Assembly rules vary seasonally in stable phytoplankton associations of the Gulf of Naples (Mediterranean Sea)
Abstract
Coastal phytoplankton present remarkable variability because of complex patterns and relationships of hydrological parameters at the land–sea interface. We analysed a time series of 917 phytoplankton samples collected in the period 1984–2010 at the coastal station LTER-MC (Gulf of Naples, Mediterranean Sea) with the aims of determining: (i) whether recurrent species associations are recognizable in this variable coastal environment; (ii) what are their composition and seasonal patterns; (iii) what are the environmental variables driving their distributions, and (iv) to what extent those associations are functionally homogeneous. Using a cluster analysis of a dataset of the 87 more frequent and abundant species in the samples, we identified seven main groups of species. Five of them were recurrent over the years in the same seasons, while two other groups showed a bimodal occurrence or no clear seasonality, respectively. Differences in the occurrence of those assemblages were primarily associated with the season (i.e., temperature), and the coastal nature of the site (mainly salinity). Except for the winter cluster, which included mostly coccolithophores and silicoflagellates, diatoms were present in all associations, along with species belonging to diverse phylogenetic groups. The diversity of morphological features defining ecological traits varied across the different associations, with a maximum divergence in the spring association and a minimum in winter. We conclude that the stability of the associations is related to the marked phenological recurrence of their individual species and their overlap in specific periods of the year, while convergent or divergent trait compositions in the different associations indicate a variable strength of the environmental filtering across the seasonal succession.
1 INTRODUCTION
Assembly processes in marine phytoplankton and their relationships with environmental variability are widely debated in the ecological literature (Rojo, 2021). Where, when, and why different species co-occur, whether co-occurring species regularly share similar environmental space, and whether they compete or mutually benefit from each other, are questions whose answers are limited by the still incomplete knowledge of the ecology of plankton and individual species. From a more practical viewpoint, the high number of microalgae occurring together in the natural plankton has always represented a challenge to ecologists in terms of analysis and prediction. Different approaches to ecological classification and the identification of species associations have been proposed to reduce the redundancy of in situ phytoplankton datasets, with the goals of linking phytoplankton dynamics to environmental drivers and predicting changes imposed by anthropogenic and climatic variability.
Phytoplankton species are often aggregated within broad phylogenetic clusters in ecological descriptions and modelling exercises based on the supposed similarity of main ecophysiological requirements and functional characteristics among phylogenetically related species. For example, dependence on silica, motility, or formation of calcite plates are respectively distinctive of taxonomic groups such as diatoms, dinoflagellates, and coccolithophores. However, these groups encompass a great variety of morpho-functional adaptations related to size, shape, coloniality, life cycles, and so on, which make these phylogeny-based classifications too coarse and scarcely effective for the ecological interpretation of phytoplankton assemblages.
As an alternative to the grouping of species by high-level taxonomic categories, a more natural way to classify phytoplankton species is based on aggregating different and even genetically distant taxa within functionally homogeneous groups (e.g., Padisák et al., 2009; Reynolds, 1997; Reynolds et al., 2002). These efforts are based on the view that optimal growth conditions for individual species exist under specific ranges of environmental and biological variables of great impact—that is, temperature, salinity, light, nutrients, water column stability, and grazing (Reynolds, 1998; Smayda & Reynolds, 2001). The optimal ranges of these variables broadly overlap in species possessing similar characteristics and ecophysiological requirements; these species, therefore, tend to be recorded in the environment in association and under certain environmental conditions.
Whether the species found in the same associations mutually benefit from their coexistence or simply respond to environmental conditions in a similar but independent way is summarised in the Clementsian/Gleasonian duality (Clements, 1916; Eliot, 2007; Gleason, 1926). In the Clementsian paradigm, inspired by Alexander von Humboldt's views (Nicolson, 2013), positive interactions among species contribute to the success of the community they belong to, a vision that parallels the one accepted for terrestrial communities, including plants and animals, in which the interspecific relationships are often easily detectable (Eliot, 2007). In the Gleasonian view, each species is distributed according to its own physiological requirements, with species assembly being determined by habitat filtration, which matches the well-known Reynolds' intaglio (Reynolds, 1997, 1998) and Margalef's mandala (Margalef, 1978). In the latter paradigmatic schemes, species in clusters or associations co-occur sharing the same space and time windows because each of them responds similarly to environmental conditions.
The actual composition of the associations of phytoplankton species sharing similar characteristics can vary from place to place and along the year, depending on local biological diversity and stochastic environmental factors that can promote the growth of one versus another species within a given association. Consequently, it is possible to predict which association (i.e., a major cluster of species) will be found under certain environmental conditions, but it is not predictable which individual species belonging to that association will be present or most abundant (Reynolds, 1997). The view of trait convergence in species associations is contrasted by an opposite rule of assembly predicting that communities are made of species that respond to different driving forces that are active at the same time (Klais et al., 2017; Padisák et al., 2010). Functional diversity within a species association is explained by the limiting similarity principle, based on which a limited number of similar competing species can share their niche and coexist in the same environment (MacArthur & Levins, 1967). It is now recognized that both environmental filtering leading to trait convergence and limiting similarity leading to trait divergence may operate simultaneously in phytoplankton and phytobenthos communities (Ács et al., 2019; Borics et al., 2020; Várbíró et al., 2020).
