Confirmation of guideline-defined hepatitis C screening strategies within the ‘Check-Up35+’ examination in the primary care setting
Handling Editor: Alessio Aghemo
Abstract
Background and Aims
Screening strategies for undiagnosed infections are fundamental for hepatitis C virus (HCV) elimination. We previously investigated HCV prevalence and screening strategies in an urban primary care setting. IV drug abuse, blood transfusion before 1992, immigration, or elevated ALT were identified as risk factors in a post hoc analysis and diagnosed 83% of unknown HCV-RNA-positive cases by screening only 26% of the population.
We aimed to validate prospectively the proposed screening algorithm in two independent urban and rural cohorts and to analyse for potential differences.
Methods
Anti-HCV and ALT were included in a routine check-up together with a questionnaire covering risk factors. HCV-RNA was analysed in anti-HCV-positive individuals.
Results
In urban and rural areas, 4323 and 9321 individuals were recruited. The anti-HCV prevalence was 0.56% and 0.49%, and 0.1% of patients were HCV-RNA-positive in both regions. Fifty-two anti-HCV positive patients including eight HCV-RNA-positive cases were unaware of the infection (number needed to screen to detect one unknown anti-HCV-positive individual: 262). At least one of the three aforementioned risk factors or elevated serum ALT was present in 3000 patients (22%). Restricting HCV screening to only those with risk factors, 52% and 75% of all anti-HCV and HCV-RNA-positive undiagnosed patients were identified (number needed to screen: 111).
Conclusions
We confirm prospectively the efficiency of a risk-based HCV screening. The risk-based algorithm should be evaluated in other countries with similarly low HCV prevalence as in Germany to achieve WHO HCV elimination goals.
Key Points
Screening strategies for undiagnosed infections are fundamental for hepatitis C virus (HCV) elimination. We prospectively validated a screening algorithm in independent urban and rural cohorts in a country with low HCV prevalence (Germany). If population-based screening is not possible, a risk-based screening algorithm including the risk factors IV drug abuse, blood transfusion before 1992, immigration, or elevated ALT can evaluate 22% of the target population and still detect 75% of all HCV-RNA-positive undiagnosed patients.
1 INTRODUCTION
In 2016, the World Health Organization announced the strategy to eliminate chronic hepatitis C virus (HCV) infection by the year 2030.1 In order to do so, political will, financing a national programme, implementing monitoring of existing programmes, screening, awareness, and linkage to care have been identified as important key factors in high-income countries.2 In Germany, the political will to eradicate the infection has been defined by the BIS2030 strategy.3 After models had shown the importance of treatment uptake with modern direct antiviral agents to eliminate HCV infections,4 interferon-free antiviral therapies were widely implemented in clinical practice since the year 2014: In the following 5 years, approximately 34% of the 267 000 viremic HCV infections estimated at the end of 2013 were treated.5 German registry data report sustained virologic response rates of 97% and excellent tolerability of various treatment regimens.6 However, rates of screening did not keep pace with fewer than 32 000 infections notified to health care authorities in the same time interval. Among prevalent viremic infections in the year 2020, only an estimated 37% were diagnosed, leaving 63% HCV-RNA-positive individuals undiagnosed. Achieving the WHO targets by 2030 will therefore require increased screening efforts with 81 200 individuals newly diagnosed and 118 600 cases initiated on treatment between 2021 and 2030.5
In order to strengthen other screening approaches, we performed a clinical study at the primary care level in the metropolitan area of Dortmund/Gelsenkirchen (North Rhine Westfalia), which proved the efficacy of guideline-defined screening efforts within an established German healthcare programme named ‘Check-Up’.7 Considering these study results, healthcare authorities recently decided to include an anti-HCV screening routinely within this ‘Check-Up’ programme, which strengthens the importance of primary care physicians in the HCV elimination strategy and underlines the political commitment to HCV elimination programmes.8
Our initial project including about 21 000 patients proved that undiagnosed HCV infections frequently exist in the primary care setting and guideline-based screening strategies help to diagnose previously unknown infections: A screening strategy including the presence of at least one of three risk factors for HCV infection (IV drug use, blood transfusion before 1992, and ‘immigration’) or elevated ALT levels diagnosed 83% of unknown HCV-RNA-positive cases by screening only 26% of the population.7
However, ‘training data’ are known to overestimate the potential of risk factors, meaning that a validation cohort is needed to confirm the screening strategies of the initial ‘Check-Up’ project. Moreover, it is unclear if the screening approach performs comparatively well in a non-metropolitan area. Additionally, a re-evaluation of the ‘Check-Up’ screening strategy is important, because the WHO has defined indicators to monitor the progress in the HCV elimination efforts, which however are not systematically captured in Germany, leaving uncertainty whether the national programme is or is not ‘on track’ for HCV elimination by 2030.5
Therefore, we designed a study within the “Check-Up” to validate the risk factors of the first study and verify results regarding the prevalence of anti-HCV and unknown diseases. In addition to a study cohort in the metropolitan area of Hamburg, we included a second cohort in the rural part of Schleswig-Holstein to analyse potential differences between metropolitan and rural areas and to investigate whether screening strategies have to be adapted according to the area in which they will be applied.
