Clinical history of cancer-associated splanchnic vein thrombosis
Manuscript handled by: Marc Carrier
Final decision: 07 December 2020
Funding information
This study was promoted through the International Society on Thrombosis and Haemostasis and was supported by a research grant from Pfizer Canada. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. There was no other funding source for this study.
Abstract
Background
Cancer represents a risk factor for splanchnic vein thrombosis (SVT) and usual site venous thromboembolism (VTE).
Objectives
To compare characteristics and outcomes of patients with cancer-associated SVT and usual site VTE.
Patients/Methods
Patients with solid cancer and SVT were enrolled in an international, prospective registry between May 2008 and January 2012. The comparison cohort included (1:1 ratio) patients with solid cancer and usual site VTE treated at two thrombosis centers who had a minimum of 12 months follow-up at December 2019 or experienced one of the outcomes within 12 months follow-up. Recurrent VTE, major bleeding, and all-cause mortality were evaluated at 12-month follow-up.
Results
A total of 264 patients (132 in each cohort) were enrolled. Patients with SVT were less likely to have metastatic disease (36.1% vs 72.5%) or receive cancer therapy at thrombosis diagnosis (29.6% vs 64.9%). The most frequent cancer types were hepatobiliary and pancreatic in the SVT cohort and gastrointestinal in the usual site VTE cohort. Fewer patients with SVT received anticoagulation (68.9% vs 99.2%), and treatment duration was shorter (6.0 vs 11.0 months). The cumulative incidence of major bleeding (2.3% vs 4.7%) was nonsignificantly lower in the SVT cohort, whereas recurrent thrombosis (4.7% vs 5.5%) and all-cause mortality (41.7% vs 39.4%) were comparable between the two cohorts.
Conclusions
The risk of recurrent thrombosis and bleeding appears to be similar in cancer patients with SVT and cancer patients with usual site VTE, despite some differences in baseline characteristics and anticoagulant treatment. Further prospective studies are warranted to confirm these findings.
Essentials
- Limited data are available on outcomes of patients with cancer and splanchnic vein thrombosis (SVT).
- Outcomes of cancer patients with SVT and usual site venous thromboembolism (VTE) were compared.
- Clinical history of cancer patients with SVT is comparable to that of patients with usual site VTE.
- Mortality was mostly related to cancer progression, with few fatal thrombotic or bleeding events.
1 BACKGROUND
Patients with cancer have a four-fold higher risk of venous thromboembolism (VTE) compared with patients without cancer and the VTE incidence may be as high as 68 per 1000 person-years.1
In cancer patients, VTE usually includes deep vein thrombosis (DVT) of the lower extremities or pulmonary embolism (PE) and less commonly unusual site thrombosis such as cerebral or splanchnic vein thrombosis.2, 3 This latter complicates the clinical course of cancer in about 1% to 12% of cases depending on differences in study population and diagnostic protocols, and up to one-third of thrombosis are detected incidentally.4-7 About 20% of patients with splanchnic vein thrombosis have solid cancer as underlying provoking risk factor.2, 8-10 Splanchnic vein thrombosis may also represent an early marker of cancer, mostly liver and pancreatic cancer, with the highest incidence reported in the first 3 months after the diagnosis of the thrombotic event.11
Most studies focused on the incidence of splanchnic vein thrombosis in cancer patients providing limited information on clinical outcomes and anticoagulant treatment.3, 7 In a recent retrospective study including 122 patients with advanced pancreatic cancer and splanchnic vein thrombosis, anticoagulant treatment, mostly low molecular weight heparin, was provided to less than one-half of patients and seemed to increase the risk of major bleeding by two-fold.4
The objective of this study was to evaluate the characteristics, anticoagulant treatment, and clinical outcomes of patients with solid cancer and splanchnic vein thrombosis and compare them with a cohort of cancer patients with usual site VTE.
2 MATERIALS AND METHODS
This study is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement for observational studies.
