Plasminogen-induced foam cell formation by macrophages occurs through a histone 2B (H2B)-PAR1 pathway and requires integrity of clathrin-coated pits
Manuscript handled by: Patricia Liaw
Final decision: Patricia Liaw, 12 January 2021
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding information
This research was supported in part by grant 5P01HL0764915 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD. This work used the Leica SP8 confocal microscope purchased with funding from National Institutes of Health SIG grant 1S10OD019972-01.
Abstract
Objective
Plasminogen/plasmin is a serine protease system primarily responsible for degrading fibrin within blood clots. Plasminogen mediates its functions by interacting with plasminogen receptors on the cell surface. H2B, one such plasminogen receptor, is found on the surface of several cell types including macrophages. Both basic and clinical studies support the role of plasminogen in the process of foam cell formation (FCF), a hallmark of atherosclerosis. Growing evidence also implicates serine protease-activated receptors (PARs) in atherosclerosis. These receptors are also found on macrophages, and plasmin is capable of activating PAR1 and PAR4. The goal of this study was to determine the extent of H2B's contribution to plasminogen-mediated FCF by macrophages and if PARs are involved in this process.
Approach and Results
Treating macrophages with plasminogen increases their oxidized low-density lipoprotein uptake and plasminogen-mediated foam cell formation (Plg-FCF) significantly. The magnitude of Plg-FCF correlates with cell-surface expression of the H2B level. H2B blockade or downregulation reduces Plg-FCF, whereas its overexpression or high endogenous levels increases Plg-FCF. Modulating PAR1 level in mouse macrophages affects Plg-FCF. Activation/overexpression of PAR1 increases and its blockade/knockdown reduces this response. Confocal imaging indicates that both H2B and PAR1 colocalize with clathrin coated pits on the surface of macrophages, and reducing expression of clathrin or interfering with the clathrin-coated pits integrity reduces Plg-FCF.
Conclusion
Our data indicate that the magnitude of Plg-FCF by macrophages is proportional to the H2B levels and demonstrate for the first time that PAR1 is involved in this process and that the integrity of clathrin-coated pits is required for the full effect of Plg-induced FCF.
Essentials
- Plasminogen is implicated in atherosclerosis, but the mechanism of its involvement is unclear.
- Factors which alter plasminogen-dependent oxLDL uptake by macrophage-like cells were assessed.
- Plasminogen receptor H2B and PAR1 influenced oxLDL uptake into clathrin-coated pits.
- An H2B and PAR1 pathway within clathrin-coated pits mediates lipid uptake by macrophages.
1 INTRODUCTION
Plasminogen (Plg), a 92-kDa glycoprotein synthesized primarily by the liver, circulates in the blood at 1 to 2 μmol/ml.1 Plg can be activated to plasmin, a broad-spectrum serine protease by urokinase plasminogen activator and tissue plasminogen activator, and this transformation is facilitated by interaction of Plg with COOH-terminal lysines that are present in extracellular matrix proteins and on cell surfaces. Plg-receptors (Plg-Rs) are broadly distributed, are heterogeneous, and frequently interact with the lysine binding sites associated with the kringle domains of Plg and plasmin. Plg-Rs not only facilitate the conversion of Plg to plasmin but also prolong plasmin activity by sheltering it from inhibitors.2-7 Several of the identified Plg-Rs are atypical membrane proteins because they lack signal sequences and transmembrane domains and are often more widely known for their intracellular functions.5, 8-10 H2B falls into this category of Plg-Rs: it has a COOH-terminal lysine and it is most often considered to be a nuclear protein but has been found on the surface of several cell types, including lymphoid cells and macrophages.6, 11, 12 Using antibodies to H2B that block its binding to Plg, we demonstrated that H2B made a substantial contribution to the Plg-binding capacity to monocytoid cells and exerted a profound effect on recruitment of macrophages in a peritoneal inflammation model in mice.11, 12
Plg and Plg-Rs have been implicated in a variety of physiological and pathological processes, including wound healing, inflammatory cell recruitment, growth factor, and hormone processing.13-16 Evidence supports a central role of Plg in atherosclerosis17, 18 based on mouse models of atherosclerotic lesion development,19 effects of Plg on inflammatory responses,20 an underpinning of atherosclerotic lesion development, and on studies correlating atherosclerotic disease with Plg in humans.21, 22 Numerous in vitro studies demonstrating a role of Plg in cell migration are consistent with its influence on atherosclerotic lesion development. Recent studies have directly implicated Plg in foam cell formation (FCF), a hallmark process in atherosclerosis23-25 in which oxidized phospholipids accumulate within cells in developing atherosclerotic lesions. Plg and Plg-Rs have been shown to influence FCF by enhancing cholesterol uptake and/or efflux from monocytic cells, and an antibody to H2B diminished Plg-dependent oxidized low-density lipoprotein (ox-LDL) uptake.24-26
How H2B, which lacks a transmembrane domain, could affect not only ox-LDL uptake but also other atherogenic processes ranging from gene expression to arachidonic acid metabolism, was enigmatic. Knowing that the enzymatic activity of plasmin was involved in Plg-dependent FCF24 led us to consider the role of protease-activated receptors (PARs) in Plg-dependent FCF. The PAR family, PAR1, PAR2, PAR3, and PAR4, are seven-transmembrane G-protein–coupled receptors.27-31 Myeloid cells are reported to differentially express these receptors.32 PARs are activated by serine protease cleavage in their NH2-terminal region to expose a tethered ligand, which then binds intramolecularly to the receptor and triggers downstream signaling across the transmembrane helices.33 In addition to the well-established PAR-activating proteases, such as thrombin and other coagulation proteases, plasmin is reported to activate PAR1 and PAR4.34, 35 For example, plasmin activates human glial cells and recruit monocytes through the activation of PAR1.35, 36 In addition, plasmin can “desensitize” PAR1 through cleavages that delete the tethered ligand segment.37 On the basis of these observations, the present study has been focused on the relationship between H2B and PARs and their roles in mediating Plg-dependent lipid uptake by monocytoid cells.