Trait-based approaches have been largely used over the last years to reduce the taxonomic complexity of phytoplankton associations and highlight the functional role of the species. However physiological traits such as response to light, temperature, and nutrients have been described for a restricted number of species and a limited number of strains within them. An alternative classification is based on morpho-functional traits of phytoplankton species, which partly depend on their phylogenetic affiliation (like in the case of silica requirement) but vary substantially within groups of related species (Salmaso & Padisak, 2007). Morpho-functional traits, such as size, surface-to-volume ratio, shape, and coloniality, are related to physiological characteristics such as growth rates, nutrient assimilation, and buoyancy, and their use is a practical approach to circumvent the lack of physiological information for most phytoplankton species (Kruk et al., 2010; Naselli-Flores et al., 2007). As a matter of fact, in Margalef's mandala (Margalef, 1978), the schematic representation of phytoplankton occurrence under seasonally changing conditions is based on the names of phytoplankton genera in brackets, standing for the different “life forms” they represent.
Ecological classification of microalgae based on their traits has been widely applied in freshwater environments, both for planktonic and benthic associations (Abonyi et al., 2021; Ács et al., 2019; Caputo et al., 2008; Padisák et al., 2010). This categorization has been successfully used in the description of both seasonal succession and biogeography, showing a higher predictive value than taxonomy in terms of relationships with environmental conditions (e.g., Kruk et al., 2011). In the marine realm, a “trait-based” approach with a good level of prediction of phytoplankton functional groups has also been introduced (e.g., Edwards et al., 2013).
Time series of phytoplankton data offer good opportunity to understand plankton succession and community assembly rules as well as to assess to what extent morpho-functional traits are determinant variables in the structuring of phytoplankton associations across the seasons. From year to year, similar environmental conditions re-occur in the same period of the year for those variables that are driven by astronomical phenomena, while other variables that are subject to meteorological and hydrographic influences change in a less predictable way (Cloern & Jassby, 2010). Therefore, long-term ecological research (LTER) sites can be used as “laboratories” where natural experiments occur under some fixed and some variable conditions (Zingone et al., 2010b). At the coastal Mediterranean site LTER MareChiara (LTER-MC), regular sampling performed since 1984 may allow extracting robust ecological signals out of the noise of the considerable environmental variability of the site (Longobardi et al., 2022). In this study, we analysed the distribution of the 87 most frequent taxa identified in 917 samples collected in the surface waters of the LTER-MC site in the period 1984–2010. We aimed at assessing whether recurrent and robust species associations are recognizable in such a variable coastal environment and at depicting their taxonomic composition and seasonal patterns, as well as their relationships with environmental variables. Finally, we used a morpho-functional trait-based approach to investigate the functional coherence of the species' associations. The overall goal of our research was to shed light on the rules of assembly of phytoplankton associations in a variable coastal environment.
2 METHODS
2.1 Study area
The sampling station LTER-MC (40°48.5′ N; 14°15′ E) is situated in the Gulf of Naples, Tyrrhenian Sea, two nautical miles offshore over the 75 m isobath (Figure S1). There, the transition between waters influenced by land runoff with its nutrient charge from a densely populated area and offshore oligotrophic conditions determines a high hydrographic variability at seasonal and interannual scales (Longobardi et al., 2022; Ribera d'Alcalà et al., 2004). Over the year, variable circulation patterns modulate the impact of nutrient charges of terrestrial origin, governing flushing rates and hence the accumulation or dispersion dynamics of plankton populations (Cianelli et al., 2017). Particularly nutrients of terrestrial origin drive intense summer blooms in the inner part of the Gulf, with a high variability of phytoplankton populations at small temporal (weekly) and spatial (<1 km) scales (Zingone et al., 1990). The exchange with the oligotrophic open Tyrrhenian waters can be very fast in some cases, causing abrupt changes in the innermost area, with drastic reductions in biomass and a reshaping of the food web configurations (D'Alelio et al., 2015). In addition to these largely unpredictable events, interannual trends and fluctuations of environmental variables have been detected in the area (Longobardi et al., 2022).
2.2 Phytoplankton and environmental data
We performed our analysis on species abundance data from a total of 917 samples collected from 1984 to 2010 in the surface waters of the sampling site. The time series consisted of two periods, 1984–1991 and 1995–2010, the former with fortnight sampling and the latter with weekly sampling, separated by a period of no sampling from the mid of 1991 to the beginning of 1995 (Zingone et al., 2019).
Phytoplankton samples were collected with Niskin bottles at the surface (0.5 m) and fixed with neutralized formaldehyde (0.8%–1.6% final concentration). Depending on cell concentration, variable sample volumes (1–100 ml) were allowed to settle in combined sedimentation chambers, and cells were counted on fields or transect at the inverted light microscope at 400× magnification, using the Utermöhl method (Edler & Elbrächter, 2010). At least 200 cells of the most abundant taxon were enumerated in each sample. Phytoplankton counts and identifications across the time series were performed by AZ and/or DS, and the dataset was periodically revised for auto-consistency and nomenclatural changes. For selected diatom species, the identification was checked with transmission and/or scanning electron microscopes as described in Sarno et al. (2005).
Physical and chemical data of surface waters were obtained through in situ measurements or from samples collected simultaneously to phytoplankton sampling. Temperature data were obtained with reversing thermometers in the years 1984–1991 and continuous multiparametric profilers from 1995 onwards. Salinity was determined with a salinometer (Beckman mod. RS7C and subsequently Autosal Guildline Instruments) until 2002; thence temperature, salinity, and pressure data were obtained by a CTD multiparametric profiler (Sea-Bird Electronics, 9-11 plus V2). Inorganic nutrient samples were collected from the same Niskin bottles as phytoplankton, placed into 20ml polyethylene vials and immediately frozen. The concentrations of ammonia, nitrates, nitrites, phosphates, and silicates were determined following Hansen and Grasshoff (1983). For chlorophyll a, a variable volume of seawater was filtered under low vacuum and extracted in 10 ml of acetone, which was neutralized by adding MgCO3 for 24 h and then filtered. Concentrations were determined spectrophotometrically till 1991 and fluorometrically afterwards. All methods for the in-situ data acquisition procedures and the analyses of nutrient and other environmental variables are described in detail in Sabia et al. (2019). Phytoplankton and environmental data used in this paper are publicly available (Sarno et al., 2022).