2.1 Patients and study characteristics
The design and main results of the multicenter, international, prospective registry on splanchnic vein thrombosis of the ISTH have been previously published.12 Briefly, consecutive patients aged >16 years with an objectively diagnosed splanchnic vein thrombosis within 6 months before inclusion were enrolled from May 2008 to January 2012. There were no exclusion criteria.
In the current analysis, we evaluated all patients with solid cancer as an underlying risk factor for splanchnic vein thrombosis who experienced one of the outcomes of interest within 12-months follow-up or who had a minimum follow-up of 12 months. The comparison cohort included patients with solid cancer and usual site VTE (ie, DVT of the lower and upper limbs and/or PE) who were followed at two specialized Thrombosis centers in Chieti and Varese, Italy. Patients with solid cancer and usual site VTE were included in a 1:1 ratio and were required to have a minimum of 12 months follow-up at December 2019 or to have experienced one of the outcomes of interest within 12 months after the diagnosis of VTE.
The ethics committees or institutional review boards of each participating center approved the study and all patients provided written informed consent, where needed.
The following data were collected: demographic and VTE risk factors (eg, age, sex, race, personal or family history of VTE), type and stage of cancer, cancer treatment, clinical presentation of splanchnic vein thrombosis and usual site VTE (ie, symptomatic or incidentally detected thrombosis), site and extension of thrombosis (ie, portal, mesenteric, splenic, suprahepatic veins, multiple veins involvement, lower and upper limbs DVT, PE), diagnostic tests (ie, doppler ultrasound, computed tomography, magnetic resonance imaging, angiography, and laparoscopic or open surgery), laboratory parameters (eg, blood count), type of anticoagulant therapy (ie, low molecular weight heparin, unfractionated heparin, fondaparinux, vitamin K antagonists, direct oral anticoagulants, antiplatelet therapy, and thrombolysis) and duration of treatment.
2.2 Clinical outcomes
The primary outcomes were recurrent VTE, major bleeding, and all-cause mortality. Recurrent VTE included objectively diagnosed recurrent splanchnic vein thrombosis (ie, thrombus extension or thrombosis in a previously patent splanchnic segment), PE, and/or DVT of the lower and upper limbs. Major bleeding was defined according to the ISTH criteria as fatal bleeding, bleeding leading to surgery or hospitalization, occurring in a critical organ (intracranial or intraspinal, retroperitoneal, intraocular resulting in visual impairment), overt bleeding associated with a drop in hemoglobin levels of 2 g/dL or more or requiring ≥2 units of red blood cell transfusion.13
2.3 Statistical analysis
Continuous variables were expressed as mean (standard deviation) or median (interquartile range), according to data distribution after applying the Wilk-Shapiro test; categorical variables were expressed as counts and percentages. Continuous variables were compared using the Student's t test or the Mann-Whitney U test, and categorical variables were compared using the chi-squared or Fisher's exact tests, as appropriate.
In the primary analysis, follow-up time was calculated from the diagnosis of vein thrombosis until the occurrence of recurrent VTE, major bleeding, death, or 12 months follow-up, whichever came first. The frequency of bleeding or thrombotic events and all-cause mortality was expressed as cumulative incidence with 95% confidence intervals (CIs). Thrombotic and bleeding events were further analyzed in competing risk analysis, considering death as a competing event.14 The competing risk survival analysis was performed using the following commands in Stata: stcrreg, stcurve, stcompet, and stcomlist.15-17 The Gray's test was calculated with the R package “cmprsk",18, 19 and used to compare the curves. Subsequently, the Fine and Gray proportional hazards model was used for modelling the cumulative incidence curve with the covariates age and sex, and the adjusted sub-hazard ratios (HR) were calculated.20
Kaplan-Meier curves were created to estimate the overall survival and compared using the log-rank test. To evaluate whether the site of thrombosis (splanchnic vs usual site VTE) influenced mortality rates, a Cox regression was fitted using age and sex as covariates and the adjusted HR was calculated. Subsequently, multivariable Cox proportional hazards models were used to identify potential predictors for mortality, separately for the two cohorts, using backward stepwise elimination (with levels of P < .05 for inclusion and P > .10 for exclusion).21 The following variables were chosen a priori and included in the model: age, sex, extent of vein involvement (single vs multiple veins for the splanchnic vein thrombosis cohort; PE with or without DVT vs DVT alone for the usual site VTE cohort), anticoagulant treatment (yes vs no), clinical presentation (symptomatic vs incidentally detected venous thrombosis), cancer site (hepatobiliary and pancreas vs gastrointestinal vs genitourinary vs others), and presence of metastasis.
