Hepatitis B Virus Variants and Cytokine Patterns in Acute Liver Failure and Transplant-Free Survival
Handling Editor: Dr. Alejandro Forner
Funding: This study was sponsored by NIH—NIDDK (U-01 58369). NHP was supported by Cumming School of Medicine and Canadian Institutes of Health Research (CIHR) Graduate Studentship.
ABSTRACT
Background & Aims
Only 25% of hepatitis B-related acute liver failure (HBV-ALF) patients survive without liver transplantation (transplant-free survival, TFS). There is limited study of immunological and virological profiles in these patients. We analysed the association between hepatitis B viremia and cytokine patterns on TFS of HBV-ALF patients.
Methods
We identified 48 acute and 20 history of HBV infection ALF patients from the US ALF Study Group registry (> 3400 patients). The inclusion criteria were age > 18 years, diagnosis of HBV-ALF with hepatic encephalopathy and INR ≥ 1.5. Data were collected from ICU admission through day 21. Serum collected at admission and at days 3–5 were used for cytokine quantification by Luminex and novel HBV biomarkers, genotypes and variants.
Results
In 48 acute (50% F, median age 40 years) and 20 history/reactivation (40% F, median age 53 years) HBV-ALF patients, there were 26 (54%) and 5 (25%) TFS, respectively. Detectable HBV DNA by clinical PCR assay (median 3.39 log10IU/mL, aOR 5.308; 95% CI: 1.217–23.155, p = 0.026) and qAHBc levels (median 4.5 log10IU/mL, aOR 4.466, 95% CI: 0.968–20.608, p = 0.050) were associated with TFS in acute HBV-ALF patients. HBV variants associated with anti-viral immune escape were more frequently detected in acute HBV-ALF TFS patients compared to non-TFS (p < 0.05). TFS with acute HBV-ALF had higher angiogenic factors (PDGF-AA, p = 0.008; PDGF-BB, p = 0.0006; VEGF-A, p = 0.014) and lower pro-inflammatory cytokine levels (IL-1α, p = 0.031; IL-2, p = 0.014; IL-6, p = 0.039). Significant differences in HBV viremia were not observed in history/reactivation of HBV-ALF patients.
Conclusions
Acute HBV-ALF patients with TFS were often viremic with immune escape variants, increased angiogenic factors and decreased pro-inflammatory cytokines.
Trial Registration: ClinicalTrials.gov identifier: NCT00518440
Summary
- Despite the high mortality rate of hepatitis B-related acute liver failure (HBV-ALF), there is limited research on virological and immunological profiles associated with transplant-free survival.
- Acute HBV-ALF transplant-free survivors were viremic with increased HBV immune escape variants and had increased levels of blood vessel growth factors and a reduction in pro-inflammatory related proteins (cytokines).
- This study provides novel insight on the potential influence of HBV and inflammatory immune responses (cytokines) on transplant-free survival of patients with HBV-ALF.
Abbreviations
-
- ALF
-
- acute liver failure
-
- ALFSG
-
- Acute Liver Failure Study Group
-
- ALT
-
- alanine transaminase
-
- Anti-HBc (IgM)
-
- Anti-hepatitis B core antibody (IgM if indicated)
-
- anti-HBs
-
- antibody to HBV surface antigen
-
- HBeAg
-
- HBV E antigen
-
- HBsAg
-
- hepatitis B virus surface antigen
-
- HBV
-
- hepatitis B virus
-
- HE
-
- hepatic encephalopathy
-
- ICU
-
- intensive care unit
-
- INR
-
- international normalised ratio
-
- KCC
-
- King's College Criteria
-
- LT
-
- liver transplantation
-
- MELD
-
- Model for End-Stage Liver Disease
-
- NGS
-
- next-generation sequencing
-
- NRAg
-
- HBV nucleic acid-related antigen
-
- qAHBc
-
- quantitative anti-hepatitis B core antigen
-
- TFS
-
- transplant-free survival
1 Introduction
Acute liver failure (ALF) is a rare syndrome with a prevalence of less than 0.1% in Western countries including Canada and the United States of America [1]. As a multisystemic illness, ALF has a high rate of morbidity and a mortality rate of 30% [2]. The manifestation of ALF is rapid with severe liver injury, hepatic encephalopathy and coagulopathy (a bleeding disorder, international normalised ratio (INR) > 1.5) in individuals without pre-existing liver disease such as cirrhosis [3]. Causes of ALF vary geographically across the globe [4]. While acetaminophen-induced ALF is the leading cause of ALF in Western countries, viral hepatitis (i.e., hepatitis B and E viruses (HBV and HEV)) is the predominant aetiology of ALF in Southeast Asia and Sub-Saharan Africa. The HBV causes ALF in two forms: true acute (de novo) HBV infection and reactivation in those with chronic HBV [5]. ALF due to hepatitis B infection has been identified to be associated with systemic inflammation and immune cell paralysis [6]. Specific factors identified include pro-inflammatory cytokine storm (i.e., interferon (IFN)-γ and tumour necrosis factor (TNF)-α), and elevated hepatitis B core IgM and IgG antibodies [7, 8]. Higher viral replication, increased HBV core protein (HBc) expression and genotypes B and D have also been reported in association with ALF onset [9, 10].