2 MATERIALS AND METHODS
2.1 Materials
Penicillin, streptomycin, bovine serum albumin (Millipore-Sigma, Burlington, MA), FSLLRY,
RWJ65110, ML354, TFLLR-NH2 (Tocris, Minneapolis, MN), plasminogen (Glu-Plg), thrombin, and M-CSF were from Hematologic Technologies Inc. VT. Dil stain (1,1′-dioctadecyl-3,3,3′,3′tetramethylindocarbocyanine perchlorate [DiI]; DiIC18(3) was from Thermofisher, Waltham, MA, and Nutridoma from Roche, Basel, Switzerland. Active and inactive human α-thrombin were obtained from Enzyme Research Laboratories (South Bend, IN). Anti-sodium potassium ATPase polyclonal antibodies (ab167390) and anti-histone H3 polyclonal antibodies (ab21357) were from Abcam (Cambridge, MA, USA); caveolin-1 (D46G3) rabbit monoclonal antibody was from Cell Signaling Technology (Danvers, MA, USA). PAR1+/+ and PAR1−/− mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA).
2.2 Cell culture
J774A.1, RAW264.7 cells, murine macrophage-like cell lines, were obtained from ATCC and maintained in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum, 4 mM l-glutamine, 1.5 μg/L sodium bicarbonate, 4.5 g/L glucose, and 1 mM sodium pyruvate and 100 mg/ml penicillin/streptomycin.
Cells were incubated at 37°C in a humidified atmosphere of 5% CO2 and 95% air and grown to 70% to 80% confluence. For lipid uptake experiments, the cells were switched to DMEM containing 1% Nutridoma and the indicated treatment in presence of 50 μg/ml ox-LDL for 18 h before quantifying their lipid uptake. For selected experiments, peritoneal-derived macrophages were isolated from mice 72 h after thioglycolate injection and cultured as described for the cell lines.
2.3 Plasminogen activation
Macrophages have been reported to activate plasminogen into plasmin.38 To confirm this observation, cells were cultured to confluency washed them with phosphate buffered saline (PBS), then incubated with serum-free media containing 0.1% bovine serum albumin (BSA) supplemented or not with plasminogen (200 nM) for 30 min. After that, 200 μM S-2251, a chromogenic substrate for plasmin, was added and the incubation continued for another 1 h. The plasmin in the media from these treatments was determined by measuring the absorbance at 405 nm.
2.4 Oil Red O staining
Cells were incubated with media containing or not 50 μg/ml human ox-LDL in the presence or absence of 200 nM plasminogen for 24 h. To assess the cell foam formation, macrophages on slides were fixed with 4% paraformaldehyde, stained with 0.5% Oil Red O, and mounted using DAP-containing mounting solution. Confocal images were obtained using a Leica microscopy system.
2.5 Complementary DNA construct expression
Mouse wild-type H2B (H2B-WT) or H2B lacking the C-terminal lysine (H2B-∆C) were cloned into a pEGFP-C2 vector (Takara, Mountain View, CA). Human PAR1 complementary DNA (cDNA) construct was cloned and characterized as previously described.39 Cells were nucleofected with the indicated constructs using a nucleofection Kit (VCA-1003) obtained from Lonza, Basel, Switzerland, according to the manufacturer's protocol. The cells were cultured in serum-containing media for 24 h before use in experiments. Stable cell lines expressing H2B-WT or H2B∆C were established using 500 µg/ml G418 for selection.
2.6 Subcellular fractionation
Cells were harvested and lysed in cold hypotonic lysis buffer (10 mM Tris-HCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 10 mM sodium fluoride, 200 mM sucrose, pH 7.4, plus an EDTA-free protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN) for 20 min at 4°C followed by 10 strokes in a Teflon-glass homogenizer. The homogenate was centrifuged for 10 min at 1000g at 4°C. The supernatant was then centrifuged for 1 h at 100,000g at 4°C to yield a top fraction enriched in cytosolic proteins and a bottom fraction containing plasma membrane proteins. These fractions were collected and used as described in the following section.