Sensitivity analyses were performed to evaluate the cumulative incidence of clinical outcomes (a) in the two cohorts at the 6-month follow-up and (b) in treated versus untreated patients with splanchnic vein thrombosis at the 12-month follow-up.
In another sensitivity analysis, the outcomes (recurrent VTE and major bleeding in the competing risk of death analysis, and all-cause mortality) were also calculated after matching the two cohorts by sex, age categories (20-29 years, 30-39 years, 40-49 years, 50-59 years, 60-69 years, 70-79 years, 80-89 years), and cancer site categories (hepatobiliary and pancreas, gastrointestinal, genitourinary, others).
The statistical software Stata/SE (version 12, StataCorp LP, College Station, TX, USA) and R (version 3.6.3, R Core Development Team, Vienna, Austria)22 were used for the analysis. P values <.05 were considered statistically significant.
3 RESULTS
A total of 264 patients (132 for each cohort) were included in the primary analysis. The main patient characteristics are shown in Table 1. Patients with splanchnic vein thrombosis were younger (61 vs 70 years, P < .01) and more often male (67.4% vs 50.0%, P < .01) compared with those in the usual site VTE cohort. The most frequent cancer types were hepatobiliary and pancreatic cancer for the splanchnic vein thrombosis cohort (57.6%) and gastrointestinal cancer (31.1%) for patients in the usual site VTE cohort. A lower proportion of patients with splanchnic vein thrombosis had metastatic disease (36.1% vs 72.5%, P < .01) and was receiving cancer-specific therapy at the time of thrombosis (29.6% vs 65.4%, P < .01). Additional information on cancer and cancer treatment is available in Table S1.
| Variables | Overall n = 264 | Splanchnic Vein Thrombosis n = 132 | Usual Site VTE n = 132 | P Value |
|---|---|---|---|---|
| Age, y (IQR) | 67.0 (55.0, 73.0) | 61.0 (52.0, 70.5) | 70.0 (59.5, 75.0) | <.01 |
| Male, n (%) | 155 (58.7) | 89 (67.4) | 66 (50.0) | <.01 |
| Incidentally detected, n (%) | 106 (40.2) | 59 (44.7) | 47 (35.6) | .13 |
| Personal history of VTE, n (%) | 23/262 (8.8) | 10/130 (7.7) | 13/132 (9.9) | .54 |
| Family history of VTE, n (%) | 6/260 (2.3) | 5/128 (3.9) | 1/132 (0.8) | .12 |
| Laboratory values | ||||
| Hemoglobin <10 g/dL, n (%) | 56/226 (24.8) | 32/109 (29.4) | 24/117 (20.5) | .12 |
| Platelet <100 x 109/L, n (%) | 30/228 (13.2) | 24/110 (21.8) | 6/118 (5.1) | <.01 |
| Cancer characteristic at thrombosis diagnosis | ||||
| Cancer type, n (%) | <.01 | |||
| Gastrointestinal | 75 (28.4) | 34 (25.8) | 41 (31.1) | |
| Genitourinary | 42 (15.9) | 28 (21.2) | 14 (10.6) | |
| Hepatobiliary and pancreatic | 84 (31.8) | 76 (57.6) | 8 (6.1) | |
| Other sites | 63 (23.9) | 8 (6.1) | 55 (41.7) | |
| Cancer therapy at diagnosis, n (%) | 114/228 (50.0) | 29/98 (29.6) | 85/130 (65.4) | <.01 |
| Locally advanced cancer, n (%) | 121/219 (55.3) | 60/98 (61.2) | 61/121 (50.4) | .11 |