Following infection, the HBV establishes a long-lived intranuclear reservoir called covalently closed circular DNA (cccDNA) that serves as a template for all viral messenger RNA. Consequently, individuals who recover from prior infection despite clearance of circulating viral surface proteins (HBV surface antigen, HBsAg) and development of so-called natural immunity with protective HBV antibodies may remain at risk for reactivation. Recent novel serum HBV biomarkers that more accurately reflect HBV cccDNA transcriptional activity and host-anti-viral immune response have been evaluated for utility in various clinical settings, but not utilised in the context of extremely rare cases of hepatitis B-related ALF (HBV-ALF). A qualitative enzyme-linked immunosorbent assay (ELISA) for detection of HBV nucleic acid-related antigen (NRAg) consisting of pre-Surface (S) 1 antigen and HBV core antigen (HBcAg) has been described in association with residual serum HBV DNA and differentiation of active vs. inactive chronic hepatitis B [11, 12]. Antibody to HBV core protein (Anti-HBc) is a diagnostic marker of past exposure to HBV infection. Since the humoral immune response is targeted towards the HBcAg, quantification (qAHBc) may be useful in monitoring the natural history of HBV infection and has been shown to differentiate in people with occult vs. overt HBV infection [13].
The management of ALF is complex, requiring intensive supportive care and life-saving liver transplantation is limited due to the donor organ shortage, risk of graft rejection and surgical risks [14]. Only 25% of HBV-ALF patients survive spontaneously without liver transplantation [15]. Refinement of established prognostic models (i.e., King's College Criteria, KCC), Model for End-Stage Liver Disease (MELD), Acute Liver Failure Study Group (ALFSG), may help predict HBV-ALF outcomes [16, 17]. The objective of this study is to characterise the immune and viral factors, using next-generation viral sequence analysis and novel serum biomarkers assays, associated with spontaneous survival of acute HBV-ALF patients. We hypothesize that an anti-inflammatory immune profile and/or presence of unique viral variants are associated with clinical outcomes.
2 Materials and Methods
2.1 Study Design, Patient Recruitment and Clinical Evaluation
In this observational cohort study, we identified 68 HBV-ALF patients from the US-ALFSG prospective registry between 1998 and 2015 (> 3400 patients). All participating sites within the US-ALFSG were tertiary academic liver transplant referral centers. This study was approved by the institutional review boards or health research ethics boards of all US-ALFSG enrolling sites. All participants or next of kin provided informed consent following the principles of the Declaration of Helsinki. The NIH guidelines for the inclusion of women and minorities as participants in clinical research were also observed. All authors had access to the study data and reviewed and approved the final manuscript.
The inclusion criteria included age of > 18 years and diagnosis of ALF, defined as presence of 1) hepatic encephalopathy of any degree, 2) evidence of coagulopathy with INR ≥ 1.5, 3) acute illness onset of < 26 weeks and 4) no evidence of cirrhosis and hepatocellular carcinoma (HCC). Transplant hepatology expert clinical decision makers verified absence of cirrhosis in enrolled patients in this study. In total 68 individuals tested HBsAg positive and were diagnosed with HBV-ALF, and further sub-classified as de novo acute hepatitis B associated ALF (HBsAg and anti-HBc IgM positive, n = 48) vs. history of hepatitis B and reactivation (HBsAg positive, anti-HBc IgG positive n = 20). HBsAg positive HBV-ALF patients with acetaminophen overdose, alcohol misuse disorder, antibodies to human immunodeficiency virus (anti-HIV), hepatitis C virus (HCV) RNA, anti-HCV, antibodies to hepatitis D virus (anti-HDV) and anti-HEV positivity were excluded (n = 4) (Figure S1).
Demographical, clinical, virological and outcome data were collected for all enrolled patients from admission (baseline) up to day 21 follow-up, including age, sex, ethnicity (race), biochemistry, viral serology (HBV, HCV, HDV, HEV and HIV), hepatic encephalopathy grade (West Haven criteria, on admission), MELD score, KCC on admission, therapy including previous use of immunosuppressive treatments, day 21 transplant-free survival (TFS) and liver transplantation. Serum samples were collected on admission to hospital (baseline) and days 3–5 follow-up and stored long-term at 80°C and shipped by overnight courier on dry ice to the investigator for further analysis.
2.2 Novel HBV Biomarkers and Next-Generation Sequencing Analysis
Quantification of novel NRAg and qAHBc biomarkers was performed using an alternative enzyme-linked immunosorbent assay-based method (Beijing Wantai Biological, Beijing, China) [11]. HBV sequencing of the HBV pre-core (C)/C and pre-surface (S)/S regions was done as previously described [18-20]. In brief, total DNA was isolated using a commercial kit (DNeasy Qiagen, Hilden, Germany) in parallel with negative mock and positive controls. HBV DNA was PCR-amplified (sensitivity of 10 IU/mL or 50 virus copies/mL), and gel purified (QIAquick gel extraction kit, Qiagen, Hilden, Germany) amplicons were analysed by Sanger sequencing (University of Calgary Sequencing Services, Calgary, AB, Canada). HBV genotype was determined using the National Center for Biotechnology Information Basic Local Alignment Search Tool (NCBI BLAST) HBV genotyping tool. Next-generation sequencing adaptors were added to HBV preS/S and preC/C amplicons using high-fidelity polymerase (Phusion, New England BioLabs, Whitby, Canada), and then gel amplicons were purified and sequenced using the Illumina MiSeq (San Diego, USA), along with preS/S and preC/C PCR clones as an internal control to determine the error rate (mean error rate calculated as < 1%). Sequence alignment and mutational analysis were performed using MEGA 10.0 with Clustal W alignment.
