflagellates), and that peaks in egg production always anticipated phytoplankton abundance, seems to confirm that C. typicus may preferentially feed on non-authotrophic and/or mixotrophic food items (ciliates and dinoflagellates) (Broglio et al., 2004; Traboni et al., 2021), and that microzooplankton diets can promote higher fecundity and/or hatching success in this species (Bonnet & Carlotti, 2001). Recently, it was demonstrated that mixotrophic-related improvement of food quality in protists increased C. typicus EPR and naupliar recruitment (Traboni et al., 2021), thus suggesting that this feeding strategy may help this species to overcome unfavorable food environments.
Egg production rate was inversely correlated with SST over the 20-year period. Although spawning females were always kept at a constant temperature of 20°C, which was the optimal temperature for maximal reproduction of C. typicus in the laboratory for Mediterranean populations (Halsband-Lenk et al., 2002), their fecundity reflected the previous condition experienced in situ (Carotenuto et al., 2006; Ianora et al., 1992; Saiz et al., 1999). This inverse fecundity–temperature relationship has been already reported for C. typicus during the 2-year survey 1989–1990 (Ianora et al., 1992). We also observed that larger C. typicus females occurred in spring, when SST was at a minimum (15°C) compared to summer (25°C), and these bigger females also produced more eggs (body size positively correlated with EPR). A similar inverse temperature–size relationship was observed for C. typicus elsewhere in the North Sea and the Mediterranean Sea (Bonnet et al., 2007; Halsband-Lenk et al., 2004), with larger individuals in spring than in summer populations. Although relationships between temperature, prosome length, and egg production have not been reported for C. typicus, maximal reproduction rates in several calanoid species are usually achieved at lower temperatures (Halsband & Hirche, 2001). A body size–EPR relationship in C. typicus has been reported previously (Smith & Lane, 1985); thus, it is possible that in colder months, when females were bigger and had likely invested more in ovary tissues, invididual EPRs were higher. Considering the predicted increase of SST in marine coastal areas (IPCC, 2022), and the fact that C. typicus has been suggested as a key species for monitoring climate changes (Beaugrand et al., 2002; Lindley & Daykin, 2005), more studies are needed to investigate the impact of future global warming scenarios on reproduction and population dynamics of this species in situ.
On a multi-annual 20-year scale, significant positive correlations between C. typicus abundance and biotic environmental parameters (phytoplankton abundance and Chl a) were reported in the present study. Similar relationships were observed in the North Sea (Helgoland Roads) during 1975–2004 (Bonnet et al., 2007) and in the Mediterranean Sea on a basin-scale survey (Molinero et al., 2009), thus suggesting that this copepod species may respond to the same seasonal signals promoting phytoplankton growth.
Significant decreases in EPR, fecal pellet production, and adult abundances were observed in the studied area from 1995 to 2015. Conversely, egg hatching success increased, in terms of monthly, mean and minimum annual values, over the multidecadal period. Hatching success therefore seems to have improved, if we compare our results with those reported previously during 1989–1990 (Ianora et al., 1992), when even lower hatching success was observed (40%). However, this improvement does not seem to have compensated the observed diminution in population abundances of C. typicus. Numerous studies have provided experimental and field evidence that copepod population growth largely depends on recruitment of larval stages (Carotenuto et al., 2006, 2011; Ianora et al., 2004, 2015). Therefore, it is possible that a lower survival of late developmental stages may have induced the overall decrease in the C. typicus population.
It is not easy to disentangle the drivers of the observed long-term trends in reproductive traits and population abundances of C. typicus at the LTER-MC site. Reduced grazing and fecundity could be related to reduced phytoplankton abundance or changes in community composition. Previous studies at this site have reported reduced Chl a concentration and mean phytoplankton size in the period 1995–2002, followed by a reversed trend for both parameters from 2003 onward (Morabito et al., 2018; D. Sarno and A. Zingone, personal communications), therefore resulting in an overall significant increase in surface Chl a (Longobardi et al., 2022; present study, personal observations). Moreover, overall abundances of main phytoplankton groups have not changed at LTER-MC during the period 1995–2015 (Longobardi et al., 2022). Given that hatching success was positively correlated with surface Chl a during the 20-year period, in the future, further studies will be necessary to understand whether a change in the abundance/species composition of the phytoplankton community may have impacted the reproductive potential of C. typicus.
In conclusion, the seasonal dynamics of C. typicus reproductive traits and population abundances suggests that the high EPR and hatching success in February–March, likely boosted by an omnivorous wintertime diet, drive the CIII-adult maximum in April–May, and that a combination of low egg production and hatching success in July–August could be responsible for the drop in the C. typicus population in those months. More importantly, in the long term from 1995 to 2015, our data indicate a decreased fecundity, coupled with a reduced adult population of C. typicus at LTER-MC, despite a significant increase in egg hatching success. It is not possible to unequivocally establish which environmental factor affects C. typicus reproductive traits and whether these, in turn, are responsible for the reduction of the in situ population. But, we could hypothesize that a combination of low individual fecundity coupled with a high in situ mortality of larvae acted sinergistically to reduce overall adult abundances. Mortality of larval stages well-explained the seasonal population dynamics of the copepod T. stylifera in the Gulf of Naples (Mazzocchi et al., 2006). More effort, therefore, should be paid on investigating natural mortality of copepod larval stages in situ, which can be at times very high (Di Capua & Mazzocchi, 2017; Russo, Lauritano, et al., 2020), to better explain copepod population dynamics on a seasonal and interannual scale. The outcomes of this two-decadal analysis shed new light into the ecology of this widespread species, improving our understanding of the driving role of different environmental factors and highlighting the importance of studying the species reproductive biology and population dynamics in the long term.
AUTHOR CONTRIBUTIONS
YC, AM, MGM, and AI: conception and design; IDC, MDP, IP, AM, MGM, and AI: acquisition of data; YC, FP, MU, MGM, and AI: analysis and interpretation of data; YC, MGM, and AI: drafting the manuscript; all authors revising it critically.
ACKNOWLEDGMENTS
The research program LTER-MC is supported by the Stazione Zoologica Anton Dohrn. We thank the crew of the R/V Vettoria for their help in zooplankton sampling. We also thank Andrea Montalbano for technical help in copepod size measurement, Francesca Margiotta for environmental data and Diana Sarno for phytoplankton data. MU thanks Andreas Bryhn and Peter Dimberg for guidance on the SMT analysis. MU acknowledges the support of NBFC to Stazione Zoologica Anton Dohrn, funded by the Italian Ministry of University and Research, PNRR, Missione 4 Componente 2, “Dalla ricerca all'impresa”, Investimento 1.4, Project CN00000033.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