2.3 The dataset
The initial phytoplankton abundance dataset was arranged in a matrix of 917 samples (rows) and 344 phytoplankton species (columns). Because one of our aims was the taxonomic characterization of the species associations, we focused on a subset of taxa reliably identified at the species or in some cases at the genus level. We excluded such groups as undetermined flagellates or dinoflagellates that are present in almost all samples and would have hence weakened the results, as well as other groups lumping many species such as unidentified pennate and centric diatoms and coccolithophores. In addition, to avoid large errors caused by low species frequency we used a species-rank frequency plot to exclude rare species and considered as a threshold value the last value before the slope changes in the frequency curve. Based on this threshold, 82 species with more than 8% of occurrence in the whole dataset were selected. With the same criterion, we ranked species by their abundance, thus identifying eight more species having a frequency lower than 8% but relatively high density values.
Following the first descriptive analysis, three species were discarded from the analyses because of their irregular seasonal patterns (Figure S2). These were Emiliania huxleyi, which showed a unique seasonal cycle, with a very high frequency at low concentrations and an increase in winter, but with occasional peaks in other seasons and no other species associated. Two more species, Chaetoceros minimus and Pseudo-nitzschia cf. pseudodelicatissima, were excluded from the analysis based on their irregular seasonal and interannual pattern of occurrence. In the latter case, this morphotype is shared by several cryptic species spread over different periods of the year (Ruggiero et al., 2015, 2022). For similar reasons, Pseudo-nitzschia galaxiae was split into two based on the different seasonal occurrences of at least two genetically distinct morphotypes (Cerino et al., 2005; Ruggiero et al., 2022). All choices described above aimed at obtaining the most robust associations by limiting identification and quantification errors due to unknown cryptic diversity or data weakness, which are unavoidable in routine phytoplankton analysis for hardly identifiable and less abundant taxa. The final reduced dataset consisted of 917 samples and 87 species, namely, 55 diatoms, 9 dinoflagellates, 12 coccolithophores, and 11 other flagellates. Because the selection was biased towards identifiable and frequent species, dinoflagellates, and flagellates were under-represented in the list despite their high diversity and relatively high abundance. Indeed the larger identifiable taxa (e.g., thecate dinoflagellates in the genera Tripos, Protoperidinium, and Dinophysis) were scarcely abundant and poorly enumerated in our samples, while most of the small naked forms of these groups cannot be identified in light microscopy in fixed material.
2.4 Species associations
Species associations were identified using hierarchical agglomerative clustering (Everitt et al., 2011) based on the Hellinger distance (Legendre & Gallagher, 2001), which gives low weights to rare species and is well adapted to abundance data, and Ward's linkage, which minimizes the within-cluster variance.
Hierarchical clustering can be affected by small changes in the data, whereby including new species or excluding some other species can change the final partition. To validate the clusters obtained, we applied a bootstrap resampling technique (Hennig, 2007). The validation procedure aims to find a stable association in the sample. The method consists in extracting 1000 subsamples of 65 species each (75% of the total number) without reintroduction, with each species appearing in the subsamples only once. The same hierarchical clustering technique was then applied to each subsample. The species that were always in the same clusters have a strong association. An overall measure of stability was obtained using the Adjusted Rand Index (ARI; Hubert & Arabie, 1985), which compares two partitions based on pairwise agreement. The ARI has an expected value of 0 under random classification and it is equal to 1 when there is perfect class agreement. ARI values close to one indicate similar partitions and therefore cluster stability. For all the 1000 partitions we computed the ARI index.
For each association obtained in the clustering, the linear trend was estimated independently on the two continuative periods of the time series (1984–1991 and 1995–2010). The significance of each trend was tested separately using an F-test on the regression model.
2.5 Relationship of associations with environmental variables
The relationships among species, hydrographic parameters (temperature and salinity), and nutrients were assessed using canonical correlation analysis (CCA, Hotelling, 1936); given two sets of variables, CCA aims to find a basis vector such that the correlation between the projections of the variables onto these basis vectors are mutually maximized.
2.6 Functional trait analysis
- Equivalent sphere diameter (ESD), eleven categories, from minimum to maximum values.
- Surface to volume ratio (S/V), eleven categories, from minimum to maximum values.
- Cell shape, eleven categories.
- Trophism, three categories, that is, autotrophy, mixotrophy, and heterotrophy.
- Silica requirement, two categories, that is, absence and presence.
- Spore formation, two categories, that is, absence and presence.
- Motility, two categories, that is, absence and presence.
- Presence of spines, two categories, that is, absence and presence.
- Coloniality, four categories, that is, the absence of colonies, the permanent presence of colonies, colonies absent in summer, and variable coloniality = random.
- Type of junction, four categories, that is, the absence of junction, rigid organic junction, flexible organic junction, and siliceous junction.
- Colony shape, four categories, that is, linear colonies, colonies growing mainly in one dimension but also slightly growing in a second dimension, colonies growing in a plane, and globular colonies.
Traits 1–3 were evaluated considering cellular forms listed in the Phytoplankton Bio-Imaging framework by Università del Salento, (Stanca et al., 2013) and formulas included therein.
Quantitative analyses of trait distributions within the species associations were carried out taking into account the average abundance of phytoplankton species. The analysis consisted of grouping species found in the same seasonal cluster based on the variants of a specific trait that they shared. The fraction of individuals (independent of the species to which they belong) bearing a specific trait-variant in a seasonal cluster was divided by the total of individuals detected in the same seasonal cluster. The derived quantitative trait–variant matrix with data grouped per seasonal clusters was inspected with multivariate analysis (cluster analysis, Euclidean distance, and single linkage) to detect parenthood among phytoplankton seasonal clusters based on their functional fingerprints.