2.3 Serum Cytokine Quantification
Multiplex Luminex assay was used to measure a panel of 42 serum cytokines, chemokines and growth factors (Luminex Eve Technologies, Calgary, AB, Canada). The panel analytes include soluble CD40 ligand (sCD40L), epidermal growth factor (EGF), Eotaxin, fibroblast growth factor (FGF)-2, FMS-like tyrosine kinase 3 ligand (FLT-3 L), Fractalkine, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), growth-related oncogene (GRO)-α, IFN-α2, IFN-γ, interleukin (IL)-1α, IL-1β, IL-1 receptor antagonist (RA), IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-17A, IL-18, interferon gamma-induced protein (IP)-10, monocyte chemoattractant protein (MCP)-1, MCP-3, macrophage-derived chemokine (MDC), macrophage inflammatory protein (MIP)-1α, MIP-1β, platelet-derived growth factor (PDGF)-AA, PDGF-BB, regulated on activation, normal T expressed and secreted (RANTES), transforming growth factor (TGF)-α, TNF-α, TNF-β and vascular endothelial growth factor (VEGF)-A.
2.4 Statistical Analysis
TFS at day 21 was used as the primary outcome. Mann Whitney U-test was performed for continuous variables, a chi-square test or Fisher's exact test for cells < 5 was performed for categorical variables. Wilcoxon signed-ranked test was performed to compare cytokine and chemokine levels of HBV-ALF patients from baseline to follow-up. Comparisons between unpaired groups were performed using Mann Whitney U-test. Logistic regression was used for univariate analysis. Given the exploratory nature of this study, adjustment for multiple comparisons was not performed. GraphPad Prism 9.0.0 and IBM SPSS Statistics 27.0.1.0 were used to perform all the statistical tests.
3 Results
3.1 Demographic, Clinical and Viral Characteristics of Patient Cohorts
From the ALFSG registry, we identified 48 de novo acute HBV-ALF patients and 20 patients that were classified with viral reactivation and/or history of hepatitis B (Figure S1). In 48 acute HBV-ALF individuals (median age 40.0 years, 50% Female), 26/48 (54%) were transplant-free survivors (median age 42.0 years, 50% female and 54% White) compared to 22/48 (46%) that received liver transplantation and/or died (non-TFS) at day 21 post-admission (median age 39.0 years, 50% female and 68% White) (Table 1). Data on 20 individuals with ALF due to history of HBV and reactivation are provided in Table 2 [(5 were TFS with median age 51.0 years, all male and 40% White) and (15 received liver transplantation and/or died at day 21 post-admission with median age 53.0 years, 53% female and 46.7% Asian)]. As expected, all de novo acute HBV-ALF patients that survived spontaneously required less organ support including mechanical ventilation (11.5% vs. 59.1%, p = 0.0007), vasopressors (7.7% vs. 36.4%, p = 0.029), renal replacement therapy (3.9% vs. 31.8%, p = 0.017) and sedatives (15.4% vs. 54.6%, p = 0.006) as reflected by established prognostic scores (i.e., KCC and MELD), Table 1. No significant changes in organ support, intracranial pressure (ICP) direct therapies and blood products (except for fresh frozen plasma, 20% TFS vs. 80% LT/died, p = 0.015) were observed for ALF patients due to reactivation of known hepatitis B infection (Table 2). We performed univariate logistic regression analysis to analyse possible predictors of transplant-free survival in HBV-ALF patients (Tables 3 and S1). As expected, poor KCC, 3/4 admit coma grade, high MELD score and increased INR were predictors for non-TFS in acute HBV-ALF patients.
| TFS (N = 26) | LT/died (N = 22) | p | |
|---|---|---|---|
| Age (years) | 42.0 (29.8–53.3) | 39.0 (29.3–50.0) | 0.521 |
| Sex (% female) | 13 (50.0%) | 11 (50.0%) | > 0.999 |
| Race | |||
| White | 14 (53.9%) | 15 (68.2%) | 0.312 |
| African American | 11 (42.3%) | 5 (22.7%) | 0.152 |
| Asian | 0 (0.0%) | 2 (9.1%) | 0.205 |
| Other | 1 (3.9%) | 0 (0.0%) | > 0.999 |
| Weight (kg) | 77.8 (68.5–98.5) | 75.5 (62.4–101.5) | 0.608 |
| Organ support (Days 1–7) | |||
| Mechanical ventilation, N (%) | 3 (11.5%) | 13 (59.1%) | 0.0007*** |
| Vasopressors, N (%) | 2 (7.7%) | 8 (36.4%) | 0.029* |
| Renal replacement therapy, N (%) | 1 (3.9%) | 7 (31.8%) | 0.017* |
