International pediatric thrombosis network to advance pediatric thrombosis research: Communication from the ISTH SSC subcommittee on pediatric and neonatal thrombosis and hemostasis
Manuscript handled by: Joost Meijers
Final decision: Joost Meijers, 01 February 2021
Abstract
Pediatric thromboembolism is a rare and heterogenous disease. As a result, there is a paucity of knowledge with regard to natural history, management, and outcomes of most types of pediatric venous and arterial thromboembolism. International research collaboration is needed to fill these knowledge gaps. Not only randomized controlled trials, but also representative observational studies are required to answer all research questions. Therefore, the ISTH SSC Subcommittee on Pediatric and Neonatal Thrombosis and Hemostasis initiated the International Pediatric Thrombosis Network (IPTN). The aims of the IPTN include (1) development of the Throm-PED registry to facilitate international prospective observational studies, and (2) establishment of a network of pediatric thrombosis centers experienced in effectively conducting clinical trials and observational studies. The IPTN needs dedicated clinicians all over the world and several funding sources to obtain high-quality research data to reach its ultimate goal of improving care in children with thrombosis. The aim of this communication is to call for active participation in the IPTN to all physicians taking care of children with thrombosis worldwide.
1 INTRODUCTION AND RATIONALE
The overall incidence of thrombosis in children is very low. In adults, the overall incidence in the general population is 1 to 2 per 1000 person-years.1 In children, a recent Danish nationwide pediatric population study reported incidences of 2.1 and 0.3 per 100,000 person years for venous and noncerebral arterial thrombosis, respectively.2 However, thromboembolism (TE) is increasingly diagnosed in hospitalized children, as result of the increased number of children with underlying medical conditions and hypercoagulability, medical and technologic developments, and heightened awareness. The frequency of hospital-acquired pediatric venous TE increased from 5.3 events per 10,000 pediatric hospital admissions in the 1990s to approximately 58 events in the early 2000s.3, 4 Morbidity of venous or arterial TE in children can be substantial. The recurrence rate following a venous TE is reported to be between 2% and 21%, depending on the study type and population studied; and post-thrombotic syndrome develops in approximately 30% of the patients.5-7 Hospital-acquired venous TE in children is associated with an increased length of stay and costs.8 Morbidity after arterial TE is less studied, but consists of limb or organ necrosis, reperfusion injury, hypertension, and leg growth retardation.9 Mortality due to venous TE varies between 0 and 2.2%.10
Optimal management and prevention strategies are crucial to decrease the occurrence of these potentially life-threatening complications in children. Unfortunately, in the last decades little progress has been made in generating evidence to substantiate these strategies. Due to the rarity and heterogeneity of pediatric TEs, international research collaboration is indispensable to take a step forward in creating evidence-based management and prevention guidelines. Therefore, the ISTH SSC Subcommittee on Pediatric and Neonatal Thrombosis and Hemostasis has initiated the International Pediatric Thrombosis Network (IPTN). The IPTN aims (1) to enable large, prospective international observational studies using the Throm-PED registry, a prospective international disease-based registry, and (2) to provide a framework for a network of pediatric thrombosis centers with clinical trial experience and infrastructure to efficiently conduct large trials. The goal of this SSC communication is a call for dedicated participation in the IPTN to all physicians taking care of children with thrombosis worldwide. The successful outcome of the IPTN, and as a consequence of international research collaboration in the field of pediatric thrombosis, depends on active collaboration of many centers from all continents.
