Von Willebrand disease type 2N: An update
Manuscript handled by: David Lillicrap
Final decision: David Lillicrap, 14 January 2020
Abstract
Quantitative or qualitative defects of von Willebrand factor (VWF) are responsible for the most common inherited bleeding disorder, von Willebrand disease (VWD). Type 2N VWD is an uncommon recessive disorder that results from gene mutations located in the region coding for the binding site of VWF for factor VIII (FVIII). This narrative review describes the pathophysiology, diagnostic procedures and treatment as well as the molecular biology of type 2N VWD. Although other VWF-dependent functions like binding to platelets and collagen are preserved, FVIII plasma levels are low due to the rapid clearance of this moiety in the absence or reduction of its binding to VWF. The diagnosis of type 2N should be considered in patients with low FVIII coagulant activity (FVIII:C) and disproportionally higher VWF antigen, especially when they present with an autosomal recessive pattern of inheritance. Because an accurate diagnosis is essential for genetic counseling and optimal treatment, type 2N must be distinguished from mild/moderate hemophilia A and its carrier state. This differential diagnosis can be obtained by using the laboratory assay of the FVIII binding capacity of VWF (VWF:FVIIIB) or analysis of the FVIII binding site on the VWF gene.
1 INTRODUCTION
Von Willebrand factor (VWF) is a large, multimeric plasma glycoprotein produced in endothelial cells and megakaryocytes. Following synthesis, VWF is transported and stored in the Weibel--Palade bodies and platelet α-granules.1 VWF plays a crucial role in both primary and secondary hemostasis by supporting platelet adhesion/aggregation at sites of vascular injury and acting as plasma carrier and stabilizer for coagulation factor VIII (FVIII).2 Defects in VWF, quantitative or qualitative, are responsible for the inherited bleeding disorder von Willebrand disease (VWD), which is classified into three main types. VWD types 1 and 3 are due to the quantitative deficiency of VWF, type 2 to functional abnormalities of this protein.3 Type 2N VWD is an uncommon recessive disorder first described almost 30 years ago and caused by the defective capacity of VWF to bind FVIII, leading to the accelerated clearance of unbound FVIII, shortening of its half-life and thus to reduced FVIII levels in plasma.4 Because patients with type 2N exhibit clinical and laboratory manifestations similar to those of male patients with mild/moderate hemophilia A or female carriers of hemophilia A, 2N VWD may be misdiagnosed, but a differential diagnosis is warranted because genetic counseling and treatment are different.5, 6 In this narrative review, we discuss the pathophysiologic mechanisms, diagnostic approach, molecular biology, clinical presentation and treatment of type 2N VWD.
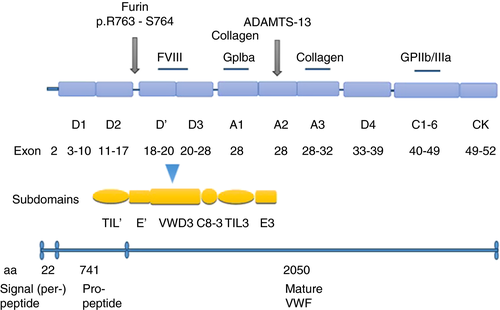
2 FROM FVIII--VWF INTERACTIONS TO TYPE 2N PATHOPHYSIOLOGY
Although FVIII and VWF are products of two separate genes, their life cycles in the circulation are inextricably associated. FVIII circulates in plasma as a noncovalent complex with VWF, this association being crucial for the half-life of FVIII.7 VWF is a mosaic protein with different domains orderly arranged in D1-D2-D′-D3-A1-A2-A3-D4-C1-C2-C3-C4-C5-C6-CK (Figure 1).8 The D′D3 domain at the N-terminal region of the mature VWF molecule is responsible for FVIII binding but for this to occur cleavage of the VWF propeptide (D1 and D2) from the mature polypeptide is needed.9, 10 Zhou et al.11 showed that the D′D3 domain can be further divided into the TIL′-E′-VWD3-C8_3-TIL3-E3 subdomains (Figure 1). As discussed below with more details, most gene mutations responsible for 2N VWD are located at the N-terminal of D′D3 near the furin cleavage site (TIL′-E′).8 Studies dealing with the FVIII--VWF complex association showed that a VWF fragment within residues 763–1035 harbors the site of interaction with FVIII,12-14 that approximately 95% to 98% of FVIII is in dynamic equilibrium complexed with VWF, and that this association is constant with a 50: 1 molar ratio of FVIII:VWF.15
The interaction of FVIII with VWF has a number of functional consequences. VWF protects FVIII from phospholipid-dependent proteolysis by activated protein C and activation by factor IX (FIXa), thereby prolonging FVIII survival in the circulation. This mechanism has been substantiated by clinical studies evaluating the plasma half-life of FVIII following the administration of factor concentrates or desmopressin (DDAVP) in patients with type 3 VWD or 2N compared to hemophilia A patients: half-life was only 1 to 2 h in the former contrasting with 12 to 14 h in the latter.16, 17 Furthermore, binding of FVIII to VWF is relevant for an enhanced rate of association of the FVIII heavy and light chains.18 Because the FVIII--VWF life cycle has clinical implications for both hemophilia A and VWD, a number of studies chose to elucidate the mechanisms of plasma clearance of FVIII, VWF, and the FVIII--VWF complex, showing that the FVIII--VWF association modulates the cellular interactions and clearance of FVIII.19 In addition, a definite role for FVIII bound to VWF pertains to the regulation of the immune recognition of FVIII.20