2 PATIENTS AND METHODS
The ‘Check-Up 35+’ is a preventive medical examination for adults >35 years and is covered by German health care insurance. It is performed by primary care physicians and includes the patient's medical history, an evaluation of risk factors, a physical examination, a cholesterol and blood glucose test, a spot urine test, and medical counselling about the results. In the year 2018, 34% of 46 316 601 insured patients participated in the test.9
In the present study, the ‘Check-Up 35+’ was amended by central analysis of alanine and aspartate aminotransferase (ALT, AST; upper limit of normal 50 U/L for males, 35 U/L for females), gamma-glutamyltransferase (GGT; upper limit of normal 60 U/L for males, 40 U/L for females), and anti-HCV (Cobas, Roche Diagnostics). If anti-HCV was positive, HCV-RNA was analysed by PCR (Cobas Amplicor version 2.0, Roche Diagnostics, Mannheim, Germany, lower limit of detection 15 IU/ml).
- Do you have elevated liver enzymes which have not normalized and whose cause has not yet been established?
- Do you suffer from fatigue, impaired concentration, or upper abdominal pain?
- Are you a member of the medical profession?
- Have you received blood transfusions prior to the year 1992?
- Have you received any kind of organ transplantation?
- Have you had any surgery in the past? (question not covered by guidelines).
- Do you have a piercing?
- Do you have a tattoo?
- Have you ever consumed illicit drugs, if so intravenously, snorting, smoking?
- Do you suffer from an already known hepatitis C virus infection?
- Does your mother, your partner, or any household member have a hepatitis C virus infection?
- Have you been treated against hepatitis C in the past?
- Do you need haemodialysis for end-stage kidney disease?
- Have you or your parents immigrated from Asia, Africa, Eastern Europe, South America or the Mediterranean area?
- Do you often travel to Asia, Africa, Eastern Europe, South America or the Mediterranean area? (question not covered by guidelines).
Our previous study showed that questions 4, 9, and 14 were the most relevant risk factors and are referred to in the remainder of the paper as ‘transfusion’, ‘IVDU’ and ‘immigration’ for brevity. Question 1 is referred to as ‘reported elevated liver values’.
All patients provided written informed consent. The study was prospectively performed between November 2018 and September 2020 in Hamburg and Schleswig-Holstein, Germany. It was approved by the Ethics Committees of the University of Leipzig (ethics vote 361/17-ek) and of the medical association Hamburg (ethics vote MC-033/18) and Schleswig-Holstein (ethics vote 007/18 m). It was registered within the German Clinical Trial Register (DRKS) with the code DRKS00017705.
2.1 Study objectives
2.1.1 Primary objective
Anti-HCV prevalence overall and in Hamburg and Schleswig-Holstein
2.1.2 Secondary objectives
Association between anti-HCV and the risk factors IVDU, transfusion and immigration for ‘pooled’ data and urban/rural data separately.
Association between anti-HCV and further HCV risk factors for ‘pooled’ data and urban/rural data separately.
HCV-RNA prevalence in Hamburg and Schleswig-Holstein.
Prevalence of elevated ALT values in Hamburg and Schleswig-Holstein including age and sex dependence.