2 PEDIATRIC THROMBOSIS: A GROUP OF RARE DISEASES
Pediatric thrombosis is not only a rare, but also a heterogeneous disease. The incidence of venous TE peaks in early infancy and adolescence with about 20% and 50% of venous thromboembolism (VTE) events occurring in children 0 to 1 and 11 to 18 years of age, respectively.3, 11 More than 90% of neonatal thrombi are catheter-related, whereas in adolescence 50% of thrombi are associated with indwelling catheters. Incidences and types of thrombotic risk factors in adolescents differ widely depending on the study design and studied patient population, but include surgery, immobility, cardiac disease, thrombophilia, sepsis, malignancy, surgery, and combined oral contraceptive pills.11-13 Thrombosis locations vary accordingly: most neonatal thrombi are located in the portal venous hepatic system, right atrium, and renal or caval vein, while the majority of the adolescent thrombi are located in the lower and upper extremities. In children between 2 and 12 years of age, almost 50% of venous TE are located in the cerebral sinus, as a complication of mastoiditis, asparaginase therapy for treatment of acute lymphoblastic leukemia, or trauma.6, 14
While arterial thrombosis in adults usually results from atherosclerotic disease, pediatric non-cerebral arterial thrombosis mainly develops as result of indwelling catheters or cardiac catheterization in neonates and young infants.15 Arterial ischemic stroke (AIS) occurs in both neonates and children with an estimated incidence between 1 per 1600 to 5000 live births, and about 1.2 to 7.9 per 100,000 children per year, respectively.16 Risk factors for neonatal AIS include maternal, antenatal, intrapartum, and postnatal factors, such as maternal smoking, male sex, low Apgar scores, and sepsis. Pediatric AIS has a heterogeneous etiology as well, including arteriopathy, sickle cell disease, congenital heart disease, coagulopathy, and metabolic disorders.17
Each type of pediatric venous or arterial TE, such as neonatal renal vein thrombosis, catheter-related arterial thrombosis, or estrogen-associated pulmonary embolism (PE), has its own acute and long-term complications. Thus, studies of renal vein thrombosis should include renal function and hypertension, and studies of arterial thrombosis should include systemic blood pressure, limb or organ necrosis, and extremity length and circumference.15, 18 In addition, the risk–benefit ratio of anticoagulant treatment varies according to thrombosis location and patient age. For example, due to increased risk of intraventricular hemorrhage the bleeding risk of anticoagulation in preterm neonates with sinovenous thrombosis is probably higher than that in adolescents with estrogen-associated deep vein thrombosis.
Hence, compared to adults, pediatric thromboembolic events are rare and differ significantly in epidemiology. They are heterogeneous with regard to patient age, comorbidity, risk factors, and anatomical location and have their unique pathophysiology, and short- and long-term consequences.19 One could consider each type of pediatric thromboembolic event an individual rare disease.19
3 RESEARCH IN PEDIATRIC THROMBOSIS
As result of the rarity and heterogeneity of pediatric TE, high-quality evidence for the management of most types of pediatric TE is not available, as reflected in the recent American Society of Hematology (ASH) 2018 guideline for treatment of pediatric venous thromboembolism.20 Almost all recommendations are based on scarce and low-level pediatric evidence and are mostly extrapolated from adult guidelines. Until recently, knowledge about the epidemiology and management of pediatric TE has mainly been based on national registries, case-series, and retrospective or prospective cohort studies. Most of the hitherto key anticoagulants, including unfractionated heparin, low molecular weight heparin (LMWH), and vitamin K antagonists, are not approved by regulatory agencies for use in children due to lack of randomized clinical trials (RCTs). The only US Food and Drug Administration (FDA)-approved anticoagulant for use in children >1 month of age is the LMWH dalteparin. FDA approval was based on evidence from two single-arm multi-center clinical trials conducted in a total of 52 pediatric patients—including one industry-sponsored registrational trial and one investigator-initiated trial.21, 22
Performing RCTs on anticoagulants in children has proven to be very challenging. The low incidence of pediatric TE has been an important hurdle. Furthermore, in contrast to adults, TE predominantly occurs in sick children, and consequently, many affected patients are excluded from studies. Moreover, parents are reluctant to give consent, particularly when their children are young and very sick. Thus, pediatric anticoagulation RCTs require multiple centers around the world to include sufficient numbers of children. This is reflected in the industry-sponsored pivotal trials on direct oral anticoagulants (DOAC), which are currently ongoing or have just been completed.6, 23-27 For example, the rivaroxaban phase 3 study required 107 pediatric hospitals in 28 countries to recruit 500 patients.6 Still, these trials are not fully powered to demonstrate safety and efficacy and build upon extrapolation from adult data. Furthermore, patients enrolled in these interventional studies are not necessarily representative of the clinical heterogeneity of all children with TE.6, 23
Although these DOAC trials are generating much valuable information about pharmacokinetics of the new drugs, epidemiology, and management of pediatric venous TE, many important knowledge gaps in pediatric thrombosis remain.20 In the ASH Pediatric VTE treatment guidelines of 2018, several research questions for various types of pediatric TE have been identified20 (Table 1). Unfortunately, due to the rarity of all those specific types of pediatric TE, RCTs will not be able to answer most of these identified research questions. It will be important to take other approaches to generate pediatric evidence, for example by representative multicenter longitudinal observational studies. As the number of patients per center is limited, international collaboration is essential to enable high-quality research to improve the evidence on the natural history and the appropriate management of all types of pediatric thrombosis.