With this mechanistic background, it can be understood that type 2N VWD is a bleeding disorder associated with a markedly decreased affinity of a dysfunctional VWF for FVIII. Other VWF functions such as binding to platelets and collagen are preserved and the corresponding plasma measurements are usually normal, but FVIII levels are reduced due to the rapid clearance of this moiety in the absence of its binding to the dysfunctional VWF, usually present at normal concentrations in plasma.4 Type 2N VWD was first reported in families in which the inheritance of low FVIII levels was autosomal rather than X-linked and was originally referred to as VWD Normandy from the French region of origin of the first reported patient.21, 22
3 PREVALENCE AND CLINICAL FEATURES
Different surveys have addressed the prevalence of type 2N VWD among all VWD cases, with values ranging from 1% to 10% in different geographical areas.23-28 Among the VWD cases diagnosed as type 2 the estimated prevalence rates of type 2N were between 10.6% and 13%.23, 24 In our large Milan cohort of patients with inherited bleeding disorders, type 2N patients are 5% of those with a diagnosis of VWD and 12% of those with type 2 VWD (unpublished data).
Type 2N shows a mild to moderate hemophilia-like phenotypic presentation with a clinical expression that depends on plasma levels of factor VIII coagulant activity (FVIII:C), usually ranging between 5 and 40 IU/dl. A few patients show a more marked decrease (1–5 IU/dl) but so far never below 1 IU/dl.4 Patients who are homozygotes or compound heterozygotes for type 2N VWF defects, with two different type 2N mutations or a combination of 2N and another VWD type, are those presenting with clinical manifestations, whereas heterozygotes are generally asymptomatic when they have normal or only mildly reduced FVIII:C.24, 29 However, when type 2N heterozygosity is co-inherited with such other bleeding disorders as hemophilia A or Glanzmann thrombasthenia, clinical symptoms are more prominent and may even be life-threatening.30-33
Bleeding is mainly related to surgery (such as tonsillectomy and tooth extraction) but may also occur as mucocutaneous bleeding (menorrhagia, epistaxis) or following trauma. More severe bleeding manifestations in the gastrointestinal tract. Postpartum hemorrhages have been reported in affected women.34, 35
Two relatively large cohorts of type 2N patients were described by Casonato et al. and van Meegeren et al.24, 35 The bleeding score (BS) was higher in genetically confirmed 2N homozygotes than in heterozygotes (median BS was 6 vs. 3).35 Genetically confirmed type 2N patients may seldom suffer from hemarthroses and muscle hematomas, with frequencies similar to those observed in patients with mild hemophilia A.35, 36 Higher BS were reported from the Northeast of Italy for both heterozygotes and homozygotes with type 2N gene defects.24 The patients who were compound heterozygotes for type 2N and type 1 VWF defects had a higher BS than homozygotes (mean BS was 10.7 ± 2.1 vs. 8.3 ± 2.5). Low borderline plasma levels of VWF antigen (VWF:Ag) influence the appearance of bleeding symptoms in heterozygotes expected to be asymptomatic on the basis of their FVIII:C levels.24
4 DIAGNOSTIC APPROACH
The diagnosis of type 2N is mainly pursued following the finding of low plasma levels of FVIII:C out of proportion of normal or low borderline VWF:Ag.37, 38 Low FVIII:C levels are obtained using the 1-stage functional assays or the 2-stage chromogenic assay and are employed to calculate the FVIII:C/VWF:Ag ratio, which usually yields values below 0.6 to 0.7.3 However, some genetically confirmed 2N patients who are compound heterozygotes for type 2N and quantitative VWF defects present with FVIII:C/VWF:Ag ratios ≥0.7 and thus would be misdiagnosed using these criteria, so that some investigators recommend the straightforward measurement of the capacity of VWF to bind FVIII (VWF:FVIIIB).29, 35, 39 VWF:FVIIIB is an ELISA method that measures in patient plasma the binding affinity to VWF of added recombinant FVIII (rFVIII). Even though in-house and commercial assay kits are available,40, 41 this diagnostically crucial assay is performed only in a relatively small number of specialized laboratories.