3 RESULTS
3.1 Seasonal cycle of phytoplankton and environmental variables
Surface temperature variations over the year followed a typical sinusoidal pattern, with minimum values ≥12.37°C at the beginning of March and maxima (≤28.92°C) in July–August (Figure 1a). From mid-March the temperature increase in surface waters drove the establishment of a thermocline shoaling to ca. 10 m depth from May through August and progressively deepening from September to December, the water column being homothermic and progressively cooling until next March. Salinity values in surface waters also followed the sinusoidal pattern typical of the Mediterranean Sea, with minima (≥36.38) in May and maxima (≤38.32) in autumn, with frequent spikes caused by lateral advection of freshwater of terrestrial origin (Figure 1b).
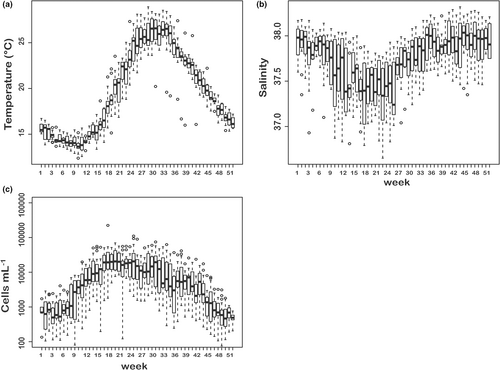
Average concentrations of dissolved inorganic nitrogen (DIN), phosphates and silicates were 2.46 (±2.81), 0.10 (±0.08), and 2.04 (±1.87) mmol m−3, respectively. Both DIN and silicates showed maximum values in spring and minima in summer, while phosphates did not show a clear seasonal pattern (Figure S3). The contribution of nutrients of terrestrial origin was testified by the numerous outlier values recorded in any period of the year.
Chlorophyll a concentrations (1.72 ± 1.94 mg m−3) started to increase in March, generally peaked in May and stayed relatively high in summer and autumn. During this whole period occasional short-lived peaks were also recorded (Figure S3). Total phytoplankton populations in surface waters showed minimum values (≥102 cells ml−1) in early winter, followed by a gradual increase starting in late February–March and a peak in May (Figure 1c). Density values were relatively high throughout the summer (≤105 cells ml−1), with a decrease in the autumn months.
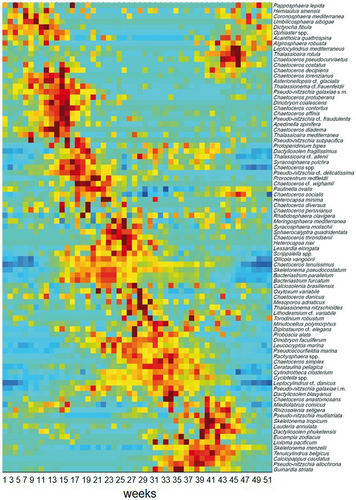
The weekly average abundance of the species selected for the analysis over the period 1984–2010 showed a clear seasonal signal, with distinct phenological signatures (Figure 2), that is, occurrence windows and maximum abundance times, spread over the entire year. Although distinct species groups were seen in the different seasons, and peak times coincided in some cases, no two species showed identical occurrence patterns.
3.2 Species associations
Cluster analysis identified seven groups of species (Figure 3) named and described in the following paragraphs based on their temporal sequence of occurrence and distinct patterns of abundance across the seasons (Figure 4).
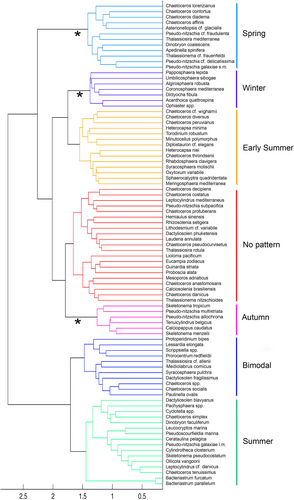
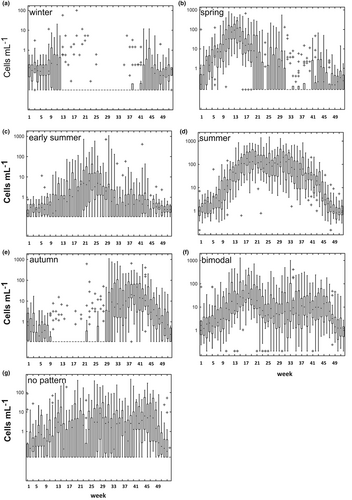
The winter cluster (Figure 4a) was present from November through February with abundance rarely exceeding 10 cells ml−1 and almost disappeared in summer. Species in this group were all coccolithophores, except for the silicoflagellate Dictyocha fibula (Figures 3 and S4). The species in this cluster, however, constituted a minor part of the whole winter phytoplankton community. In this season of completely mixed water column, small undetermined flagellates belonging to several classes (avg: 103 cells ml−1) dominated. This whole group was excluded from our analyses along with all other undetermined groups.
The spring cluster (Figure 4b) began to increase in winter and peaked (103 cells ml−1) at the onset of the thermal stratification period in mid-March, contributing to the annual spring peak of the whole phytoplankton (ca. 104–105 cells ml−1). It was dominated by Pseudo-nitzschia cf. delicatissima, followed by other colonial diatoms of the genera Chaetoceros and Thalassiosira, but included also the tiny non-colonial diatom Pseudo-nitzschia galaxiae small morphotype (s.m.), exclusive of this period of the year, along with two chrysophytes, the silica scaled Apedinella spinifera and the colonial Dynobrion coalescens, the latter also only found in spring (Figures 2 and S4). Several species (e.g., Asterionellopsis cf. glacialis and colonial Chaetoceros spp.) almost disappeared in summer but were found again in autumn and winter with lower densities. Some other colonial Chaetoceros not included in the spring cluster also peaked in this season, but since they occurred again later in the year they were placed in the bimodal group (see below) in the cluster analysis. Small unidentified flagellates excluded from the analysis were also relatively abundant in this period of the year.