| Poor KCC, N (%) | 2 (7.7%) | 8 (36.4%) | 0.029* |
| Coma grade 3/4 (admit), N (%) | 2 (7.7%) | 10 (45.5%) | 0.006** |
| ICP directed therapies (Days 1–7) | |||
| ICP monitor, N (%) | 1 (3.9%) | 5 (22.7%) | 0.081 |
| Mannitol, N (%) | 1 (3.9%) | 8 (36.4%) | 0.007** |
| Sedatives, N (%) | 4 (15.4%) | 12 (54.6%) | 0.006** |
| Blood products (Days 1–7) | |||
| Red blood cells, N (%) | 1 (3.9%) | 9 (40.9%) | 0.003** |
| Fresh frozen plasma, N (%) | 5 (19.2%) | 15 (68.2%) | 0.0006*** |
| Platelets, N (%) | 1 (3.9%) | 9 (40.9%) | 0.003** |
| ICU complications (Days 1–7) | |||
| Arrhythmias, N (%) | 2 (7.7%) | 10 (45.5%) | 0.006** |
| GI bleeding, N (%) | 0 (0.0%) | 4 (18.2%) | 0.038* |
| Laboratory tests | |||
| Prothrombin time (s) | 24.7 (20.6–30.3) | 36.3 (22.0–62.8) | 0.026* |
| INR | 2.2 (2.0–2.7) | 3.2 (2.2–5.7) | 0.024* |
| ALT (IU/L) | 1671.0 (604.5–3074.0) | 668.0 (207.5–3426.0) | 0.335 |
| Bilirubin (mg/dL) | 18.1 (12.5–25.8) | 21.3 (13.2–28.4) | 0.349 |
| Creatinine (mg/dL) | 0.9 (0.7–1.4) | 1.4 (0.7–3.1) | 0.280 |
| MELD | 30.0 (26.0–34.0) | 38.5 (33.0–43.5) | 0.002** |
| Hepatitis B | |||
| HBeAg, N (%) | 11 (42.3%) | 11 (50.0%) | 0.594 |
| Anti-HBc (total and/or IgM), N (%) | 26 (100%) | 21 (95.5%) | 0.458 |
| Anti-HBs, N (%) | 9 (34.6%) | 7 (31.8%) | 0.838 |
| Detectable HBV DNA levels, N (%) | 23 (88.5%) | 13 (59.1%) | 0.042* |
| HBV-NRAg (A/CO, 450/630 nm) | 16.5 (5.8–26.3) | 21.1 (0.5–24.6) | 0.611 |
| qAHBc (log10 IU/mL) | 4.5 (4.3–5.2) | 4.2 (3.5–4.5) | 0.044* |
| HBV genotype, N (%) | |||
| A | 8 (30.8%) | 11 (50.0%) | 0.239 |
| B | 0 (0.0) | 0 (0.0%) | > 0.999 |
| C | 1 (3.9%) | 2 (9.1%) | 0.587 |
| D | 5 (19.2%) | 3 (13.6%) | 0.710 |
| E | 1 (3.9%) | 0 (0.0%) | > 0.999 |
| Nucleos(t)ide analoguea, N (%) | 8 (30.8%) | 3 (13.6%) | 0.189 |
- Note: Continuous data are shown as median (IQR). Categorical data are shown as mean % (n/n known). For continuous data, Mann Whitney U-test was performed. Chi-square test or Fisher's exact test (for cells < 5) were performed for categorical data.
- Abbreviations: ALT, alanine transaminase; Anti-HBc, antibody to hepatitis B core antigen; Anti-HBs, antibody to hepatitis B surface antigen; HBeAg, hepatitis B virus e antigen; HBV-NRAg, hepatitis B virus nucleic acid-related antigen; ICP, intracranial pressure; INR, international normalised ratio; KCC, King's College Criteria; LT, liver transplantation; MELD, Model for End-Stage Liver Disease; qAHBc, quantitative anti-hepatitis B core antigen; TFS, transplant-free survival.
- a The timing of nucleos(t)ide analogue duration is unknown, likely started post-admission to study.
- * p < 0.05.
- ** p < 0.01.
- *** p < 0.001.
| TFS (N = 5) | LT/died (N = 15) | p | |
|---|---|---|---|
| Age (years) | 51.0 (39.5–58.0) | 53.0 (37.0–58.0) | 0.567 |
| Sex (% female) | 0 (0.0%) | 8 (53.3%) | 0.055 |
| Race | |||
| White | 2 (40.0%) | 4 (26.7%) | 0.613 |
| African American | 1 (20.0%) | 3 (20.0%) | > 0.999 |
| Asian | 2 (40.0%) | 7 (46.7%) | > 0.999 |
| Other | 0 (0.0%) | 1 (6.7%) | > 0.999 |
| Weight (kg) | 74.0 (59.1–117.2) | 74.0 (66.4–79.8) | 0.877 |
| Organ support (Days 1–7) | |||
| Mechanical ventilation, N (%) | 1 (20.0%) | 9 (60.0%) | 0.121 |
| Vasopressors, N (%) | 1 (20.0%) | 6 (40.0%) | 0.417 |
| Renal replacement therapy, N (%) | 0 (0.0%) | 6 (40.0%) | 0.260 |
| Poor KCC, N (%) | 1 (20.0%) | 7 (46.7%) | 0.292 |
| Coma grade 3/4 (admit), N (%) | 0 (0.0%) | 7 (46.7%) | 0.058 |
| ICP directed therapies (Days 1–7) | |||
| ICP monitor, N (%) | 0 (0.0%) | 3 (20.0%) | 0.540 |
| Mannitol, N (%) | 0 (0.0%) | 6 (40.0%) | 0.260 |
| Sedatives, N (%) | 2 (40.0%) | 10 (66.7%) | 0.347 |
| Blood products (Days 1–7) | |||
| Red blood cells, N (%) | 1 (20.0%) | 8 (53.3%) | 0.195 |
| Fresh frozen plasma, N (%) | 1 (20.0%) | 12 (80.0%) | 0.015* |
| Platelets, N (%) | 0 (0.0%) | 2 (13.3%) | > 0.999 |
| ICU complications (Days 1–7) | |||
| Arrhythmias, N (%) | 1 (20.0%) | 3 (20.0%) | > 0.999 |
| GI bleeding, N (%) | 1 (20.0%) | 0 (0.0%) | 0.250 |
| Laboratory tests | |||
| Prothrombin time (s) | 23.9 (22.3–25.3) | 50.7 (37.6–78.0) | 0.027* |
| INR | 2.2 (1.9–2.4) | 5.4 (3.8–336.4) | 0.010* |