2.7 LDL labeling
LDL, kindly provided by Dr. Richard Morton (Cleveland Clinic, Cleveland, OH), was isolated from fresh plasma of healthy donors by sequential ultra-centrifugation as described previously.40, 41 LDL was labeled with fluorescent probe (Dil stain) using a modified procedure.42 Briefly, a stock solution of DiI was prepared by dissolving 3 mg of DiI in 1 ml dimethyl sulfoxide and then added to the LDL solution to yield a final ratio of 150 μg DiI/mg LDL protein. After incubation in the dark for 18 h at 37°C, the DiI-labeled LDL (DiI-LDL) was oxidized by incubation for 24 h at 37°C with 5 μM CuCl2. The final preparation was dialyzed against saline solution containing 0.01% EDTA for 48 h, then filtered (0.45 μm), and the protein content was determined.
2.8 Ox-LDL uptake by macrophages
Cells cultured to confluency in serum-containing media were washed three times with PBS and then pretreated with media containing 1% Nutridoma and the indicated compound for 30 min. The medium was removed and serum-free media containing 50 μg/ml of Dil-ox-LDL, supplemented or not with 200 nM Plg and incubated for 18 h at 37°C. The cells were washed three times with cold PBS and fixed with 1% paraformaldehyde. Samples were analyzed by flow cytometry using a FACS Calibur instrument (BD Biosciences, San Diego, CA) and in some cases with SpectraMax ID5 plate reader (Molecular Devices, San Jose, CA).
2.9 Effect of H2B antibody on Plg-FCF by J77A.1 macrophage-like cells
J774A.1 cells were grown in complete media to confluency and then incubated with serum-free media alone or supplemented with 10 nM nonimmune Fab (NI-Fab) or H2B-Fab for 1 h. The cells were switched to serum-free media containing 50 μg/ml of Dil-oxLDL with or without 200 nM of Plg for 18 h, and then their fluorescent lipid content was determined as describe previously.
2.10 Effect of PAR1-siRNA, PAR2-siRNA, or clathrin-siRNA on the uptake of ox-LDL
Indicated cells were cultured overnight in 100-mm culture dishes and then collected and counted for nucleofection. The cells were treated with nucleofection reagent alone or containing 300 nM nontargeting RNA (On-TARGET plus, D-001810-01-05), small interfering RNA (siRNA) against mouse PAR1 (si-PAR1, ON-TARGET plus, L-054176-00-0005), siRNA against mouse PAR2 (si-PAR2, ON-TARGET plus, L-061445-02-005), or siRNA against clathrin (ON-TARGET plus mouse Cltc siRNA) from Dharmacon, Lafayette, CO, using Amaxa mouse macrophage nucleofection kit (VP-1009) according to the manufacturer's protocol (Lonza, Koln, Germany). The nucleofected cells were then plated in serum-containing medium overnight. The next day, cells were washed three times with PBS and then incubated with serum-free media containing Dil-oxLDL (50 μg/ml) in presence or absence of Plg (200 nM) for 18 h, and their lipid uptake was determined as described previously. To assess the effect of the siRNA treatment on the protein expression level, an equal amount of cell lysates from the treated cells was analyzed by western blots using specific antibodies against PAR1, PAR2, or clathrin.
2.11 Ex vivo foam cell formation
PAR1+/+ and PAR1−/− mice were injected intraperitoneally with 0.5 ml of 4% thioglycolate. Blood was collected via the inferior vena cava, and peritoneal cells (90% macrophages) were collected by lavage after 3 days. The peritoneal macrophages were incubated with 200 μM tranexamic acid followed by three washes with PBS. This step was performed to remove surface-bound Plg from the macrophages and did not affect cell viability as assessed by trypan blue exclusion.43 Cells were treated with ammonium-chloride-potassium lysis buffer for 2 min on ice, washed three times with PBS, and then distributed onto 24-well plates (Corning) in DMEM supplemented with 10% serum and 100 μg/ml Pen/strep. For FCF, cells were incubated in medium containing 1% Nutridoma supplemented either with 50 μg/ml Dill-ox-LDL or unlabeled ox-LDL for 18 h in the presence or absence of the selected compounds. The cells were washed three times with PBS, dislodged from the plate using nonenzymatic dissociation solution (Sigma), pelleted by centrifugation at 1500g for 2 min, homogenized in PBS containing 1% PFA, and analyzed by flow cytometry. Mean fluorescence intensity values were obtained using the Cell Quest Pro software (BD Biosciences, Mississauga, Canada). As a second approach, the mass of total cholesterol contained in cells after incubation with unlabeled ox-LDL was measured using an enzymatic assay.44 The values of total cholesterol were normalized to the total protein content of the extracts used in the assay.
2.12 Effect of PAR agonists or antagonists on the uptake of ox-LDL by J774A.1 cells
Confluent cells grown in serum-containing media were washed three times with PBS and then treated with serum-free culture medium containing: active thrombin (0.5, 1, or 5 U/ml), PAR1-activating peptide TFLLR-NH2 (10 μM), PAR1 antagonist RWJ56110 (30 μM), PAR2 agonist AC5541 (10 μM), PAR2-blocking peptide FSLLRY-NH2 (10 μM), and PAR4 antagonist ML354(10 μM) for 1 h. The cells were then incubated with serum-free media containing Dil-ox-LDL (50 μg/ml) in presence or absence of 200 nM Plg for 18 h. The cells were washed and their fluorescent lipid content was measured as described previously.