The early-summer cluster showed its maxima (10–102 cells ml−1) in June–July (Figure 4c) but began to grow in spring and was still present in autumn. This group of species developed at the surface of the stratified water column, mainly depending on nutrient inputs associated with low salinity waters of terrestrial origin. From the phylogenetic point of view, this was the most heterogeneous cluster, including species from five different algal classes. Differently from the spring ones, most diatoms of this assemblage were small and/or non-colonial (e.g., Chaetoceros throndsenii and C. peruvianus) or formed short chains (C. diversus, C. cf. wighamii, and Minutocellus polymorphus). Several dinoflagellates (Heterocapsa niei, H. minima, Torodinium robustum, and Oxytoxum variabile) were also in this group, along with other autotrophic, mixotrophic, and heterotrophic flagellates (Meringosphaera mediterranea and Diplostauron elegans), and several coccolithophores (Figure S4). Most of these species were also found in other periods of the year although with lower concentrations.
Species of the summer cluster attained together high densities (<103 cells ml−1) from late spring across the whole summer (Figure 4d). This group included non-colonial (Chaetoceros tenuissimus, Cyclotella spp., and Cylindrotheca closterium) or colonial diatoms present as single cells or short chains (Skeletonema pseudocostatum, Pseudo-nitzschia galaxiae large morphotype (l.m.), Leptocylindrus cf. danicus, and Cerataulina pelagica), along with several flagellate species, such as the prasinophytes Pachyspaera spp. and Pseudoscourfieldia marina, the non-colonial chrysophyte Dinobryon faculiferum and the heterotrophs Leucocryptos marina and Ollicola vangorii.
The autumn cluster (Figure 4e) was comprised of species with density levels lower than the ones observed in spring and summer (10–102 cells ml−1). Their abundance began to increase in late summer, peaking in the following months and persisting at lower concentrations until early winter. Along with the tiny single-celled Skeletonema menzelii, two larger chain-forming diatoms that are suspected to be aliens in the Mediterranean Sea were found in this cluster: Skeletonema tropicum, not seen in the area before 2002 (Zingone et al., 2003), and the toxigenic Pseudo-nitzschia multistriata, found since 1996 (D'Alelio et al., 2010). The recently described cryptic species Pseudo-nitzschia allochrona, genetically distinct from P. delicatissima and P. arenysensis, was recurrently detected with molecular methods only in this period of the year (Percopo et al., 2022; Ruggiero et al., 2022). Typical to autumn was also a colonial diatom previously identified as Leptocylindrus minimus and subsequently attributed to the newly erected genus, Tenuicylindrus, as T. belgicus (Nanjappa et al., 2013). The coccolithophore Calciopappus caudatus had already been noticed to be recurrent in this season in previous investigations in the area (Ribera d'Alcalà et al., 2004). Emiliania huxleyi, which is the most abundant and frequent coccolithophore at LTER-MC, would also probably belong to the autumn species association, as it is often found with relatively high abundance (103 cells ml−1) in this season (Figure S2). However, the species was excluded from the cluster analysis because it frequently extended its occurrence throughout winter and in some years in spring, sporadically attaining bloom concentrations of 104 cells ml−1, for example, in summer 1985 and 1986.
The species in the bimodal cluster (Figure 4f) had two phases of higher abundances (102 cells ml−1) in spring and early autumn, respectively, partly overlapping with the spring and autumn clusters but separated by a period of lower density or no detection. The most abundant species in this cluster were Chaetoceros socialis and other colonial Chaetoceros species, followed by diatoms of the family Thalassiosiraceae (Mediolabrus comicus and Thalassiosira cf. allenii), the cercozoan Paulinella ovalis, several autotrophic and heterotrophic dinoflagellates of the genera Scrippsiella, Protoperidinium, Prorocentrum and Lessardia, and the coccolithophore Syracosphaera pulchra.
The last cluster (no pattern, Figure 4g) was a species-rich and taxonomically heterogeneous group that showed a less clear seasonality and a lower density compared to the other clusters, with occasional increases along the seasons. Except for the dinoflagellate Mesoporos adriaticus and the coccolithophore Calciosolenia brasiliensis, this group was constituted by large-sized, colonial diatoms, such as Thalassionema nitzschioides, Thalassiosira rotula, Guinardia striata, Dactyliosolen phuketensis, Lauderia annulata, Proboscia alata, and several Chaetoceros species, which were all less frequent and abundant over the year than the other diatoms. Despite the lack of a well-defined seasonal pattern of the whole cluster, the individual species showed at times defined periods of high abundance, mostly in autumn but in some cases also in summer (Figures 2 and S4).
The bootstrap analysis showed the high stability of the different clusters, with an average ARI of 0.898. In a more detailed analysis the autumn and winter associations, followed by the spring one, were the most stable, that is, had the highest number of species still associated upon the resampling procedure of the bootstrap analysis. Nevertheless, within each association individual species showed differences in their high abundance period and peak timing, and generally their temporal distribution did not coincide strictly (Figure S4).
All seven associations identified in our analysis showed wide interannual variability in their abundance (Figure 4). In the second, longest part of the time series (1995–2010) none of the seven groups showed a significant trend (data not shown). In the first period (1984–1991), the spring and no-pattern associations had a slight decreasing trend (−0.002, p < .005) while the early summer association slightly increased (0.002, p < .001).