| ALT (IU/L) | 661.0 (354.0–1208.0) | 1861.0 (700.8–2646.0) | 0.087 |
| Bilirubin (mg/dL) | 17.5 (12.4–30.3) | 19.4 (14.3–24.3) | 0.823 |
| Creatinine (mg/dL) | 1.2 (−0.9–6.8) | 0.9 (0.6–1.3) | 0.084 |
| MELD | 28.0 (26.5–43.5) | 38.5 (28.8–42.5) | 0.637 |
| Hepatitis B | |||
| HBeAg, N (%) | 2 (40.0%) | 5 (33.3%) | 0.787 |
| Anti-HBc (total and/or IgM), N (%) | 2 (40.0%) | 3 (20.0%) | 0.371 |
| Anti-HBs, N (%) | 0 (0.0%) | 3 (20.0%) | 0.540 |
| Detectable HBV DNA levels, N (%) | 4 (80.0%) | 11 (73.3%) | 0.766 |
| HBV-NRAg (A/CO, 450/630 nm) | 24.1 (21.9–26.4) | 17.3 (0.1–26.4) | 0.197 |
| qAHBc (log10 IU/mL) | 4.9 (4.5–5.2) | 4.8 (4.0–5.1) | 0.667 |
| HBV genotype, N (%) | |||
| A | 0 (0.0%) | 5 (33.3%) | 0.266 |
| B | 1 (20.0%) | 4 (26.7%) | > 0.999 |
| C | 2 (40.0%) | 1 (6.7%) | 0.140 |
| D | 1 (20.0%) | 0 (0.0%) | 0.250 |
| E | 1 (20.0%) | 0 (0.0%) | 0.250 |
| Nucleos(t)ide analoguea, N (%) | 2 (40.0%) | 2 (13.3%) | 0.249 |
- Note: Continuous data are shown as median (IQR). Categorical data are shown as mean % (n/n known). For continuous data, Mann Whitney U-test was performed. Chi-square test or Fisher's exact test (for cells < 5) were performed for categorical data.
- Abbreviations: ALT, alanine transaminase; Anti-HBc, antibody to hepatitis B core antigen; Anti-HBs, antibody to hepatitis B surface antigen; HBeAg, hepatitis B virus e antigen; HBV-NRAg, hepatitis B virus nucleic acid-related antigen; ICP, intracranial pressure; INR, international normalised ratio; KCC, King's College Criteria; LT, liver transplantation; MELD, Model for End-Stage Liver Disease; qAHBc, quantitative anti-hepatitis B core antigen; TFS, transplant-free survival.
- a The timing of nucleos(t)ide analogue duration is unknown, likely started post-admission to study.
- * p < 0.05.
| Variable | Univariate logistic regression analysis adjusted odds ratio (95% CI) |
|---|---|
| Age (years) | 1.017 (0.975–1.060) |
| Sex (% female) | 1.000 (0.321–3.113) |
| Ethnicity | |
| White | Ref |
| African American | 2.493 (0.704–8.832) |
| Asian | 1.337 (0.560–3.192) |
| Poor KCC | 0.146 (0.027–0.785)*, p = 0.025 |
| Coma grade 3/4 (admit) | 0.143 (0.026–0.799)*, p = 0.027 |
| Laboratory tests | |
| MELD | 0.866 (0.782–0.958)**, p = 0.005 |
| INR | 0.627 (0.407–0.965)*, p = 0.034 |
| Bilirubin (mg/dL) | 0.978 (0.922–1.036) |
| Creatinine (mg/dL) | 0.783 (0.550–1.114) |
| Hepatitis B | |
| HBeAg | 0.733 (0.234–2.297) |
| Detectable HBV DNA levels | 5.308 (1.217–23.155)*, p = 0.026 |
| HBV-NRAg (A/CO, 450/630 nm) | 1.009 (0.942–1.080) |
| qAHBc (log10 IU/mL) | 4.466 (0.968–20.608)*, p = 0.050 |
| HBV genotype | |
| A | 0.519 (0.120–2.248) |
| B | N/A |
| C | 0.500 (0.041–6.166) |
| D | 2.167 (0.415–11.302) |
| E | N/A |
- Note: Adjusted odds ratios shown with 95% confidence intervals (CI).
- * p < 0.05.
- ** p < 0.01.
3.2 HBV Viral Load and qAHBc Was Associated With TFS in Acute HBV-ALF Patients
Assessment of hepatitis B virus in 48 patients with acute HBV-ALF showed all were HBsAg positive, 22 (46%) were HBeAg positive, with a median HBV DNA of 3.71 log10 IU/mL and predominant HBV genotype A (40%). Overall, detectable DNA levels and higher levels of qAHBc were predictors for TFS (Tables 1 and 3). A higher number of TFS HBV-ALF patients had detectable HBV DNA levels compared to non-TFS HBV-ALF patients (n = 23/26, 88.5% vs. 13/22, 59.1%, p = 0.042). Additionally, higher qAHBc levels were observed for TFS HBV-ALF patients (median 4.5 log10 IU/mL vs. 4.2 log10 IU/mL, p = 0.044). No significant changes in other HBV biomarkers tested (i.e., HBeAg status, anti-HBc total and/or IgM, anti-HBs and HBV-NRAg) and HBV genotypes A–E in TFS vs. non-TFS were observed. Univariate logistic regression analysis showed that detectable HBV DNA levels (adjusted odds ratio of 5.308 with 95% Cl of 1.217–23.155, p = 0.026) and increased qAHBc (adjusted odds ratio of 4.466 with 95% Cl of 0.968–20.608, p = 0.050) were predictors of transplant-free survival acute in HBV-ALF patients. ALF patients due to reactivation and/or history of hepatitis B did not show any significant changes in HBV biomarkers and genotype per primary outcomes of TFS (p > 0.05). (Tables 2 and S1).