2.13 Colocalization of H2B, PAR1, and clathrin on the surface of live J774A.1 cells
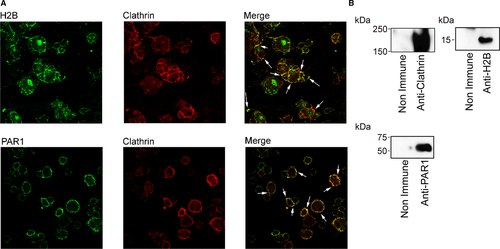
J774A.1 cells were cultured on coverslips in serum-containing media until they reached ~70% confluence, and the medium was replaced with serum-free medium supplemented with 5% lipoprotein-deficient serum for 24 h. The cells were washed three times with PBS and then incubated with nonimmune rabbit, mouse, or goat IgG for 45 min in 1.5% BSA-Hanks balanced salt solution. Cells were washed and then incubated overnight with mouse anti-H2B, rabbit anti-clathrin, or goat anti-PAR1 at 4°C. Cells were washed and then stained with Alexa-488 anti-mouse, Alexa-568 anti-rabbit, or Alexa-647 anti-goat antibodies (Invitrogen) for 30 min at room temperature. Cells were washed, fixed in 2% PFA, and mounted in Vectashield DAPI mounting solution (Vector Laboratories, Burlington, CA). Confocal images were captured on a Leica Microsystem TCS SPE (Wetzlar, Germany) using a 40X/125 NA oil-objective lens and analyzed using Leica software.
2.14 Immunoprecipitation and western blotting
J774.A1 cells were grow to confluence in serum-containing medium and then placed in medium containing 5% lipo protein deficient serun for 24 h. Cell lysates were prepared according to a modification of a previously described method.45 Briefly, cells were washed three times with ice-cold PBS followed by ice-cold buffer A (0.1 M MES, pH 6.5, 0.2 mM EGTA, 0.5 mM MgCl2, 0.02% NaN3, 0.2 mM PMSF, 0.5% Triton X-100) and then scraped into 1 ml buffer A supplemented with protease inhibitor cocktail (Roche, Basel, Switzerland). Homogenization was carried out with 10 strokes of a Potter glass homogenizer. The homogenates were centrifuged at 3000g for 30 min at 4°C to remove nuclei and cell debris. The supernatant was brought to 35% sucrose, placed at the bottom of an ultracentrifugation tube, and 5% to 30% discontinuous sucrose gradient was layered on top of it and centrifuged at 100,000g at 4°C for 18 h. Fractions were collected and analyzed by western blotting suing antibodies against clathrin, H2B, PAR1, caveolin-1, histone H3, and Na-ATPase. For immunoprecipitation, fractions from sucrose gradient ultracentrifugation were precleared and then incubated with mouse monoclonal antibody against H2B at 4°C overnight and then with protein A/G for 60 min at room temperature. The immunoprecipitates were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis on 4% to 20% gradient gels and analyzed by western blotting using a mouse monoclonal antibody against PAR1 (Santa Cruz), a rabbit polyclonal against clathrin (Santa Cruz), and a mouse monoclonal against H2B (made in house).24 Western blot analysis was used to determine the protein expression levels of H2B-WT, H2B-∆C, and PAR1 in cells overexpressing these proteins, and when cells are treated with siRNA.
2.15 Effect of clathrin integrity on ox-LDL uptake
J774A.1 macrophage-like cells were grown to confluence in serum-containing media then washed with warm PBS and treated with either chlorpromazine (10 μg/ml) or methylamine (100 nM), chemicals that interfere with clathrin-coated pit assembly,46 for 1 h. The cells were then incubated with serum-free media containing Dil-ox-LDL (50 μg/ml) supplemented or not with Plg (200 nM) for 18 h. The cells were washed three times with cold PBS and their fluorescent lipid content measured as describe previously.
2.16 Protein content measurement
The protein content of cell homogenates was determined using Pierce BCA protein assay kit (Thermo Fisher Scientific, Rockland, IL) according the manufacturer's instructions.
2.17 Statistics
Data represent mean ± standard error where applicable. Statistical significance was calculated with paired Student's t-test. Differences were considered significant at p < 0.05.