3.3 Relationship with environmental variables
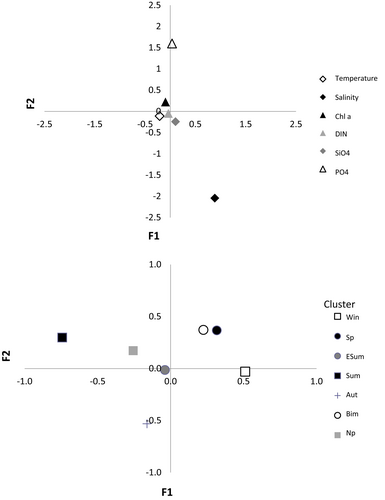
The seven phytoplankton assemblages identified by the cluster analysis showed different correlations to the environmental variables (Figure 5). In the CCA, the first axis explained 39% of the variability and showed a high negative correlation with temperature. The second axis explained 32% of the variability and was correlated positively with chlorophyll a and phosphate concentrations and negatively with salinity. The latter feature reflected the impact of land runoff on the coastal waters of the sampling site as well as the seasonal variations driven by the precipitations/evaporation regime throughout the year. The assemblages were distributed along the first axis according to their frequency in the different seasons, the summer association opposite to the winter and spring ones, while the others had intermediate positions. The most abundant assemblages (spring, summer, and bimodal) and the no-pattern one showed a negative relationship with the salinity axis, reflecting the influence on the coastal area of waters of terrestrial origin, which generally are associated with high biomass values. The autumn assemblage was the only one positively related to salinity, which showed its highest annual values (Figure 1b) concurrent with maximum evaporation in this season.
3.4 Trait diversity
The functional diversity (Fun Div index) of the seven associations, based on 11 morpho-functional traits of the species, was highest in spring (0.73) and decreased progressively across the seasons, reaching a minimum (0.34) in the winter association (Figure 6a). The two associations that showed non-univocal seasonal patterns had intermediate Fun Div values, that is, 0.65 and 0.40, for the bimodal and no-pattern clades, respectively.
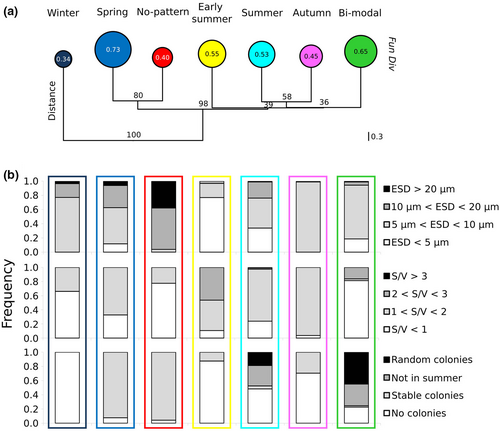
Based on the traits' variants in the various seasonally aggregated clusters, the winter association was significantly distinct from the others (Figure 6a). The spring and no-pattern associations were fairly separated from the others, while they were not robustly distinguishable one from another.
We focused on the traits ESD and S/V, which can affect the physiology of phytoplankton cells, and coloniality, which is related to the defense strategy against grazing as well as to sinking rate and response to turbulence (Figure 6b). Concerning ESD, the early summer clade was dominated by small-sized species (<5 μm), with very few cells between 10 and 20 μm and no cells exceeding 20 μm. The autumn association was dominated by species between 5 and 10 μm. The winter clade was dominated by non-colonial cells of spherical shape (S/V < 1). Species with a shape close to the spherical one but slightly larger (5–10 μm) were also found in the no-pattern and bimodal associations. Larger species (>20 μm) showing stable coloniality were relatively abundant only in the cluster of species lacking a seasonal pattern.
4 DISCUSSION
4.1 General characteristics of species associations
In the highly dynamic coastal Gulf of Naples, characterized by relatively high interannual variability in hydrographic parameters, seven well-defined and stable phytoplankton associations are identified over a span of ca. 20 years, five of which re-occurring each in a specific period of the year throughout the time series. These associations are clearly related to environmental variables, and especially to the seasonal temperature signal, supporting the high predictability of species assemblages proposed for freshwaters (Reynolds, 2006; Reynolds et al., 2000). The influence of coastal conditions is visible in the inverse relationship with salinity shown by all associations, except for the autumn cluster, which occurs in the period of maximum annual salinity caused by intense evaporation of relatively warm water masses in this season (Ribera d'Alcalà et al., 2004). The signal of nitrates and silicates and their influence on the phytoplankton associations is less clear, which is understandable based on excess concentration values in many cases and the variable outcome of nutrient consumption. The only nutrients showing a clear negative correlation with salinity are phosphates, which are constantly low but rarely limiting at the study site (Ribera d'Alcalà et al., 2004).
Quite important differences in terms of abundance characterize the different associations, reflecting the general seasonal trend for the whole phytoplankton in the area. The spring association is the first to increase after the winter minima, and in some years forms blooms across the isopycnal water column even before mid-March, favoured by occasional conditions of mild and calm weather (Zingone et al., 2010a). The summer association is quite abundant for a longer time (from May through September), with species often forming intense blooms that succeed and overlap one the other, supported by nutrient inputs from municipal discharges (Zingone et al., 1990). The distinctive feature in the bimodal cluster is the attainment of similar density values in two different and non-contiguous periods of the year, spring and early autumn, which differ in many aspects including day length, salinity, and nutrient availability (Ribera d'Alcalà et al., 2004). The other associations are all less abundant. Possibly, the scarce definition of the seasonality of the no-pattern cluster resides in the low density of its components, which increases the probability to miss them in the observed fraction of the samples and weakens the statistics.
The distinct seasonality and stability over the years of the species associations identified in this study fully match the remarkably recurrent patterns of the individual species and whole phytoplankton assemblages of the area, which have recently been shown to be linked to photoperiod as the most important variable (Longobardi et al., 2022). In fact, slight but consistent differences in the occurrence and peak times of species belonging to the same association suggest that they fit the Gleasonian concept of independent species behaviour, whereby strong phenological signatures of individual species cause the more or less complete overlap of their occurrence time in the year.