3.3 Elevation of Growth Factors and Decline in Pro-Inflammatory Cytokines Observed in TFS
A significant increase in growth factors was observed in acute HBV-ALF patients who were transplant-free survivors from baseline to days 3–5 follow-up (Figure 1). These angiogenic factors included PDGF-AA (p = 0.008), PDGF-BB (p = 0.0006) and VEGF-A (p = 0.014), Figure 1A. In comparison, non-TFS acute HBV-ALF patients that received liver transplantation and/or died showed little to no increase/no change or declining levels of angiogenic factors during follow-up. Additionally, non-TFS acute HBV-ALF patients showed an increase in serum pro-inflammatory cytokines and chemokines levels from baseline to days 3–5 follow-up, including interleukin IL-12p70 (p < 0.0001), TNF-α (p = 0.0004), IFN-α2 (p = 0.0006), G-CSF (p = 0.002), RANTES (p = 0.018), IL-6 (p = 0.034), IL-8 (p = 0.035) and fractalkine (p = 0.039), Figures 1B and S2. Whereas TFS acute HBV-ALF patients had decreasing levels of pro-inflammatory IL-8 (p = 0.0003), IL-1α (p = 0.031), IL-2 (p = 0.014) and IL-6 (p = 0.039) compared to non-TFS acute HBV-ALF patients. In addition, an increase in anti-inflammatory IL-10 was observed in both TFS (p = 0.036) and non-TFS (p = 0.0009) acute HBV-ALF patient cohorts, Figure 1C. Analysis of other cytokines showed no difference between groups (Figure S2). Collectively, the multiplex Luminex data indicate that despite an increase in pro-inflammatory cytokine response, acute TFS HBV-ALF patients exhibited heightened angiogenic response and a lower pro-inflammatory cytokine response compared to non-TFS acute HBV-ALF patients.
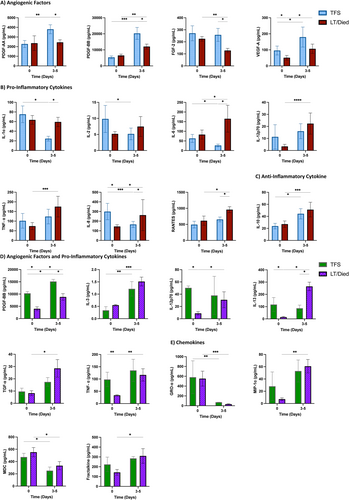
Serum cytokine and chemokine levels in HBV-ALF patients with history and/or reactivation of HBV showed elevation of pro-inflammatory cytokines such as IL-3 (p = 0.0005), MIP-1α (p = 0.004), TNF-α (p = 0.002), TGF-α (p = 0.016), IL-12p70 (p = 0.016), fractalkine (p = 0.029) and IL-13 (p = 0.031) in non-TFS compared to TFS patients from baseline to days 3–5 follow-up (Figure 1D,E). Only the angiogenic factor PDGF-BB (p = 0.027) was higher in TFS in this group. No significant difference in analysis of other angiogenic factors, cytokines and chemokines were observed (Figure S3).
3.4 Higher Frequency of HBV Immune Escape Variants in TFS Acute HBV-ALF Patients
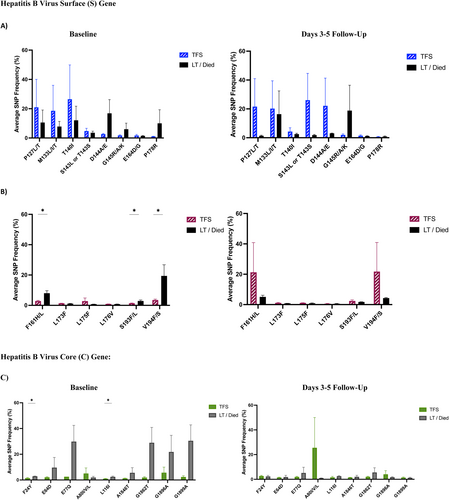
Next-generation sequencing analysis of HBV surface and core genes showed that TFS acute HBV-ALF patients had higher levels of HBV variants associated with immune escape phenotype compared to non-TFS acute HBV-ALF patients at baseline and days 3–5 follow-up (Figure 2A). These variants include P127L/T (p = 0.030 baseline, p = 0.007 d 3–5), T140I (p = 0.035 baseline) and S143L or T143S (p = 0.041 baseline) and E164D/G (p = 0.046 d 3–5) (Table S2). In TFS compared to non-TFS acute HBV-ALF patients, the overall frequency of HBV immune escape variants (D144A/E) declined from baseline to days 3–5 follow-up p = 0.047. HBV surface gene variants associated with the development of anti-viral therapy resistance were not statistically different between groups (Figure 2B). As well, HBV core gene variant at baseline only, F24Y levels (associated with increased risk of cirrhosis and HCC development) were greater for non-TFS than TFS acute HBV-ALF patients (p = 0.037, Figure 2C).