3 RESULTS
3.1 Role of H2B in plasminogen-mediated foam cell formation
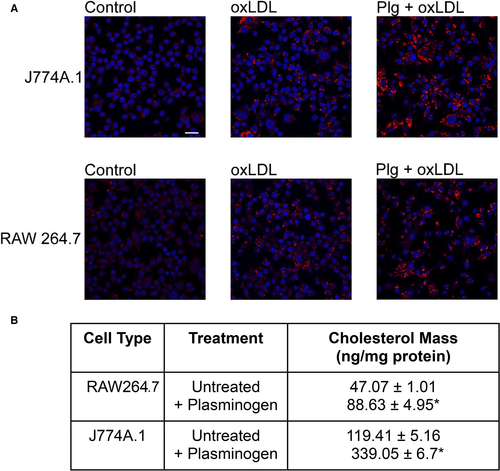
A prior study24 had suggested a role of H2B in Plg-dependent oxLDL uptake by macrophages. To substantiate this interpretation, we used two murine macrophage-like cell lines, RAW264.7 and J774A.1, which are models widely used to study lipid metabolism by macrophages.47-49 The Plg-dependence of oxLDL uptake by these cells is shown in Figure 1A. The cells were cultured in the presence or absence of 50 μg/ml OxLDL with or without Plg for 18 h and then stained with Oil Red O. The analysis of the slides indicated that in the absence of oxLDL very little staining was observed in both cell lines, and staining increased when the cells were incubated with oxLDL. The addition of Plg to the oxLDL induced a dramatic increase of the Oil Red O staining, indicating an increased oxidized lipid uptake by the cells. As an independent validation, the mass of total cholesterol accumulated by these cells, determined by fluorometric assay, was performed. As indicated in Figure 1B, J774 accumulated 2.5-fold more cholesterol than RAW264.7 cells in presence of oxLDL alone. When cells were stimulated with Plg, the amount of cholesterol accumulated by J774A.1 cells was 3.8-fold more than that of RAW264.7 cells. Thus, Plg induced substantially more oxLDL uptake in J774A.1 compared with RAW264.7.
Dil-ox-LDL (50 μg/ml) was used to quantify ox-LDL uptake by these two cell lines. At 18 h, Plg enhanced the oxLDL uptake lipid by twofold in RAW264.7 cells and threefold in J774A.1 cells (Figure 2A), consistent with the cholesterol mass measurements. Two independent approaches were then used to show that H2B is involved in Plg-dependent uptake of oxLDL. First, we treated J774A.1 cells with either NI-Fab or Fab of a monoclonal antibody raised to a peptide corresponding to the C-terminal amino acids of H2B (H2BFab) including its C-terminal lysine to which Plg binds11 and measured their ability to accumulate ox-LDL in presence and absence of Plg (Figure 2B). The Plg-mediated ox-LDL uptake by cells treated with NI-Fab was not significantly different from that of cells treated with Plg alone and was significantly higher than oxLDL taken up by cells in the absence of Plg. However, Plg-mediated lipid uptake was reduced by ~50% by anti-H2B Fab. Second, we overexpressed mouse H2B-WT and a mutant H2B lacking its C-terminal lysine to which Plg binds (H2B∆C) in Raw264.7 cells using nucleofection. The RAW264.7 cells were selected for this set of experiments because of the lower H2B surface expression so that changes in surface expression would be more dramatic. Membrane fractions of the transduced cells analyzed by western blotting with an H2B monoclonal antibody that reacts equally with both forms of the protein, showed that H2B-WT and H2B-∆C protein levels in the membrane fractions of these cells was increased substantially for both H2B forms compared with the vector control (Figure 2D). Densitometry indicated that the levels of H2B-WT and H2B-∆C were 53% and 30% higher, respectively, compared with the vector control cells. When measuring the lipid uptake of these cells, H2B-WT increased their Plg-mediated uptake by 1.8-fold compared with vector control cells (Figure 2C). However, the H2B-∆C cells exhibited the same level of Plg-mediated oxLDL uptake as the vector control or the nontransfected cells.

3.2 Role of PAR1 and PAR2 in Plg-mediated cell formation
The requirement for active plasmin50, 51 and involvement of PAR1 in the proinflammatory endothelial cell phenotype52 raised the possibility that a PAR might be involved in Plg-dependent oxLDL uptake because macrophages express PAR1, PAR2, and PAR3.32 Accordingly, we tested the effects of specific agonist and antagonists of these PARs on Plg-dependent lipid uptake. As shown in Table 1, J774A.1 macrophage treated with Plg alone increased their lipid uptake by ~twofold compared with cells in the absence of Plg. PAR1-activating peptide increased the uptake by more than 60% compared with control. On the other hand, RWJ56110, a specific PAR1 antagonist, reduced Plg-mediated oxLDL uptake by 1.9-fold and even reduced that of untreated cells by 21%, suggesting a role of PAR1 in basal lipid uptake. In contrast, PAR2 and PAR4 antagonists failed to interfere with Plg-driven lipid uptake. FSLLRY-NH2, a PAR2-specific blocking peptide, and ML354, a specific PAR4 antagonist, had no effect on Plg-enhanced lipid uptake as indicated by a more than 70% and 50% respective increase of lipid uptake even after treatment with these antagonists. We also tested the PAR2 agonist AC55541 and observed no increase lipid uptake. We confirmed the effect of a PAR1 antagonist with a non-peptide inhibitor (SCH79797). This inhibitor also completely suppressed Plg-dependent ox-LDL uptake (data not shown). When cells were treated with thrombin, the most widely recognized protease activator of PAR1, their lipid uptake also increased significantly (42%-60%). Taken together, these results indicate that PAR1 but not PAR2 nor PAR4 is a contributor to Plg-mediated oxLDL uptake.