4.2 Taxonomic characterization of species associations
Phytoplankton associations in the Gulf of Naples are composed of phylogenetically diverse species, as they include members of all main taxonomic groups, fitting the idea of functional convergence of distantly related taxa (Reynolds, 2006; Reynolds et al., 2000, 2002). The highest taxonomic divergence is found in the summer association, which includes diatoms (both centric and pennate taxa), dinoflagellates, coccolithophores, and some identifiable prasinophyte flagellates. On the other hand, species within the same genus, for example, Chaetoceros, Pseudo-nitzschia, Dinobryon, and Syracosphaera, are often not found together in the same clusters but rather are present with various representatives in the different associations that alternate over the seasonal cycle. This pattern supports the idea of functional divergence even between species that are very close phylogenetically. This is conceivable in the spatially homogeneous marine plankton, where the birth of a new species could more easily occur by temporal segregation of sibling populations (allochronic speciation) in ecologically different seasons, as it has been proposed for the two sympatric species of the Pseudo-nitzschia delicatissima group, P. arenysensis, and P. allochrona (Percopo et al., 2022), found in the spring and autumn associations, respectively.
The result of a relatively high phylogenetic homogeneity in some seasons, for example, in the diatom-dominated spring association and the coccolithophore-dominated winter one, is biased by the exclusion of unidentified dinoflagellate, coccolithophore, and small flagellate taxa from our analysis. Because these groups that lump undetermined species are present and often abundant across the whole year, their inclusion would not help in the characterization of individual associations. Yet, unidentified flagellates are dominant in the scarcely abundant late autumn-winter community and relatively abundant also in spring and other seasons, when they are represented by species of algal classes that are quite distant phylogenetically, such as cryptophyte, mamiellophytes, prasinophytes, haptophytes, and pelagophytes (McDonald et al., 2007). Like diatoms and coccolithophores, also these microalgal groups are not exclusive to a single phase of the seasonal succession. For example, cryptophytes can reach high concentrations in spring and summer, but they include species that may show peaks in other seasons of the year (Cerino & Zingone, 2006). Dinoflagellates are generally associated with a later phase of the annual phytoplankton succession, namely, the warm and stable conditions from late spring to autumn, when they are second only to diatoms in terms of biomass (Ribera d'Alcalà et al., 2004). However, a clear seasonal alternation of different taxa and a quite distinctive and diversified association in the winter season have been identified also for this group by applying a metabarcoding approach (Mordret, 2018).
In summary, based on present results and other studies mentioned above, different microalgal groups seem to be present, albeit with different species and in various proportions, in any period of the year in the Gulf of Naples, with the only exception of diatoms, which appear to be virtually absent from the winter community, when they may be limited by low light conditions under complete water column mixing (Zingone et al., 2010a). In this respect, marine phytoplankton assemblages are not different from those thriving in freshwaters, in which recurrent assemblages are often composed of species belonging to only distantly related groups (Kruk et al., 2021; Reynolds et al., 2002). Similar evidence of a wide functional convergence of distantly related taxa has also been obtained by examining eukaryotic plankton of the world seas through a metagenomic approach (Delmont et al., 2022). Nonetheless, under extreme environmental conditions, for example, summer nitrate depletion and stratification, filamentous cyanobacteria that are capable to assimilate atmospheric nitrogen (e.g., Microcystis, Beversdorf et al., 2013) and possess high buoyancy (e.g., Planktothrix, D'Alelio et al., 2011) may largely dominate freshwater phytoplankton. By contrast, the Gulf of Naples coastal environment, with frequent exchanges with offshore waters and inputs of terrestrial origin, likely does not present constraints to the development of phytoplankton assemblages that include phylogenetically distant species at any time of the year.
4.3 Morpho-functional characterization of the associations
The traits selected in our investigation include both physiological properties and morphological features that have a functional role, showing multiple effects, for example, on resource competition, growth rate, and avoidance of mortality by grazing or sinking. A relatively high morpho-functional diversity characterizes most of the associations identified in our study, but some of the traits considered show to be under selection in different seasons.
In spring, coloniality is the most represented trait in the diatom-dominated association. Contrasting hypotheses on the significance and advantages of chain formation are related to (i) increased drag and hence reduced sinking velocities (Smayda & Boleyn, 1966; Takabayashi et al., 2006); (ii) enhanced nutrient uptake through advective thinning of the diffusive boundary layer (Karp-Boss et al., 1996); (iii) increased encounter rate (sexual reproduction) due to colony entanglement or confluence while sinking (Botte et al., 2013; Font-Muñoz et al., 2019); and (iv) plastic defense from predation (Bergkvist et al., 2012). It should be noticed, however, that colonial habit includes several sub-traits (e.g., colony shape junction type) that may widen the shape range and confer different selective advantages, alone or in association with other traits such as the presence of spines. The variety of patterns and forms offered by the colonial habit results in diverse shapes and mechanical properties which in turn may affect nutrient acquisition, encounter with grazers, aggregate formation, and settling. However, the different morpho-functional properties of rigid or flexible needle-like, fan-shaped, spiral, or tri-dimensional colonies in the spring association are difficult to interpret. Besides colonial diatoms, the tiny non-colonial Pseudo-nitzschia galaxiae, the colonial flagellate Dinobryon, and the spiny flagellate Apedinella spinifera all together make the spring association quite diversified in terms of functional traits.