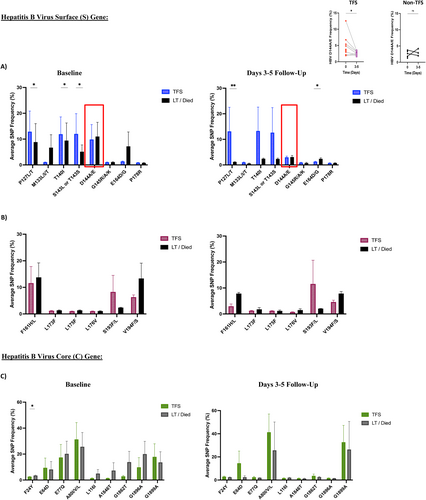
In ALF patients with history and/or reactivation of hepatitis B, there weren't significant differences in immune escape variants frequencies between TFS and non-TFS groups at baseline (Figure 3A). Increased frequency of variants associated with anti-viral therapy resistance (F161H/L p = 0.034, S193F/L p = 0.011 and V194F/S p = 0.019, Figure 3B), and variants associated with increased risk of cirrhosis and HCC (F24Y p = 0.036 and L116 I p = 0.030, Figure 3C) were observed at baseline. No significant differences in HBV surface and core gene variants were observed at days 3–5 follow-up.
4 Discussion
HBV induced acute liver failure is rare in non-HBV endemic regions and decreasing due to universal childhood vaccination programmes. Most immunocompetent adults spontaneously clear acute HBV infection with clearance of circulating viral antigens (i.e., HBsAg) and develop long-lived natural immunity with neutralising anti-HBs. Very rarely de novo HBV infection can lead to fulminant hepatitis, characterised by a cytokine storm, massive hepatocellular necrosis and liver failure. In this rare life-threatening condition, there is limited systematic investigation of the virological and immunological characteristics in people who survive or spontaneously recover. In this study, using the US-ALFSG registry and biobank, we provide detailed and serial analysis of HBV replication status, viral genome and host serum cytokine landscape.
Interestingly, detectable HBV DNA and increased qAHBc levels were associated with TFS in acute HBV-ALF patients. This data is not consistent with other studies that report higher HBV levels to be associated with HBV-induced ALF [12-14]. These findings are likely due to (secondary) reduced pro-inflammatory environment and heightened angiogenic factors observed in TFS acute HBV-ALF patients. Assessment of HBV mRNA and pre-genomic (pg) RNA will provide further insight into the role(s) of HBV in TFS of acute HBV-ALF patients.
Acute HBV-ALF TFS patients displayed increased levels of angiogenic factors and lower levels of pro-inflammatory cytokines and chemokines. A hallmark of ALF, dysregulated systemic inflammation is responsible for organ failure and mortality [7-11]. Cytokine storm and excessive inflammation are also observed in acute-on-chronic ALF (ALF patients with history of cirrhosis) [21]. Similar studies in paediatric ALF have shown that liver regenerative marker serum alpha-NH-butyric acid and IFN-γ related networks to be TFS associated [22, 23]. Thus, we can expect acute HBV-ALF TFS to display systemic inflammation to a lower a degree than non-TFS, as shown in this study.
Despite reduced levels of pro-inflammatory cytokines, we observed increased levels of HBV variants associated with host anti-viral immune escape phenotype in acute HBV-ALF TFS patients. This suggests that there is likely a functional anti-viral immune response in these patients. Functional anti-viral immune responses, particularly functional HBV-specific T cell response, are a hallmark of acute HBV infection [24]. Whereas HBV-specific T cells are exhausted, the T-cell-mediated anti-viral response is weak in chronic hepatitis B carriers. Therefore, we can expect an enhanced anti-viral immune response in acute HBV-ALF patients. However, further insight into the association between increased HBV variants related to immune escape and transplant-free survival is required in acute HBV-ALF patients.
Apart from acute HBV-ALF patients, we also examined possible viral and immune factors of TFS in HBV-ALF patients with reactivation and/or history of hepatitis B. Unlike in the acute HBV-ALF patient cohort, no significant predictors of TFS were found in the HBV-ALF patients with reactivation and/or history of HBV, likely due to small sample size. In contrast, the trend of pro-inflammatory cytokine elevation in the non-TFS reactivation and/or history of HBV-ALF patient cohort was found similarly to the acute HBV-ALF patient cohort. Lastly, increased presence of HBV variants associated with anti-viral therapy resistance and increased risk of cirrhosis and HCC development were observed for non-TFS reactivation and/or history of HBV-ALF patients, as expected given history of hepatitis B. Although data regarding initiation of anti-viral therapy within 21 days post-admission to the ALFSG study were not available, other studies have demonstrated this to have limited effect on HBV-specific markers [25-27].