| Treatment | Sample Size (N) | Dil-ox-LDL Uptake (% of Control) |
|---|---|---|
| Untreated | 3 | 100.00 ± 4.42 |
| + Plasminogen | 3 | 202.98 ± 8.81* |
| PAR1 activating peptide | 3 | 160.51 ± .51* |
| PAR1 antagonist | 3 | 79.75 ± 2.03* |
| PAR1 antagonist + plasminogen | 3 | 108.05 ± 1.53* |
| PAR2 agonist | 3 | 98.93 ± 1.00 |
| PAR2 blocking peptide | 3 | 101.78 ± 7.39 |
| PAR2 blocking peptide + plasminogen | 3 | 179.38 ± 1.38* |
| PAR4 antagonist | 3 | 89.76 ± 1.29 |
| PAR4 antagonist + plasminogen | 3 | 157.79 ± 3.84* |
| Thrombin 0.5 U/ml | 3 | 142.04 ± 17.83* |
| Thrombin 1 U/ml | 3 | 149.60 ± 5.36* |
| Thrombin 5 U/ml | 3 | 160.21 ± 4.42* |
- Cells are treated with PAR1 activating peptide (TFLLR-NH2, 10 µM), PAR1 antagonist (RWJ56110, 30 µM), PAR2 agonist (AC55541, 30 µM), PAR2 blocking peptide (FSLLRY-NH2, 10 µM), PAR4 antagonist (ML354, 10 µM), or active thrombin (0.5, 1, 5 U/ml) for 1 h, then incubated with media containing or not Plg (200 nM) and Dil-ox-LDL (50 µg/ml) for 18 h. Cells were washed and their lipid content was measured as described in Methods. The values represent the mean of triplicates ± SEM.
- Abbreviations: Dil-ox-LOL, 1,1′-dioctadecyl-3,3,3′,3′tetramethylindocarbocyanine perchlorate oxidized low-density lipoprotein; PAR, protease-activated receptor.
- * p < .05 vs untreated cells.
Several independent approaches were taken to verify the involvement of PAR1 in Plg-dependent ox-LDL uptake by macrophages. We treated J774A.1 cell with specific PAR1-activating peptide and then measured their lipid uptake in presence or absence of Plg. As shown in Figure 3A, PAR1-activating peptide alone increased oxLDL uptake by 1.4-fold and when combined with Plg the increase was twofold over the untreated cells. On the other hand, Plg alone increased lipid uptake by 2.2-fold compared with the oxLDL uptake by untreated cells.
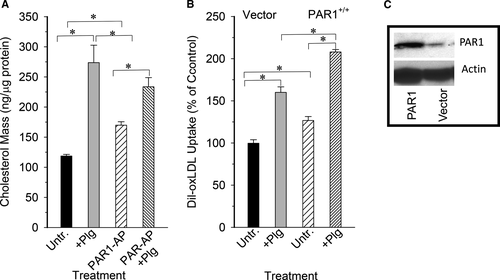
As a second approach, we transiently nucleofected J774A.1 cells with either cDNA encoding mouse PAR1 or empty vector. The level of PAR1 protein expression was measured by Western blots using anti-PAR1-specific antibody of membrane fractions prepared from the nucleofected cells. Densitometry indicated that PAR1 protein level was increase by ~fivefold over the vector control cells (Figure 3C). When these cells were incubated with ox-LDL in the absence of Plg, the cells overexpressing PAR1 accumulated 25% more lipid than control. Upon addition of Plg, cells expressing PAR1 increased their lipid uptake by more than 50% (Figure 3B).
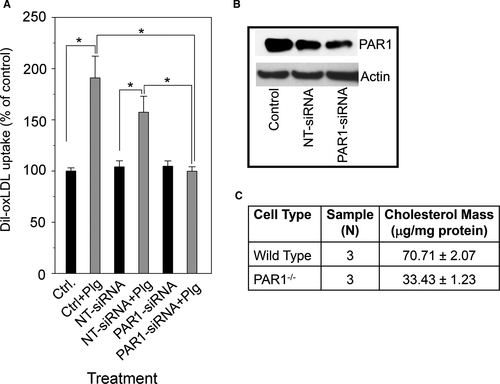
As an alternative strategy to implicate PAR1 in Plg-dependent ox-LDL uptake, we used two approaches to decrease PAR1 expression. First, we used nucleofection of J774A.1 cells with siRNA targeting the mouse PAR1 gene (PAR1-siRNA), or nontargeting siRNA (NT-siRNA). Treatment with PAR1-siRNA reduced PAR1 protein level by more than 60% compared with control and by ~55% compared with cells treated with NT-siRNA as indicated by Western blot analyses (Figure 4B). As shown in Figure 4A, no statistically significant differences were detected in Plg-mediated oxLDL uptake between control cells and cells treated with NT-siRNA because they both exhibited more than 50% increase in oxLDL uptake compared with untreated cells or untreated control cells. However, when PAR1 expression was decreased by siRNA treatment, Plg-induced oxLDL was abrogated, indicating that PAR1-siRNA treated cells lost their ability to respond to Plg stimulation. Second, we compared the ability of peritoneal macrophages from either WT PAR+/+ or PAR1−/− mice to accumulate ox-LDL in presence of Plg. As shown in Figure 4C, the mass of total cholesterol accumulated by macrophages obtained from wild-type Plg+/+ mice was >twofold higher than from PAR1−/− macrophages. In contrast, PAR2 downregulation with siRNA did not suppress the ability of Plg to increase lipid uptake by mouse macrophage-like cells. J774A.1 cells retained their response to Plg stimulation as they increase their lipid uptake by more than 50% after treatment with PAR2-siRNA as shown in Figure S2. Thus, PAR2 does not contribute to Plg-mediated oxLDL uptake by these cells.