| Topic | Key questions |
|---|---|
| Natural history |
|
| Diagnosis |
|
| Treatment |
|
| Outcome |
|
- Abbreviations: ATE, arterial thromboembolic event; DVT, deep vein thrombosis; PE, pulmonary embolism; SVT, sinovenous thrombosis; VTE, venous thromboembolic event
4 INTERNATIONAL RESEARCH COLLABORATION
To empower international research collaboration in pediatric thrombosis, the IPTN was initiated at the ISTH SSC Subcommittee on Pediatric and Neonatal Thrombosis and Hemostasis meeting in 2017.28 The ultimate mission of the IPTN is to improve the quality of care of pediatric patients with thrombosis worldwide. Any center that takes care of children with thrombosis may become a member of the IPTN. At this moment, IPTN has 74 member centers from 27 countries. The first step of the IPTN to initiate international research collaboration was the establishment of the Throm-PED registry, a prospective disease-based registry, which uses REDCap provided by the ISTH. Although RCTs are the preferred study design to address research questions about the benefits or harms of interventions, many disease registries, which can gather clinical data in larger, more heterogeneous populations than clinical trials, have shown to be invaluable sources of information, especially in rare diseases.29, 30 Data provided by disease registries are used for the development of clinical research and trials, and the improvement of clinical care and health-care management. For example, RIETE (Registro Informatizado Enfermedad TromboEmbólica), which is an ongoing, multicenter prospective registry of adult patients with acute VTE, has shown to be of value in providing a better understanding of the epidemiology, common treatment patterns, and outcomes of subgroups that are underrepresented in clinical adult trials.31, 32 Even regulatory authorities such as the US FDA and the European Medicines Agency are increasingly using registries for medication regulation.33, 34
To further stimulate international research collaboration in pediatric thrombosis, the IPTN will serve as a platform for the Throm-PED Clinical Trial Network, a network of pediatric thrombosis centers with clinical trial experience and infrastructure to effectively conduct both investigator-initiated academic trials and industry-sponsored regulatory trials in pediatric thrombosis. Pediatric experts of this network will contribute to the design of the trials by developing clinically relevant and feasible study protocols, assess the feasibility of running these trials among centers of the network, act as contact for industry to roll out these trials in the network, and collaborate with patient representatives to address patient needs.
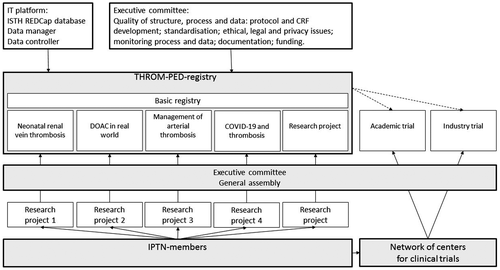
The current structure of the IPTN is shown in Figure 1. The executive committee of the IPTN consists of pediatric thrombosis experts from various continents.28 Each member of the IPTN collects data for the general Throm-PED registry and can propose substudies using the Throm-PED registry. These proposals are discussed in the general assembly meeting, which takes place at least once a year during the ISTH congress. Each approved substudy has its own scientific board, which is responsible for the design and execution of the substudy. At present, the IPTN data management team consists of data managers and controllers from the Erasmus Medical Center in Rotterdam, Royal Children's Hospital in Melbourne, and ISTH. With funding, the IPTN will be able to make use of a Data Coordinating Center that will help with study and database design, development of statistical analysis plans, data management including monitoring and quality checks, and all data analyses.