| Peritoneal carcinomatosis, n (%) | 29/213 (13.6) | 12/95 (12.6) | 17/118 (14.4) | .71 |
| Metastasis, n (%) | 130/228 (57.0) | 35/97 (36.1) | 95/131 (72.5) | <.01 |
| Anticoagulant therapy | ||||
| Anticoagulant therapy, n (%) | 222 (84.1) | 91 (68.9) | 131 (99.2) | <.01 |
| Parenteral therapy, n (%) | 178 (67.4) | 61 (46.2) | 117 (88.6) | <.01 |
| Vitamin K antagonists, n (%) | 30 (11.4) | 30 (22.7) | 0 (0.0) | <.01 |
| Direct oral anticoagulants, n (%) | 14 (5.3) | 0 (0.0) | 14 (10.6) | <.01 |
| Therapy duration, month (IQR) | 6.0 (2.0, 12.0) | 6.0 (3.5, 12.0) | 11.0 (5.6, 12.0) | <.01 |
- Abbreviations: IQR, interquartile range; SD, standard deviation; VTE, venous thromboembolism.
Splanchnic vein thrombosis included portal, mesenteric, suprahepatic, splenic, and multiple vein thrombosis in 79.6%, 31.1%, 12.9%, 9.1%, and 27.3% of patients, respectively. In the usual site VTE cohort, PE with or without DVT represented 69.7% of cases (DVT involved the lower and upper extremities in 39 and 2 patients, respectively), whereas DVT alone occurred in 30.3% (proximal DVT of the lower extremities in 35 patients and isolated distal DVT of the lower extremities in 5 patients).
Sixty-nine percent of patients with splanchnic vein thrombosis received anticoagulant therapy compared to more than 99% of those with usual site VTE (Table 1). In the former group, anticoagulant therapy consisted of parenteral anticoagulation in 46.2% and vitamin K antagonists in 22.7%; none of these patients received direct oral anticoagulants. In patients with usual site VTE, 88.6% received parenteral anticoagulation and 10.6% direct oral anticoagulants; none of these patients received vitamin K antagonists. Median anticoagulant treatment duration was significantly shorter in patients with splanchnic vein thrombosis (6.0 months vs 11.0 months, P < .01).
3.1 Recurrent VTE and major bleeding
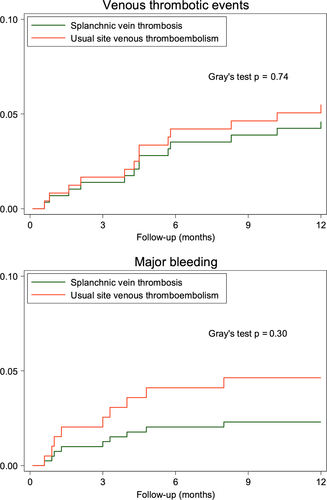
During a median follow-up of 12 months (interquartile range 4.8 to 12), recurrent VTE occurred in 13 patients, 6 in the splanchnic vein thrombosis cohort and 7 in the usual site VTE cohort, for a cumulative incidence of 4.7% (95% CI, 1.9-9.3) and 5.5% (95% CI, 2.4-10.4), respectively, in the competing risk of death analysis. Recurrent VTE at 6 months is shown in Table 2. The risk of recurrent VTE, was similar between the two cohorts (adjusted sub-HR 1.2; 95% CI, 0.4-3.3; Figure 1A). In patients with splanchnic vein thrombosis, VTE recurred in the splanchnic veins in three cases, and one recurrent event was fatal (Table 3). Three recurrent events occurred during anticoagulant therapy with low molecular weight heparin and one during treatment with vitamin K antagonist. In the usual site VTE cohort, five events recurred during treatment with low molecular weight heparin and one event occurred on direct oral anticoagulants.