Difference in prevalence of HCV and elevated ALT values in Hamburg and Schleswig-Holstein.
2.2 Sample size and power
In order to have a 95% confidence interval narrower than 1.5% points for the estimate of anti-HCV prevalence in risk subgroups, we planned to enrol 10 000 patients in Hamburg. Based on the expectation that anti-HCV prevalence would be about half in the rural area compared to the urban one, we planned to enrol 20 000 patients in Schleswig-Holstein to have roughly the same number of anti-HCV-positive cases in each region. This would provide >99% power to detect the difference in the prevalence of 1.0% (urban) and 0.5% (rural). Due to accrual and funding issues, recruitment was stopped after about 4300 (urban) and 9300 (rural) patients had been included. As a result, the 95% CI will be about 1.5 times wider than planned and the power is 89% to detect the above difference.
2.3 Statistics
Proportions and their difference were based on observed frequencies where a Wilson score was used for the construction of confidence intervals. Validation of two previous risk models (model 1: IVDU, immigration, transfusion, model 2: IVDU, immigration, transfusion, elevated ALT) was performed with logistic regression. Identification of HCV risk factors used stepwise logistic regression optimizing Akaike's information criterion. The confidence intervals from these regression models were found using profiling and the p-values by inverting the interval. All analyses were carried out with the software R version 4.1.1.
3 RESULTS
3.1 Baseline characteristics of the study cohorts
Patients were recruited in 29 and 46 primary care private practices in Hamburg and Schleswig-Holstein, respectively. The median (interquartile range) of patients per clinic was 122 (23–291) and with a range from 1 to 1312 patients. The full data set included 13 644 patients and at least 10 questions of the study questionnaire on HCV risk scenarios were answered by 12 897 (95%) of them. The characteristics of the recruited patients are listed in Table 1.
| All patients | Patients who answered ≥10 questions | |||||||
|---|---|---|---|---|---|---|---|---|
| Hamburg | Schleswig-Holstein | Hamburg | Schleswig-Holstein | |||||
| Healthy (n = 4299) | Anti-HCV (n = 24) | Healthy (n = 9273) | Anti-HCV (n = 48) | Healthy (n = 4211) | Anti-HCV (n = 24) | Healthy (n = 8620) | Anti-HCV (n = 42) | |
| Age (years) | 52.6 ± 15.2 | 58.1 ± 14.7 | 56.6 ± 15.9 | 58.1 ± 16.2 | 52.6 ± 15.2 | 58.1 ± 14.7 | 56.7 ± 15.6 | 58.1 ± 16.3 |
| ≤ 40 | 1005 (23%) | 2 (8%) | 1532 (17%) | 5 (10%) | 991 (24%) | 2 (8%) | 1369 (16%) | 5 (12%) |
| 40–60 | 1997 (46%) | 11 (46%) | 3972 (43%) | 24 (50%) | 1957 (46%) | 11 (46%) | 3749 (43%) | 20 (48%) |
| >60 | 1297 (30%) | 11 (46%) | 3769 (41%) | 19 (40%) | 1263 (30%) | 11 (46%) | 3502 (41%) | 17 (40%) |
| Male | 1905 (44%) | 17 (71%) | 4019 (43%) | 22 (46%) | 1867 (44%) | 17 (71%) | 3707 (43%) | 20 (48%) |
| Elevated ALT | 437 (10%) | 5 (21%) | 858 (9%) | 5 (10%) | 426 (10%) | 5 (21%) | 802 (9%) | 5 (12%) |
| Elevated AST | 207 (5%) | 2 (8%) | 409 (4%) | 3 (6%) | 200 (5%) | 2 (8%) | 375 (4%) | 3 (7%) |
| Elevated GGT | 478 (11%) | 4 (17%) | 1143 (12%) | 5 (10%) | 463 (11%) | 4 (17%) | 1063 (12%) | 5 (12%) |