The results of this study should be considered within the context of its study design. Given that HBV-ALF is extremely rare, we performed a pilot/exploratory study. Due to the small size of the patient cohort (related to the rarity of HBV-ALF), this study lacked the statistical power to perform multivariate analysis to adjust for multiple covariates due to the limited number of events (deaths). Second, there was a lack of longitudinal data beyond day 21 post-admission of enrolled HBV-ALF patients, which is the reflection of the overall US Acute Liver Failure Study Group registry. As such, we did not have the granularity to perform a Cox-regression model that requires longitudinal survival data. For longitudinal cytokine analysis, we were unable to secure patient serum samples beyond days 3–5 post-admission. Longitudinal analysis of immune and viral profiles of HBV-ALF patients can be an area of exploration for future studies. Third, the non-TFS de novo HBV-ALF patients compared to TFS patients required greater blood products, organ support and ICP-directed therapies and had greater ICU complications. Thus, there can be potential biases that make true comparisons between these patient cohorts challenging and require future exploration. Finally, while patients were prospectively enrolled, analyses were performed retrospectively and as such can only comment on associations between covariates and not causation. Despite these limitations, this is one of the largest novel experimental studies evaluating the viral and immune profile of TFS of HBV-ALF patients outside of Asia, an area that has not been previously explored.
In summary, we show that increased HBV replication, heightened angiogenic factor levels and reduction in pro-inflammatory cytokines can be associated with TFS in acute HBV-ALF patients. These valuable findings will inform future studies and management guidelines on transplant-free survival of acute HBV-ALF patients.
Author Contributions
Nishi H. Patel: experimental work, data contribution, data analysis and manuscript draft writing and editing. Annie Y. Chen and Carla Osiowy: experimental work, data contribution, manuscript editing and feedback. Valerie Durkalski Mauldin and William M. Lee: supervisor of All authors (U-01 Grant). Assisted in developing study design, manuscript editing and feedback. Carla S. Coffin and Constantine J. Karvellas: study conception, design, supervision, resources, manuscript editing, overall responsibility for the study conduct and coordination. US Acute Liver Failure Study Group: data review, manuscript editing and feedback.
Acknowledgements
The authors would like to thank all members and institutions participating in the Acute Liver Failure Study Group 1998–2018: W. M. Lee, M.D. (Principal Investigator); Anne M. Larson, M.D., Iris Liou, M.D., University of Washington, Seattle, WA; Oren Fix, M.D., Swedish Medical Center, Seattle, WA; Michael Schilsky, M.D., Yale University, New Haven, CT; Timothy McCashland, M.D., University of Nebraska, Omaha, NE; J. Eileen Hay, M.B.B.S., Mayo Clinic, Rochester, MN; Natalie Murray, M.D., Baylor University Medical Center, Dallas, TX; A. Obaid S. Shaikh, M.D., University of Pittsburgh, Pittsburgh, PA; Andres Blei, M.D., Northwestern University, Chicago, I.L. (deceased); Daniel Ganger, M.D., Northwestern University, Chicago, IL; Atif Zaman, M.D., University of Oregon, Portland, OR; Steven H. B. Han, M.D., University of California, Los Angeles, CA; Robert Fontana, M.D., University of Michigan, Ann Arbor, MI; Brendan McGuire, M.D., University of Alabama, Birmingham, AL; Raymond T. Chung, M.D., Massachusetts General Hospital, Boston, MA; Alastair Smith, M.B., Ch.B., Duke University Medical Center, Durham, NC; Robert Brown, M.D., Cornell/Columbia University, New York, NY; Jeffrey Crippin, M.D., Washington University, St Louis, MO; Edwin Harrison, Mayo Clinic, Scottsdale, AZ; Adrian Reuben, M.B.B.S., Medical University of South Carolina, Charleston, SC; Santiago Munoz, M.D., Albert Einstein Medical Center, Philadelphia, PA; Rajender Reddy, M.D., University of Pennsylvania, Philadelphia, PA; R. Todd Stravitz, M.D., Virginia Commonwealth University, Richmond, VA; Lorenzo Rossaro, M.D., University of California Davis, Sacramento, CA; Raj Satyanarayana, M.D., Mayo Clinic, Jacksonville, FL; and Tarek Hassanein, M.D., University of California, San Diego, CA; Constantine J. Karvellas, M.D., University of Alberta, Edmonton, AB; Jodi Olson, M.D., University of Kansas, Kansas City, KA; Ram Subramanian, M.D., Emory, Atlanta, GA; James Hanje M.D., Ohio State University, Columbus, OH. The University of Texas Southwestern Administrative Group included Grace Samuel, Ezmina Lalani, Carla Pezzia and Corron Sanders, Ph.D., Nahid Attar, Linda S. Hynan, Ph.D. and the Medical University of South Carolina Data Coordination Unit included Valerie Durkalski, Ph.D., Wenle Zhao, Ph.D., Jaime Speiser, Catherine Dillon, Holly Battenhouse and Michelle Gottfried. As well, the authors would like to thank Drs. Guido Van Marle, Laura Sycuro and Trushar Patel (PhD supervisory committee members for NHP) for their guidance and feedback on the experimental and statistical approach and formal data analysis.
Ethics Statement
This study was approved by the institutional review boards or health research ethics boards of all US Acute Liver Failure Study Group enrolling sites.
Consent
All subjects or next of kin provided informed written consent to participate according to the 1975 Declaration of Helsinki guidelines.
Conflicts of Interest
Nishi H. Patel, Annie Y. Chen, Carla Osiowy, Valerie Durkalski Mauldin, William M. Lee declare no conflicts of interest. Constantine J. Karvellas: Consulting ad hoc for Baxter, Grifols, Morphocell. Carla S. Coffin: Advisory boards and Speaker Fees: Altimmune Pharmaceuticals, Janssen, Roche, GSK (paid to the University of Calgary). Investigator Initiated Grants: Gilead, GSK, Janssen (paid to the University of Calgary the Canadian HBV Network).
Open Research
Data Availability Statement
Deidentified individual will be shared at request.