| Splanchnic Vein Thrombosis | Usual Site VTE | P Value | |
|---|---|---|---|
| Cumulative Incidence % (95% CI) | Cumulative Incidence % (95% CI) | ||
| Recurrent VTE | |||
| 6-month follow-up | 3.1 (1.0-7.2) | 4.7 (1.9-9.3) | .77a |
| 12-month follow-up | 4.7 (1.9-9.3) | 5.5 (2.4-10.4) | .77a |
| Major bleeding | |||
| 6-month follow-up | 2.3 (0.6-6.0) | 3.9 (1.4-8.2) | .31a |
| 12-month follow-up | 2.3 (0.6-6.0) | 4.7 (1.9-9.3) | .31a |
| All-cause mortality | |||
| 6-month follow-up | 30.3 (23.2-38.9) | 26.5 (19.8-34.9) | .62b |
| 12-month follow-up | 41.7 (33.8-50.6) | 39.4 (31.7-48.3) | .62b |
- Abbreviations: CI, confidence intervals; VTE, venous thromboembolism.
- a Gray's test.
- b Log-rank test.
| Clinical Outcomes | Overall | Splanchnic Vein Thrombosis | Usual Site VTE |
|---|---|---|---|
| Recurrent VTE, n | 13 | 6 | 7 |
| Splanchnic vein thrombosis | 3 | 3 | 0 |
| Proximal DVT and/or PE | 8 | 2 | 6a |
| Thrombosis in other sites | 1 | 0 | 1a |
| Fatal venous event | 1 | 1 | 0 |
| Major bleeding, n | 9 | 3 | 6 |
| Gastrointestinal | 5 | 2 | 3 |
| Intracranial/intraspinal | 2 | 0 | 2 |
| Intraperitoneal | 1 | 1 | 0 |
| Retroperitoneal | 1 | 0 | 1 |
| Death, n | 107 | 55 | 52 |
| Cancer progression | 74 | 42 | 32 |
| Vascular death (venous event) | 1 | 1 | 0 |
| Vascular death (arterial event) | 4 | 0 | 4 |
| Fatal bleeding | 1 | 1 | 0 |
| Unknown or unable to adjudicate | 24 | 8 | 16 |
| Other causes | 3 | 3 | 0 |
- Abbreviations: DVT, deep vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
- a Renal vein thrombosis.
Overall, nine patients experienced a major bleeding event during follow-up, three in the splanchnic vein thrombosis cohort and six in the usual site VTE cohort, for a cumulative incidence of 2.3% (95% CI, 0.6-6.0) and 4.7% (95% CI, 1.9-9.3; Table 2), respectively, in the competing risk of death analysis. The risk of major bleeding was not statistically different between the two cohorts (adjusted sub-HR 1.4; 95% CI, 0.3-6.3; Figure 1B). All major bleeding events in patients with splanchnic vein thrombosis occurred in the first 6 months of follow-up during therapy with vitamin K antagonists with international normalized ratio within the therapeutic range. In the usual site VTE cohort, four major bleeding events occurred during treatment with low molecular weight heparin and two on direct oral anticoagulants. Five of these bleeding events occurred within the first 6 months after thrombosis diagnosis (Table 3). No major bleeding events occurred after anticoagulant treatment discontinuation in both cohorts of patients.