| Known elevated liver blood tests | 431 (10%) | 6 (25%) | 754 (8%) | 13 (27%) | 431 (10%) | 6 (25%) | 754 (9%) | 13 (31%) |
| Fatigue, impaired concentration, upper abdominal pain | 1311 (30%) | 6 (25%) | 2312 (25%) | 17 (35%) | 1311 (31%) | 6 (25%) | 2306 (27%) | 17 (40%) |
| Medical profession | 281 (7%) | 0 (0%) | 571 (6%) | 0 (0%) | 279 (7%) | 0 (0%) | 569 (7%) | 0 (0%) |
| Blood transfusion before 1992 | 194 (5%) | 2 (8%) | 466 (5%) | 6 (12%) | 194 (5%) | 2 (8%) | 466 (5%) | 6 (14%) |
| Organ transplantation | 43 (1%) | 0 (0%) | 83 (1%) | 0 (0%) | 43 (1%) | 0 (0%) | 82 (1%) | 0 (0%) |
| Operation | 2676 (62%) | 15 (62%) | 5769 (62%) | 29 (60%) | 2674 (64%) | 15 (62%) | 5764 (67%) | 29 (69%) |
| Piercing | 340 (8%) | 1 (4%) | 686 (7%) | 3 (6%) | 340 (8%) | 1 (4%) | 680 (8%) | 3 (7%) |
| Tattoo | 699 (16%) | 8 (33%) | 1300 (14%) | 16 (33%) | 699 (17%) | 8 (33%) | 1298 (15%) | 16 (38%) |
| IV drug users | 24 (1%) | 6 (25%) | 45 (0%) | 10 (21%) | 24 (1%) | 6 (25%) | 45 (1%) | 10 (24%) |
| Known HCV | 13 (0%) | 5 (21%) | 13 (0%) | 13 (27%) | 13 (0%) | 5 (21%) | 13 (0%) | 13 (31%) |
| Household member or family with HCV | 68 (2%) | 1 (4%) | 139 (1%) | 2 (4%) | 68 (2%) | 1 (4%) | 139 (2%) | 2 (5%) |
| HCV therapy in past | 8 (0%) | 4 (17%) | 32 (0%) | 18 (38%) | 8 (0%) | 4 (17%) | 32 (0%) | 18 (43%) |
| Serious kidney disease | 29 (1%) | 0 (0%) | 51 (1%) | 1 (2%) | 29 (1%) | 0 (0%) | 51 (1%) | 1 (2%) |
| You or parents from high prevalence region | 648 (15%) | 5 (21%) | 541 (6%) | 10 (21%) | 647 (15%) | 5 (21%) | 516 (6%) | 9 (21%) |
| Travel to high prevalence region | 932 (22%) | 6 (25%) | 1218 (13%) | 11 (23%) | 932 (22%) | 6 (25%) | 1194 (14%) | 10 (24%) |
- Note: The “high prevalence regions” for HCV are Asia, Africa, Eastern Europe, South America or the Mediterranean.
3.2 Anti-HCV prevalence according to the geographical area and HCV risk factors
The overall anti-HCV prevalence was 0.53% (95% CI: 0.42%–0.66%), 0.56% (95% CI: 0.37%–0.82%), and 0.51% (95% CI: 0.39%–0.68%) in the subgroups of the metropolitan area of Hamburg and the rural area of Schleswig-Holstein, respectively (Table 2). It increased to 1.89% (95% CI: 1.37%–2.61%) in patients with at least one of the three HCV risk factors IVDU, transfusion or immigration. If one of these risk factors was present or ALT was elevated, then the anti-HCV prevalence was 1.33% (95% CI: 0.98%–1.81%).
| Hamburg | Schleswig-Holstein | |||||
|---|---|---|---|---|---|---|
| All (n = 4323) | Risk 1 (n = 852) | Risk 2 (n = 1200) | All (n = 9321) | Risk 1 (n = 1049) | Risk 2 (n = 1800) | |
| Anti-HCV-positive | 24 (0.56%) | 12 (1.41%) | 14 (1.17%) | 48 (0.51%) | 24 (2.29%) | 26 (1.44%) |
| Anti-HCV-positive and unaware of HCV status | 19 (0.44%) | 10 (1.17%) | 12 (1.00%) | 35 (0.37%) | 15 (1.43%) | 16 (0.89%) |
| Anti-HCV-positive and RNA-positive | 4 (0.09%) | 3 (0.35%) | 3 (0.25%) | 7 (0.08%) | 4 (0.38%) | 6 (0.33%) |
| Anti-HCV-positive and RNA-positive and unaware of HCV status | 3 (0.07%) | 2 (0.23%) | 2 (0.17%) | 5 (0.05%) | 3 (0.29%) | 4 (0.22%) |
- Note: Risk 1: IV drug use (“IVDU”) or blood transfusion before 1992 (“transfusion”) or the patient or patient's parents immigrated from Asia, Africa, Eastern Europe or the Mediterranean (“immigration”). Risk 2: IVDU, transfusion, immigration or elevated ALT.