In the frame of this assay, two different tests are carried out in parallel on the same plasma samples. While in the first microtiter plate the binding capacity of VWF for FVIII is measured as VWF:FVIIIB, the second plate measures the concentration of VWF (VWF:Ag). An anti-VWF antibody is coated onto the plate wells and then diluted patient plasma is added. The FVIII--VWF complex in plasma is first bound by the anti-VWF antibody but then, by incubating the plate with a high-ionic strength CaCl2 solution, patient FVIII is detached from the complex. Excess rFVIII is added and then, after well washing to remove unbound rFVIII, the amount bound to patient VWF is ultimately measured by using an anti-FVIII antibody. The values of the VWF:FVIIIB must be normalized to the VWF:Ag values, so that results are calculated as the ratio of bound rFVIII to immobilized VWF and reported as VWF:FVIIIB ratio (Figure 2).
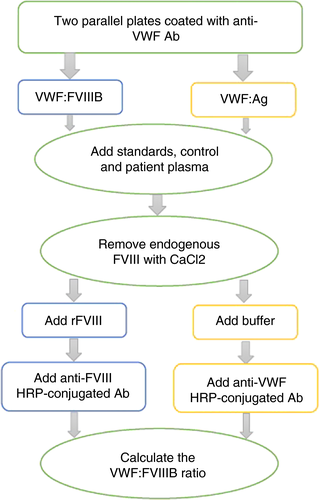
This is the only truly discriminant laboratory test for distinguishing type 2N from mild/moderate hemophilia A in males and females carriers, with lower value in the former and normal in the latter.42 Homozygotes or compound heterozygotes for type 2N exhibit no or severely reduced VWF:FVIIIB (<15%) and very low ratios of VWF:FVIIIB/VWF:Ag (<0.3). In heterozygotes VWF:FVIIIB is moderately reduced or normal but the VWF:FVIIIB/VWF:Ag ratios are below the normal range (<0.75).29, 35
In general, other VWF-related measurements such as VWF:Ag, ristocetin cofactor activity (VWF:RCo), collagen binding activity (VWF:CB), and the multimeric pattern are normal in type 2N. However, when type 2N heterozygous variants compound with quantitative VWF defects in heterozygosity, patients have low VWF:Ag levels in addition to low FVIII:C (see more below).43, 44 The clinical and laboratory parameters used to diagnose type 2N VWD are summarized in Table 1.
| Clinical parameters |
|
|
Laboratory parameters PT APTT FVIII:C FVIII:C/VWF:Ag ratio VWF:Ag VWF activity VWF multimer VWF:FVIIIB VWF:FVIIIB/VWF:Ag ratio Mutation analysis |
|
- Abbreviations: APTT, activated partial thromboplastin time; FVIIIB, factor VIII binding capacity; HMWM, high molecular weight multimers; PT, prothrombin time; VWD, von Willebrand disease; VWF, von Willebrand factor.
5 MOLECULAR BIOLOGY OF TYPE 2N VWD
Because the VWF:FVIIIB assay is not widely available, a targeted molecular analysis is beneficial for an accurate diagnosis. At variance with the majority of cases with other VWD types (type 1, most 2A, 2B, and 2M) transmitted as autosomal dominant traits, type 2N is inherited recessively and affected patients may be homozygotes for a single 2N mutation, compound heterozygotes for two distinct 2N mutations, or compound heterozygotes for a type 2N VWF mutation and a null VWF mutation.45, 46 In patients with reduced plasma levels of VWF:Ag, the molecular basis of this phenotype is the coinheritance of a 2N defect with a null VWF allele (nonsense, splice site, deletion/insertion).47 Even though in most affected patients the VWF multimeric pattern is normal, a few patients with missense VWF mutations presented with ultra large (UL) multimers or reduced high-molecular-weight (HMW) multimers (Table 2).48-53 As shown in Figure 1, the D′D3 domain of VWF, divided into the TIL′-E′-VWD3-C8_3-TIL3-E3 subdomains, is the FVIII binding site encoded by exons 18–28 of VWF. D′ is the site of the majority of the missense mutations that impair the interaction of FVIII with VWF.54 Przeradzka et al.55 showed that even though the isolated D3 fragment does not interact with FVIII it is very critical for its binding by D′D3 and thus for the formation of the FVIII--VWF complex.