3.2 Survival analysis
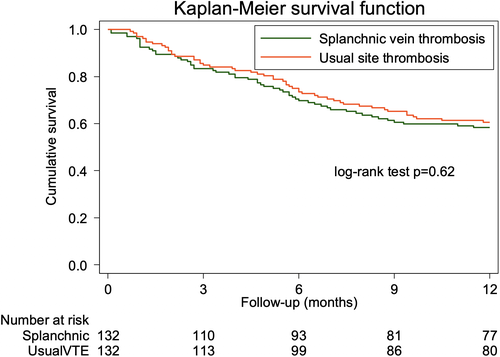
A total of 55 patients with splanchnic vein thrombosis and 52 patients with usual site VTE died during follow-up for a cumulative incidence of 41.7% (95% CI, 33.8-50.6) and 39.4% (95% CI, 31.7-48.3), respectively. All-cause mortality at 6 months is shown in Table 2. Most deaths were related to cancer progression (Table 3). In the splanchnic vein thrombosis cohort, one patient died following major variceal gastrointestinal bleeding and one patient had fatal PE. In the usual site VTE, four patients died due to an arterial event (stroke in three patients and acute myocardial infarction in one patient).
Mortality was similar between patients with splanchnic vein thrombosis and those with usual site VTE (age- and sex-adjusted HR 0.8; 95% CI, 0.6-1.2; Figure 2). In patients with splanchnic vein thrombosis, the risk of death was comparable between those receiving anticoagulant therapy and patients who received no treatment (Table S2 and Figure S1).
In multivariable analysis, the risk of death in patients with splanchnic vein thrombosis was lower in cases presenting with incidentally detected thrombosis (HR 0.3; 95% CI, 0.1-0.6), and higher in patients with hepatobiliary and pancreatic cancer (HR 2.9; 95% CI, 1.4-6.1) and metastatic disease (HR 3.5; 95% CI, 1.8-6.9). In patients with usual site VTE, hepatobiliary and pancreatic cancer (HR 6.8; 95% CI, 2.9-15.9) and metastatic disease (HR 2.6; 95% CI, 1.2-5.4) were associated with a higher risk of death.
3.3 Additional analysis
After matching the two cohorts by sex, age, and cancer site categories, a total population of 100 patients (50 for each cohort) remained in the analysis. Median age was 69.5 years in the splanchnic vein thrombosis cohort and 69.0 years in the usual site VTE cohort (P = .70); 58% of patients in both cohorts were male (P = 1.00). Primary site of cancer was hepatobiliary and pancreatic in 16%, gastrointestinal in 52%, genitourinary in 16%, and in other sites in 16% of patients in both cohorts (P = 1.00).
Cumulative incidences for recurrent VTE and major bleeding in the competing risk of death analysis and for all-cause mortality were similar for patients with splanchnic vein thrombosis and usual site VTE, both at the 6- and 12-month follow-up (Table S3).
4 DISCUSSION
The clinical history of patients with cancer-associated splanchnic vein thrombosis is similar to that of patients with cancer-associated usual site VTE in terms of recurrent VTE, major bleeding, and all-cause mortality rates. However, fewer patients with splanchnic vein thrombosis received anticoagulation and the duration of treatment was significantly shorter.
Compared with patients without malignancy, those with cancer-associated VTE have an increased risk of recurrent thrombosis, which may be as high as 11% despite anticoagulant therapy.23-26 In the presence of active cancer, the risk of recurrent VTE persists beyond the first 3 to 6 months, requiring extended anticoagulant treatment.27-31 Information on the risk of recurrence is mostly derived from studies on cancer patients with usual site VTE, whereas data for cancer patients with splanchnic vein thrombosis remain scarce.7 Because of limited information available, treatment recommendations of major international societies are based on experts’ opinion and rely on data extrapolated from studies including patients with splanchnic vein thrombosis and various underlying risk factors.32, 33 Our results are in agreement with those reported by published trials suggesting that patients with splanchnic vein thrombosis presented a not-negligible risk of recurrence and may benefit of similar anticoagulant treatment as for usual site VTE.23-25, 34 In the current study, indeed, up to one-third of patients with splanchnic vein thrombosis did not receive anticoagulation and in those who were treated, median treatment duration was 5 months shorter than in patients with usual site VTE. According to previous data of usual site VTE, a treatment duration beyond the first 3 to 6 months may further reduce the risk of recurrent VTE also in patients with splanchnic vein thrombosis.28, 31