3.3 HCV-RNA prevalence according to the geographical area and HCV risk factors
HCV-RNA (PCR) was positive in 11 patients. In three individuals, the HCV infection was already known, whilst eight cases were previously undiagnosed. Characteristics of the HCV-RNA-positive patients are listed in Table 3.
| Individual | Age | Sex | ALT (ULN) | Known HCV | Transfusion | IVDU | Immigration |
|---|---|---|---|---|---|---|---|
| 1 | 63 | M | 1.52 | N | N | N | Y |
| 2 | 82 | M | 0.58 | N | Y | N | N |
| 3 | 86 | M | 0.62 | N | Y | N | N |
| 4 | 62 | F | 0.74 | N | N | Y | N |
| 5 | 63 | M | 0.76 | N | N | N | Y |
| 6 | 74 | F | 0.43 | N | N | N | N |
| 7 | 55 | M | 2.18 | N | N | N | N |
| 8 | 37 | F | 1.46 | Y | N | Y | N |
| 9 | 61 | F | 1.26 | Y | N | Y | N |
| 10 | 95 | F | 2.80 | Y | N | – | N |
| 11 | 81 | F | 0.63 | – | – | – | – |
The prevalence of anti-HCV-positive/HCV-RNA-positive patients with previously unknown HCV infection was 0.07% and 0.05% in Hamburg and Schleswig-Holstein respectively (Table 2).
Table 4 lists different screening strategies to identify previously unknown HCV infections. If the subgroup of patients with ‘IVDU or transfusion or immigration or elevated ALT’ comprising 3000/13 644 (22%) of the total cohort is considered, then 6/8 (75%) HCV-RNA-positive cases can be detected implying a number needed to screen of 500. In the total cohort, the number needed to screen is 1706 (Table 4).
| Number screened for anti-HCV | Anti-HCV-positive and unaware of infection | Anti-HCV prevalence (%) | HCV-RNA-positive | Number needed to screen to detect one anti-HCV-positive patient (n) | Number needed to screen to detect one HCV-RNA-positive patient (n) | |
|---|---|---|---|---|---|---|
| Total cohort | 13 644 | 52 (100%) | 0.38 | 8 (100%) | 262 | 1706 |
| At least one of IVDU, transfusion or immigration | 1901 | 25 (48%) | 1.32 | 5 (62%) | 76 | 380 |
| At least one of IVDU, transfusion, immigration or elevated ALT | 3000 | 28 (54%) | 0.93 | 6 (75%) | 107 | 500 |
3.4 Prevalence of elevated ALT, AST and GGT in Hamburg and Schleswig-Holstein
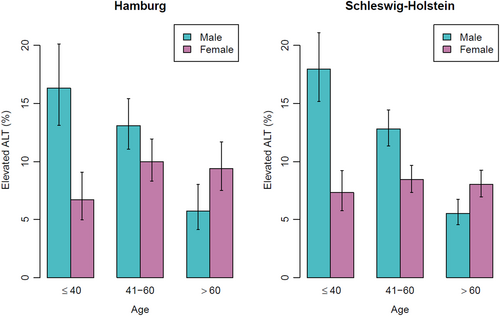
The prevalence of elevated ALT values in Hamburg and Schleswig-Holstein was 10.2% and 9.3% respectively. Age and sex dependence of elevated ALT values are depicted in Figure 1. The prevalence of elevated AST values in Hamburg and Schleswig-Holstein was 4.8% and 4.4%, respectively. For GGT the corresponding values were 11.1% and 12.3%.