3.3 Cell surface colocalization of H2B and PAR1 on macrophages
To understand the mechanism(s) that allow H2B and PAR1 to collaborate in Plg-mediated FCF, we cultured J774A.1 cells on coverslips and immunostained with antibodies for H2B, PAR1, and clathrin because PAR1 appears to reside in clathrin-coated pits.53 Images in Figure 5A obtained by confocal microscopy showed that H2B, PAR1, and clathrin are all expressed on the surface of the cells. Double staining for H2B and clathrin showed that the two proteins colocalize on the surface of the cells. Double staining for PAR1 and clathrin showed that they also colocalize on the cell surface. These results suggest that H2B, PAR1, and clathrin reside in the same surface area of the cells and are in close proximity to each other. Western blot analysis of immunoprecipitates obtained from clathrin-enriched subcellular fractions from these cells (Figure 5B) also suggested that these proteins coassociated as they were all pulled down by anti-H2B. The majority of PAR, H2B, and clathrin are found in plasma membrane and caveolin-1–enriched cellular fraction (Figure S3).
It is well documented that functional endocytic clathrin-coated pits are required for lipid internalization54 and that these structures are also important in PAR trafficking.55 To test whether the integrity of clathrin-coated pits is required for the Plg-enhanced FCF, J774A.1 cells were treated with either chlorpromazine or methylamine, compounds known to interfere with proper formation of clathrin-coated pit,s46, 56 and then measured their lipid uptake in presence of Plg. The results presented in Figure 6 show that untreated cells increased their ox-LDL uptake dramatically when treated with Plg. On the other hand, when these cells were treated with chlorpromazine or methylamine, their Plg-induced lipid uptake was reduced by 70% and by 50%, respectively. These results strongly indicate that the integrity of clathrin-coated pits is required for optimal Plg-mediated ox-LDL uptake and FCF.
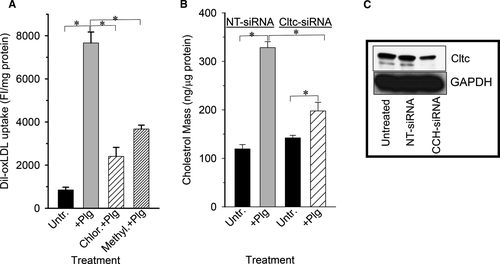
To further test the relevance of clathrin in Plg-mediated uptake, we treated J774A.1 cells with NT-siRNA or with siRNA against clathrin and then measured ox-LDL uptake. The result depicted in Figure 6B indicate that NT-siRNA does not interfere with Plg-induced oxLDL because its lipid uptake was increased 2.6-fold by Plg compared with untreated cells. On the other hand, reducing clathrin expression with Ctlc-siRNA prevented the full effect of Plg because these cells increased their lipid uptake by only 33%, a dramatic decrease compared with NT-siRNA–treated cells. To verify that the siRNA treatment was specific, we measured the level of clathrin protein in nucleofected cells by western blotting. Figure 6C indicates that NT-siRNA did not affect clathrin levels, but Ctlc-siRNA reduced its level by more than 60%.
4 DISCUSSION
It is well documented that the Plg/Plm system plays a pivotal role in the initiation and progression of atherosclerosis in mice17, 18 and humans.22, 57, 58 However, despite the increasing number of both in vivo and in vitro studies supporting this relation, the mechanism(s) by which Plg influences development of atherosclerotic lesions are not understood. A key step in atherosclerosis is the infiltration of LDL particles into the arterial wall, where they are modified (oxidized) and then taken up by recruited macrophages. The aim of the present study was to shed new light on the process by which the Plg/Plm system facilitates the accumulation of ox-LDL by macrophages, leading to FCF. Our data show that peritoneal macrophages as well as RAW264.7 and J774A.1, mouse macrophage-like cell lines, respond to Plg by increasing their capacity to accumulate ox-LDL. We show that H2B expression, a major Plg-R on macrophages,11, 12 is a key component in regulating lipid accumulation when macrophages are stimulated by Plg. This relationship was confirmed in two separate experiments: a specific antibody against the Plg-binding site in H2B (H2B-Fab) reduced the Plg-induced oxLDL uptake by J774A cells and transient expression of H2B in RAW264.7 cells increased their oxLDL uptake compared with nontransfected control cells or cells expressing a Plg-binding defective mutant H2B (H2B-∆C). Lipid uptake by RAW264.7 cells in the presence of Plg was significantly lower than that of J774.7 cells; this disparity could be explained, at least in part, by their lower levels of H2B on their surface.