| Mutationa | Multimer profile | Exon | Reference |
|---|---|---|---|
| R760C | Presence of UL, smeary pattern | 17 | 48 |
| R763G | Presence of UL, decreased intensity of satellite bands | 18 | 49 |
| Y795C | Presence of UL | 18 | 50 |
| C788Y | Absent HMWM, smeary pattern | 18 | 51 |
| C804F | Slightly decreased HMWM | 18 | 52 |
| Q1053H | Presence of UL | 24 | 53 |
| C1060R | Slightly decreased HMWM | 24 | 53 |
| D879N | Decreased HMWM | 20 | 66 |
- Abbreviations: HMWM, high molecular weight multimers; UL, ultra-large multimers; VWD, von Willebrand disease.
- Adapted from EAHAD VWF Mutation Database March 2020 [https://databases.lovd.nl/shared/genes/VWF]
Nowadays several specialized laboratories are using new and advanced technology for DNA sequencing such as next-generation sequencing (NGS), but otherwise it is recommended that mutation analysis first targets exons 18–20 of VWF because they comprise approximately 85% of reported mutations, but ultimately when no defects are found exons 17 and 24–27 should also be evaluated. When no mutations are identified following D′D3 analysis, additional analysis for a differential diagnosis should be done on the FVIII gene.47
Two mutations (p.Arg760Cys and Arg763Gly) disrupt the furin cleavage site of VWF, thus leading to the persistence of the propeptide along with the mature subunit.48, 49 Carriers of these mutations, besides having an ultra large multimeric pattern, showed a laboratory phenotype that might be due to steric hindrance of the binding of FVIII to VWF.
Since the first description of the type 2N missense mutation in the female propositus from Normandy, more than 50 mutations have been reported in the EAHAD VWF Variant Database (von Willebrand factor LOVD database) as of March 2020. Four are located at the propeptide and the rest in the mature VWF subunit. Missense mutations are more than 90% of those identified, even though nonsense mutations have also been reported.56, 57 As summarized by Hampshire and Goodeve, among 37 entries that provided details on the mutation inheritance pattern, 62% were compound heterozygotes, 30% homozygotes, and 8% had only a single heterozygous mutation.58 The heterozygous 2N VWF defect was estimated to be present in 5.2% of the general population of Northeast Italy, always in association with the original p.R854Q Normandy mutation, in clinically asymptomatic cases with a normal ISTH Bleeding Assessment Tool (ISTH-BAT) score.24 A 1.7% prevalence for the heterozygous p.R854Q mutation was reported in healthy individuals,59 so these findings show that type 2N VWF defects are not so rare.
The homozygous p.Arg816Trp (R816W) mutation is typically associated with a clinically severe type 2N VWD owing to a severe defect of the FVIII binding capacity of VWF.60 This mutation is also relatively frequent in affected European individuals and was reported in 17 entries according to the EAHAD VWF Mutation Database.
6 HOW TO HANDLE TREATMENT
The optimal and effective treatment of this VWD type depends on an accurate diagnosis and detailed information on the phenotypic severity. The intravenous or subcutaneous administration of DDAVP, a synthetic analogue of the antidiuretic hormone vasopressin, releases into the circulation endogenous VWF and FVIII from vascular endothelial cells61 and is the treatment of choice in type 1 VWD patients with a mild phenotype. In patients with type 2N VWD its administration usually produces an increase in plasma FVIII:C, but fails to correct the capacity of VWF to bind endogenous FVIII.62 Thus, DDAVP is unable to maintain for an adequate time period hemostatic FVIII:C levels owing to the short plasma half-life of this moiety.63, 64
There is evidence of a relationship among the type of VWF 2N mutation, the related functional abnormalities, and the varied response to the administration of DDAVP.65 The most common type 2N mutation, R854Q, causes a mild reduction of FVIII:C plasma levels (~25%), with an almost optimal response to DDAVP.65 However, other mutations, such as C1060R, R816W, and T791M, are associated with a more significant defect of FVIII:C (<10%) and a poor response to DDAVP.4, 65 Some type 2N variants (e.g., C858F and D879N) are associated with low VWF levels that tend to exacerbate the bleeding phenotype.51, 66 A defective secretion of VWF in association with impaired thrombus formation was also observed for the R854Q and R763A variants in mice with type 2N VWD, suggesting that primary hemostasis is normal in type 2N VWD but that secondary hemostasis is defective with decreased thrombus stability.67 In practical terms, to ensure that patients have a post-DDAVP response of a magnitude sufficient to increase FVIII at hemostatic levels and thus to avoid the use of plasma products containing both FVIII and VWF, an explorative trial is recommended, with blood samples taken at baseline, 1, and 4 h following administration in order to evaluate not only the peak but also and most importantly the clearance pattern of FVIII:C. Recently the definition of desmopressin response was proposed by the ASH ISTH NHF WFH 2021 guidelines and It was defined an increase of at least 2 times the baseline VWF activity level and the levels of both VWF and factor VIII (FVIII):C to > 0.50 IU/mL for at least 4 hours.