3.5 Difference in prevalence of anti-HCV, HCV-RNA and elevated ALT values in Hamburg and Schleswig-Holstein
A significant difference in the prevalence of anti-HCV, HCV-RNA or elevated ALT values could not be detected in Hamburg compared to Schleswig-Holstein (Table 5).
| Hamburg (%) | Schleswig-Holstein (%) | Difference in percentage points (95% CI) | p-value | |
|---|---|---|---|---|
| Anti-HCV prevalence | 0.56 | 0.51 | 0.04 (−0.21 to 0.34) | .76 |
| HCV-RNA prevalence | 0.093 | 0.075 | 0.017 (−0.081 to 0.167) | .74 |
| Elevated ALT | 10.2 | 9.3 | 0.97 (−0.09 to 2.06) | .074 |
3.6 Odds ratios for risk factors for being anti-HCV-positive
The validation of the risk model (IVDU, immigration and transfusion) for anti-HCV used logistic regression to estimate the odds ratios 54 (95% CI: 28–98, p < .001), 2.6 (95% CI: 1.4–4.6, p = .0046) and 2.1 (95% CI: 0.8–4.4, p = .11), respectively. In the second risk model to be validated, the odds ratios were 53 (95% CI: 28–97, p < .001), 2.6 (95% CI: 1.4–4.6, p = .0047), 2.1 (95% CI: 0.8–4.4, p = .11) and 1.4 (95% CI: 0.6–2.6, p = .41) for IVDU, immigration, transfusion and elevated ALT, respectively. The odds ratio for rural versus urban settings was 1.1 (95% CI: 0.6–1.8, p = .82) in the first risk model.
A stepwise procedure was performed to determine the optimal risk model and estimate the odds ratio for being anti-HCV-positive. In the total study cohort, in Hamburg, and Schleswig-Holstein, IV drug use was by far the most relevant risk factor for HCV infection (Table 6).
| Odds ratio estimate | 95% CI | p-value | |
|---|---|---|---|
| All patients | |||
| Age | |||
| 41–60 vs. ≤40 | 2.0 | 0.9–5.0 | .14 |
| >60 vs. ≤40 | 3.2 | 1.4–8.4 | |
| Reported elevated liver values vs non-elevated | 2.9 | 1.6–5.0 | <.001 |
| Tattoo vs. no tattoo | 2.2 | 1.2–3.9 | .013 |
| IVDU vs. no IVDU | 38 | 19–75 | <.001 |
| Immigration vs. no immigration | 2.8 | 1.4–5.1 | .0032 |
| Hamburg | |||
| Age | |||
| 41–60 vs. ≤40 | 1.8 | 0.5–12.0 | .11 |
| >60 vs. ≤40 | 3.7 | 0.9–24 | |
| Reported elevated liver values vs. non-elevated | 2.7 | 0.9–6.7 | .068 |
| Sex (female vs. male) | 0.33 | 0.13–0.82 | .014 |
| IVDU vs. no IVDU | 54 | 17.8–149 | <.001 |
| Schleswig Holstein | |||
| Reported elevated liver values vs. non-elevated | 3.3 | 1.6–6.3 | .0020 |
| Transfusion vs. no transfusion | 2.6 | 0.9–6.2 | .069 |
| Tattoo vs. no tattoo | 2.0 | 1.0–3.8 | .057 |
| IVDU vs. no IVDU | 32 | 13–74 | <.001 |
| Immigration vs. no immigration | 3.3 | 1.4–6.7 | .0070 |
4 DISCUSSION
- The prevalence of HCV-RNA-positive patients is low and the number needed to screen correspondingly high.
- The screening strategies according to risk factors were confirmed in a second, large, prospectively recruited patient cohort at the primary care level.
- The results apply not only to another German metropolitan area but also to a rural region.
These results are important because the WHO recommends a re-evaluation of national efforts on the way to eliminating HCV infection by the year 2030: We prove that the anti-HCV screening within the ‘Check-Up 35+’ programme of primary care physicians is a milestone in the German HCV elimination initiative.