PARs mediate both physiological (homeostasis, embryogenesis) and pathological (inflammation, metastasis) processes.27-29, 33 They are widely distributed and are tightly regulated.23 Both in vivo and in vitro studies support that enhanced PAR1 and PAR2 expression levels are proatherogenic.59-61 Multiple proteases are capable of activating PARs, and plasmin has been shown to be capable of cleaving and activating PAR1 and PAR4. The contribution of PARs to atherosclerosis has focused on their capacity to activate monocytes and their differentiation into macrophages, one of the first steps in the development of atherosclerosis.32 The involvement of these receptors in lipid uptake by macrophages is not extensively studied; the few instances where the implication of PARs in cholesterol mobilization was investigated, the data are contradictory.60, 61 Here we use molecular biology tools, chemical and biological reagents, and macrophages derived from PAR1−/− mice to show that PAR1 is directly involved in Plg-mediated ox-LDL uptake by macrophages. Specifically, we found that vector-mediated overexpression of PAR1 increased and its downregulation using specific targeting siRNA, or PAR1 antagonists decreased the extent of Plg-mediated ox-LDL uptake by J774.1 cells. A specific PAR1-activating peptide also enhanced lipid accumulation in these cells. The involvement of PAR1 in Plg-mediated uptake of ox-LDL was confirmed using macrophages derived from mice in which the PAR1 gene had been inactivated. These cells accumulated 50% less cholesterol than cells obtained from WT mice. The contribution of PAR1 to ox-LDL uptake in macrophages was also demonstrated by the increase of FCF when cells were treated with active thrombin, which activates PAR1. On the other hand, we could not demonstrate any significant contribution of PAR2, a receptor also expressed by macrophages, to the uptake of ox-LDL by macrophages. Indeed, cells treated with PAR2-blocking peptide or with PAR4-specific antagonist had the same capacity as untreated cells to accumulate lipids when treated with Plg. That neither PAR4 activating peptide nor its antagonist affected lipid taken up by the cells is consistent with the absence of PAR4 on their surface.32
The activation of Plg zymogen to plasmin is greatly facilitated by its binding to fibrin or to its receptors.62-64 The generated plasmin if released from cell surfaces is rapidly inactivated by α2-antiplasmin,64, 65 and free plasmin is only found in the circulation under pathological and therapeutic conditions. Such regulation implies that receptor-bound plasmin is the most likely activator of other receptors such as PAR1. Close physical proximity of the PAR1 target and bound plasmin should enhance efficient activation. Previous studies have demonstrated that PAR1 can be found in clathrin-coated pits.53 Our immunostaining data show that PAR1 and Plg-R H2B are present in clathrin-coated pits; they both colocalize with clathrin. The colocalization of these proteins on the surface of macrophages was confirmed by coimmunoprecipitation. PAR1, H2B, and clathrin were immunoprecipitated from sucrose gradients of isolated cellular fraction enriched in vesicle-coated pits obtained from J774A.1 cells. In addition, it appears that the integrity of these cellular structures is required for the full effect of Plg on lipid uptake by macrophages, as evidenced by reduced lipid accumulation in cells that were pretreated with chlorpromazine or methylamine, drugs that interfere with the normal formation of clathrin-coated pits.66 To investigate the importance of clathrin-coated pits in Plg-mediated oxLDL uptake, we treated J774A.1 cell with siRNA targeting clathrin and found that reduction of clathrin levels attenuated Plg-mediated lipid uptake by these cells dramatically, whereas NT-siRNA did not affect Plg-induced lipid uptake. Taken together, these results suggest that H2B-bound plasmin resides in close proximity with PAR1 and that this physical proximity allows plasmin to initiate the downstream signaling and gene regulation associated with PAR1 activation, leading to increased oxLDL uptake and foam cell formation.
ACKNOWLEDGMENTS
We thank Dr. Ricahrd E. Morton . in the Department of Cardiovascular and Metabolic Sciences of Lerner Research Institute, Cleveland, OH, for providing native low-density lipoprotein and lipoprotein-deficient serum, and to the Cleveland Clinic Lerner Research Institute Imaging Core, which provided electron microscopy services.
CONFLICT OF INTEREST
All authors declare no financial conflict-of-interest.
AUTHOR CONTRIBUTIONS
Lahoucine Izem undertook conceptualization, software, formal analysis, validation, investigation, methodology, and writing original draft, reviewing, and editing. Katazyna Bialkowska provided formal analysis, methodology, and writing, reviewing, and editing. Elzbieta Pluskota undertook formal analysis, methodology, and writing and editing. Mitali Das provided formal analysis, methodology, reviewing, and editing. Riku Das undertook concept development, reviewing, and editing. Marvin T. Nieman provided methodology, reviewing, and editing. Edward F. Plow undertook conceptualization, supervision, writing of the initial draft, and reviewing and editing.