With this background and limitations of DDAVP, in severely affected patients with type 2N, replacement therapy with VWF concentrates that also contain FVIII is often required. The majority of the currently available plasma-derived products (Humate-P, Wilate, and Alphanate) are adequate, because they contain both VWF and FVIII.68 rVWF is another possible treatment option for type 2N as well as for other types of VWD because it stabilizes endogenous FVIII,69 but clinical experiences are currently lacking. However, the infusion of rFVIII alone is ineffective, because it provides a short-term benefit owing to its rapid clearance from plasma due to lack of protection by the abnormal 2N VWF.70 In some patients FVIII replacement might be needed only initially in order to correct low FVIII:C levels at the challenging times of surgery or acute hemorrhage but could be omitted subsequently, when FVIII normally produced endogenously is stabilized by the exogenously provided VWF and plasma levels become normal. In the near future the novel rFVIIIFc-VWF-XTEN (BIVV001) may be a suitable therapeutic approach, because this rFVIII product does not bind to VWF.71 The potential hemostatic benefits of off-label emicizumab have been recently investigated in a very limited number of patients with type 2N VWD.72 Even though a higher plasma concentration (100 μg/ml) of this molecule than the traditional therapeutic levels (50 μg/ml) was employed, thrombus formation was enhanced independently of VWF in these patients.
All in all, it can be understood from these therapeutic experiences that the differentiation between type 2N from hemophilia A is crucial to implement an appropriate therapy and that misdiagnosis of type 2N might result in the ineffective treatment with rFVIII or DDAVP leading to adverse clinical consequences.73
There are a few reports on the management of pregnancy and delivery in women with type 2N VWD.74-80 Pregnant women diagnosed with VWD should be monitored in terms of their VWF and FVIII plasma levels at the time of the third trimester of pregnancy. For type 2N patients as well as for those with other VWD types, treatment should be implemented before invasive procedures and delivery when plasma levels of FVIII:C are lower than 40 IU/dl.81 Generally during pregnancy there is a plasma increase of both VWF and FVIII levels, but in 2N pregnant women FVIII levels often remain low owing to the impaired binding of this moiety by the still dysfunctional albeit increased VWF. The type of mutation in the VWF gene and the severity of the resulting binding defect might predict the effect of pregnancy on the changes of FVIII levels and the bleeding phenotype.81, 82 Thus for the clinical management of type 2N during pregnancy it is recommended to use VWF-containing products in patients with FVIII levels lower than 40 IU/dl. Postpartum factor replacement is recommended for at least 3 to 4 days after vaginal delivery and 5 to 7 days after Cesarean section.82, 83 However, three of the seven reported cases needed no replacement therapy during delivery nor in the postpartum period, because their FVIII:C plasma were high enough. Thus, in order to determine the most appropriate pregnancy management, it is very critical to consider the patient's clinical history, the bleeding phenotype as well as genotype.
7 CONCLUSIONS
Despite its rarity, an accurate diagnosis of type 2N VWD is crucial in order to provide not only correct genetic counseling but also an optimal treatment strategy. This recessive bleeding disorder may be easily misdiagnosed with hemophilia A and type 1 VWD, so the differential diagnosis should be taken into consideration in order to reduce incorrect diagnoses and inaccurate treatments. Because critical and discriminant tests such as VWF:FVIIIB and genetic analysis are not routinely performed in laboratories and are only available in specialized centers, type 2N VWD is likely still to be underdiagnosed.
CONFLICTS OF INTEREST
PMM: member of the scientific board for the Bayer Awards. He has also received from Bayer, Kedrion, and Novo Nordisk honoraria for lectures at educational symposia. FP: honoraria for participating as a speaker at educational meetings, symposia, and advisory boards for Roche, Sobi, Sanofi, Grifols, and Takeda. OS: no conflict of interest.
AUTHOR CONTRIBUTIONS
The three authors contributed equally to the analysis of the literature, to writing the manuscript, and to its revision.