The screening strategy comprised the ‘presence of at least one of the risk factors IVDU, transfusion, immigration or elevated ALT values’, which identified 83% of previously unknown HCV-RNA-positive cases by screening 26% of the first ‘Check-Up 35+’ population, also holds true in the validation cohorts and detects 6/8 (75%) previously unknown replicative individuals by screening 22% of the population. Due to the declining anti-HCV prevalence between the two study periods from 0.95% to 0.53%, the number needed to screen to detect one HCV-RNA-positive individual increased from 139 to 500. IV drug abuse is unaltered by far the most relevant risk factor for being anti-HCV-positive. However, note that only 1/8 cases with previously unknown HCV-RNA replication reported IV drug abuse, which implies that primary care physicians should be aware of alternative risk factors and potential underreporting, which is often due to stigmatization concerning HCV. The odds ratio for transfusion was lower than in the first study (5.3 vs. 2.1) but comparable for immigration (2.4 vs. 2.6). An overestimate of odds ratios is not uncommon in “training data” for one thing and the proportion of patients with a blood transfusion before 1992 clearly dwindles with time. However, stepwise procedures to identify risk factors in the current data confirmed IVDU, immigration and in-part transfusion. Furthermore, they identified that patients' reporting of elevated liver values may provide indications of increased risk. In contrast to the risk scenarios, we note that the patients with neither risk factors nor elevated ALT comprise the majority of the cohort (78%) in which the number needed to screen is 5322 to find one HCV-RNA-positive patient.
- The anti-HCV screening in the primary care physician's “Check-Up 35+” is approved for patients at the age of 35 years or older, but not for younger ones. The United States expanded their screening efforts in the year 2020 to a one-time screening of all adults over 18 years, alongside a routine screening of pregnant women and those with a risk profile.11 This was cost-effective in cohorts with an anti-HCV prevalence of >0.07%,11, 12 far below the prevalence observed in this study, though the HCV-RNA prevalence should be taken into account regarding cost-effectiveness. However, the extension of the anti-HCV screening programme to individuals between 18–35 years was not granted by the initial German Federal Joint Committee's (G-BA) recommendation8 and remains a matter of debate. An argument against a universal one-time screening in adults over 18 years is that it may not be cost-effective, which is mainly driven by the costs of the direct antiviral agents. It should be noted that the two fixed-dose combination regimens used most frequently in Germany, sofosbuvir/velpatasvir and gelaprevir/pibrentasvir, will go off-patent in the years 2026 and 2027, which will probably lead to a drop in prices.
- The preventive ‘Check-Up’ programme in Germany was amended in the year 2019: In addition to the ‘Check-Up 35+’, which can be performed every 3 years and can be combined once with the hepatitis screening, a second Check-Up programme has been established for adults between 18–34 years of age (‘Check-Up 18’).13 It covers medical counselling by the primary care physician about (amongst others) risk factors for cardiovascular diseases, chronic kidney diseases and diabetes mellitus, a blood sample for cholesterol and glucose in serum if risk factors are present, and can be performed once in this age span. The results of our previous7 and the current study have successfully proved that hepatitis screening can be established within existing preventive medical strategies and does not need additional programmes. Thus, the discussion should be re-opened as to whether the hepatitis screening should be implemented within the ‘Check-Up 18’ programme, and not only within the ‘Check-Up 35+’. This would strengthen a primary care-based hepatitis screening independent of the patients' self-reported risk status. This decision will be a matter of political will as mentioned above in the introduction.2
- It has to be acknowledged that people with lower socio-economic status and therefore potential high-risk groups like people with IV drug abuse are less likely to participate in the ‘Check-Up’ programme. Participation rates are associated with available income and professional status.14 Risk groups can be screened by point-of-care assays, which deliver HCV-RNA results on-site within a short period of time. For example, the Xpert HCV-RNA viral load fingerstick assay has been evaluated in drug and alcohol clinics, homelessness services, needle and syringe exchange programmes in people who inject drugs, prisoners, and also in the primary care setting.15-18 In combination with special cooperation between hepatology experts and directly observed therapy in opioid substitution programmes, high rates of sustained virologic response can be achieved with modern DAA treatment regimens.19, 20
- To overcome the observation, that only 34% of eligible people participate in the ‘Check-Up’ programme, primary care physicians could selectively inform patients with low economic status, that the ‘Check-Up’ examination is free of charge. In addition, automatic recall tools in the management software could improve motivation to participate in preventive examinations. However, inequality between different socio-economic communities and resulting disadvantages in medical care have to be addressed by political initiatives and cannot be solved by primary care physicians.14 The aspect of linkage to care after identification of HCV-RNA-positive patients should not only be strengthened in individuals at risk for non-adherence to DAA therapy but also in the ‘Check-Up’ programme because follow-up data of our screening programmes showed that subsequent diagnostics and initiation of antiviral therapy were very poor in newly diagnosed HCV infected patients.21, 22 It should be noted that primary care physicians are allowed to prescribe DAA therapies and do not necessarily refer the patient to secondary specialized care.5
- The low HCV-RNA prevalence means that analyses cannot be performed to identify risk factors for being HCV-RNA-positive.