5 THROM-PED REGISTRY
The multinational Throm-PED registry will offer unique research opportunities. By systemically and prospectively collecting patient data, the Throm-PED registry can be used for many purposes, including understanding the natural history of certain types of pediatric TE; identifying risk factors and groups at high risk for TE; understanding diagnostic methods; and monitoring clinical effectiveness, safety, and cost effectiveness of anticoagulant drugs in all types of TE.29, 35 Moreover, the Throm-PED registry will provide an important complement to the data of the new drug trials, as they are able to evaluate the effects of interventions in real-world settings and may collect additional effectiveness data, including patient-reported outcomes, for example, quality of life assessments.36 In addition, it may be a powerful tool to evaluate long-term clinical outcomes, especially in rare types of TE, and effects on growth and development. Furthermore, the international character of the Throm-PED registry will enable examination of geographic variations in disease etiology and treatment patterns and provide country-specific information on new drugs.37
The basic Throm-PED registry collects data about patients with venous and arterial thrombosis, including age, gender, location, risk factors, treatment, and short-term outcome. By January 2021, more than 400 subjects have been included. Current substudies focus on the management of neonatal renal vein thrombosis, the epidemiology of pediatric thrombosis in children with COVID-19 infection, management of arterial catheter-related thrombosis, and real-world efficacy and safety of DOACs in children. For the DOAC project, IPTN has joined forces with the pediatric subgroup of Venous thromboEmbolism Network US (VENUS), the United States member of the International Network of Venous Thromboembolism Clinical Research Networks (INVENT).
6 CALL FOR PARTICIPATION IN IPTN
Overall, the success of disease registries is dependent on three quality criteria: quality of structure, quality of process, and quality of data.36 The quality of structure needs to be guaranteed by a dedicated executive committee, which takes care of a professional structure of the Throm-PED registry, including the availability of information technology personnel, database managers, and experts in epidemiology and biostatistics; the overall protocol and case report form of the basic Throm-PED registry; all regulations such as data and privacy protection in cooperation with legal departments; and approvals of the research ethical boards of all participating centers. To increase the quality of data collection standardization is essential, which requires defined research protocols. A monitoring plan and regular audits are needed; not only to address deficiencies in standardization, but also to increase the quality of data by identifying outlying data and following-up on missing data. Abovementioned conditions for a successful disease registry require sufficient resources and funding to ensure long-term sustainability. In addition, development and operation of the Throm-PED registry involves long-term commitment to realize its full benefits. As a consequence, the success of the IPTN is largely dependent on the willingness, dedication, and perseverance of participating centers to contribute data to the registry, to initiate and lead new IPTN study projects, and to participate in investigator-initiated academic trials and/or industry-sponsored regulatory trials. To succeed in obtaining high-quality research data to improve care of children with thrombosis, the IPTN needs many dedicated clinicians all over the world, and multiple funding sources.
7 CONCLUSIONS
The IPTN has been initiated to enable international research collaboration, which is crucial to increase our knowledge about natural history, treatment, and outcomes of various types of pediatric TE. Filling the knowledge gaps will improve the care for all neonates and children with thrombosis. The IPTN can only succeed through the dedication and hard work of all participating members to contribute data to the Throm-PED registry, and to develop, lead, or contribute to new IPTN projects. Only together can we advance the field of pediatric thrombosis.
CONFLICTS OF INTEREST
We have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
C.H. van Ommen wrote the manuscript. All authors critically edited the manuscript and approved the final version.