In the associations of the early/late summer and autumn, a switch towards non-colonial species, or non-colonial forms of colonial species, along with the prevalence of small-sized species with relatively high s/v ratio, match the physical and chemical characteristics of the water column, whereby stratification and limited turbulence would not be favourable to larger-sized, non-motile species with low affinity for nutrients. Had we included undetermined naked dinoflagellates, which are prevalently small-sized forms in this period of the year, and small flagellates, probably the functional diversity of the summer association would have been enhanced by the presence of motile, hetero- and mixotrophic taxa.
The winter association with coccolithophores and silicoflagellates is unique and homogeneous in terms of traits, which show the minimum diversity compared to the other associations. In that period of the year, the dominance of motile, non-colonial, and non-spore former species is evident and would not be contradicted in case the undetermined flagellates were considered. This group includes taxa with a quite wide variety of functional properties, for example, in terms of trophy and pigment assets, but all generally share important traits such as relatively small size, spherical shape, absence of coloniality and spore formation, and prevalence of mixotrophy and heterotrophy.
Less clear is the interpretation of the trait distribution in the non-seasonal or bimodal species association. Interestingly the latter includes species that thrive under contrasting environmental conditions in terms of water column structure, temperature, and nutrient availability. In some cases, the bimodal distribution could correspond to different populations of morphologically identical taxa (or cryptic species), but cases of physiological plasticity or intra-specific diversity are also known in marine plankton species (Pargana et al., 2020; Pinseel et al., 2022).
Our analysis of morpho-functional properties aimed at relating ecologically aggregative processes to trait-selection processes. The clearest outcome of this analysis is that, despite some signals of convergence in specific seasons of the year, trait distribution does not explain univocally the clustering of phytoplankton into the seven identified associations. In most cases, the functional diversity of these associations would have probably been the same or even higher if we had included naked and thecate undetermined dinoflagellates of various sizes, life cycles, and trophic habits, as well as small flagellates belonging to phylogenetically distant groups, and undetermined coccolithophores and diatoms. Except for the size, morpho-functional traits cannot be assessed with optical methods for these broad groups, whose functional diversity remains largely underestimated.
5 CONCLUDING REMARKS
Based on a 20-year-long time series, seven stable phytoplankton species associations are identified in the Gulf of Naples, which show different seasonal patterns and defined relationships with environmental variables, particularly with those related to astronomical factors, that is, those strictly linked to the calendar. In contrast to the great importance attributed to nutrients as the main drivers of phytoplankton variability, the results of our study along with similar ones from research in our area point to the major importance of the biological asset of individual phytoplankton species and their interaction with the changes in the physical environment (e.g., light, turbulence, and stratification) over the seasons.
Within the associations, species often differ for phylogenetic affiliation, demonstrating that high-level, taxonomically based groups, although often defined as “functional groups,” can only provide a limited picture of the ecological role of phytoplankton populations. It also basically confirms that phylogenetic diversity is not a good indicator of ecological diversity. A strong phenological signature in phytoplankton species tends to stabilize the associations and their seasonal occurrence, while differences in their occurrence patterns fit the concept of a statistical overlap of independent species that are distributed according to their individual physiological requirements.
The decrease in functional diversity from spring to winter, that is, along a decreasing gradient of environmental resources, suggests a change in the assembly rules, from trait divergence under recurrent optimal spring conditions to convergence under limiting environmental resources, for example, hydrographic instability and light limitation in winter. This progression fits the “stress-dominance hypothesis” (Swenson & Enquist, 2007; Weiher & Keddy, 1995) which postulates that high levels of environmental stress impose a higher degree of environmental filtering and trait underdispersion, whereas more favourable conditions allow for a greater resource partitioning and trait overdispersion.
The convergence of traits as evidence of effective environmental filtering supports the idea that phytoplankton associations promptly respond to variations in external conditions. However, the extremely regular temporal patterns of traits, life forms, and individual species, rather than a response, could well be the result of adaptation, the latter term widely used by Margalef (1978) in his famous mandala paper. In this view, the environmental filter would exert its role in selecting individual species on an evolutionary basis, leading to their adaptation to distinct conditions, while biologically driven phenology would allow them to predict their occurrence in the most favourable season for their growth. Using Margalef's words, “The organism performs its own, individual Fourier analysis. When the impact occurs, it is already prepared” (Margalef, 1980, p. 179). Rather than drivers, environmental variables can be considered as predictors of species associations, which explains the link found between the two sets of variables. Nutrients seem to play a secondary role in this respect, probably also due to the characteristics of the study area where they rarely attain limiting conditions (Longobardi et al., 2022; Ribera d'Alcalà et al., 2004), but they affect the abundance of the individual species and the whole phytoplankton communities, in this way being responsible for a large amount of interannual variability.
As a final consideration, the remarkable stability of species association is the result of a balance between biological properties and environmental conditions, but this balance cannot be given for granted under important climatic and anthropogenic changes that imply changes in selective pressure on certain traits. Further studies aimed at monitoring the species associations found in this investigation over a longer time can shed light on mechanisms driving their interannual variability, thus improving our predictive capability of the future status of the planktonic system.
ACKNOWLEDGEMENTS
The authors would like to thank Marco Cannavacciuolo, Augusto Passarelli, Ferdinando Tramontano, Giancluca Zazo, and the crew of the R/V Vettoria, for sampling and data collection at sea. We also thank the Marine Research Infrastructure of the Stazione Zoologica for acquiring, processing, and managing the environmental data, and the entire LTER-MC Team for continued collaboration in the project. Thanks are due to three anonymous reviewers whose relevant comments contributed to improving the first version of our manuscript.
FUNDING INFORMATION
CT was funded by a fellowship of the EU-Enveurope project (LIFE08 ENV/IT/000399). The LTER-MC program is funded by SZN.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Phytoplankton and environmental data analysed in this paper are available in the public repository Harvard Dataverse at https://doi.org/10.7910/DVN/YDMWNQ (Sarno et al., 2022).