- Primary care private practices and their patients may not represent the average in Hamburg and Schleswig-Holstein. This limitation may to some extent be explained by the phenomenon that the recruitment period was partially overlapping with the Covid-19 pandemic in Germany. The Covid-19 pandemic had an impact on in-person outpatient visits in the primary care setting of −61% and also affected screening procedures.23 Primary care physicians were occupied by major changes in their workflow which limited resources for participation in our study group.24
- Study participation rates of patients could not be analysed.
- Funding issues prohibited us from reaching the intended sample size. However, the cohort is still large and the questionnaire was filled out by the vast majority of patients.
- Risk factors in the questionnaire were self-reported by the patients. This may lead to underreporting.
- Immigration was reported by 15.1% and 5.9% of individuals in Hamburg and Schleswig-Holstein and was, therefore, lower compared to 35.5%–36.7% of individuals with migration background in Hamburg in the years 2018–2020 and 17.2% in Schleswig-Holstein in the year 2019 as reported by the Federal Statistical Offices of these regions,25, 26 though different definitions of ‘immigration’ and ‘migration background’ make a direct comparison difficult. Moreover, we cannot distinguish between a non-representative population and mere underreporting.
In conclusion, our second independent screening prospectively verifies the main results of the first study and is an important re-evaluation of primary care-based screening. The decision of health care authorities to include an anti-HCV screening within the ‘Check-Up’ programme is a milestone toward eliminating hepatitis C in Germany during the next years. However, continuous efforts must be undertaken to further improve the national HCV elimination strategy.
In addition, the proposed risk-based algorithm should be evaluated in other countries with similarly low HCV prevalence as in Germany to achieve WHO HCV elimination goals.
ACKNOWLEDGMENT
Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
The study was funded by unrestricted research grants of Abbvie, Gilead Sciences, and MSD to T.B.
CONFLICT OF INTEREST
Nothing to disclose: DP, IW, KJ, and JK. OB: Lecturer fees from Abbvie. TB: Grants/research supports: Abbvie, BMS, Gilead, MSD/Merck, Humedics, Intercept, Merz, Norgine, Novartis, Orphalan, Sequana Medical; honoraria or consultation fees/advisory board: Abbvie, Alexion, Bayer, Gilead, GSK, Eisai, Enyo Pharma, Falk Foundation, HepaRegeniX GmbH, Humedics, Intercept, Ipsen, Janssen, MSD/Merck, Novartis, Orphalan, Roche, Sequana Medical, SIRTEX, SOBI and Shionogi; company sponsored speaker's bureau: Abbvie, Alexion, Bayer, Gilead, Eisai, Intercept, Ipsen, Janssen, MedUpdate GmbH, MSD/Merck, Novartis, Orphalan, Sequana Medica, SIRTEX and SOBI. JW: Travel grants from Abbvie and Gilead Sciences.
ETHICS APPROVAL AND PATIENT CONSENT STATEMENT
All patients provided written informed consent. The study was approved by the Ethics Committees of the University of Leipzig (ethics vote 361/17-ek) and of the medical association Hamburg (ethics vote MC-033/18) and Schleswig-Holstein (ethics vote 007/18 m).
CLINICAL TRIAL NUMBER
The study was registered within the German Clinical Trial Register (DRKS) with the code DRKS00017705.