Cancer patients with VTE have also a higher risk of major bleeding compared with the general population because of several local or systemic cancer-specific risk factors (eg, tumor invasion of the mucosa, thrombocytopenia, disseminated intravascular coagulopathy).35 The development of splanchnic vein thrombosis may further increase the risk of upper gastrointestinal bleeding by raising the pressure in the splanchnic circulation, which may lead to the rupture of gastroesophageal varices.9 The risk of bleeding with anticoagulant therapy for splanchnic vein thrombosis has been mostly evaluated in patients with liver cirrhosis, whereas little is known in patients with solid cancer.36, 37 The perceived risk of bleeding complications in this latter group may have discouraged physicians to use anticoagulation in patients with splanchnic vein thrombosis included in the current analysis. Although limited by the observational design of the study, our findings seem to be reassuring when compared with data of previous studies on usual site VTE (2% to 4%) as well as on splanchnic vein thrombosis related to other risk factors such as liver cirrhosis (about 3%).23-25, 34, 36-38 In those latter studies, anticoagulant therapy was also associated with higher recanalization rates and lower thrombosis progression, which may lead to reduced pressure inside gastrointestinal varices and lower risk of bleeding.9, 36, 39 This positive effect on splanchnic circulation hemodynamic could further support the use of anticoagulant treatment in patients with splanchnic vein thrombosis.
Whether anticoagulant therapy improves survival of patients with splanchnic vein thrombosis remains unclear.3, 4 The occurrence of splanchnic vein thrombosis may cause or worsen portal hypertension and impair liver function, which could negatively affect patients’ prognosis.9, 32 In the current study, over half of patients died within 12 months, with no survival difference between those receiving anticoagulant therapy and patients who were left untreated. The risk of death was comparable between patients with splanchnic vein thrombosis and usual site VTE, despite the proportion of patients with metastatic cancer being significantly higher in the latter than in the former group. Six-month mortality rates were similar to those reported in recent trials.23-25, 34, 40
The current study has some limitations that warrant discussion. First, the number of patients with splanchnic vein thrombosis was relatively low and may not be completely representative of this patient population. However, the current cohort of patients with splanchnic vein thrombosis is the largest reported to date with clinical outcomes evaluated over a long follow-up. Second, the type, dose, and duration of anticoagulant treatment were left at the discretion of the treating physician and heterogeneity in treatment regimens hampered to draw firm conclusions on the safety and efficacy of anticoagulation. The acute versus chronic presentation of splanchnic vein thrombosis may have affected decisions about anticoagulation. Although splanchnic vein thrombosis had to be diagnosed within 6 months and we collected information on clinical signs and symptoms, it was not possible to establish with certainty whether splanchnic vein thrombosis was acute in all cases, especially those with incidental thrombosis. Third, patients with usual site VTE differed in several characteristics, including cancer-related variables and type of anticoagulant treatment, which represents a limitation for the comparisons between the two cohorts. However, this reflects the different characteristics and different management strategies between the two populations and the analysis restricted to a smaller cohort of patients matched by sex, age, and cancer type found similar results (Table S2). The different enrollment period between the two cohorts of patients may have affected study outcomes because of the significant changes in cancer treatment and anticoagulant treatment over the years. In cancer patients with usual site VTE, recent randomized controlled trials suggested that the latter may be one of the factors contributing to the lower incidence of bleeding complications compared with earlier studies.23, 25, 41 Fourth, because of the low number of events, the analysis on recurrent VTE and major bleeding could not be adjusted for several potential confounders, which may weaken the overall strength of our conclusions. The incidence of recurrent thrombosis in patients with usual site VTE appeared to be lower compared with similar patient groups included in recent randomized controlled trials.23-25 Although cross-study comparison remains difficult, difference in VTE risk may suggest the inclusion of a lower risk group. Although the baseline patient characteristics did not suggest a different risk profile, we cannot exclude residual confounding related to unmeasured variables.
In conclusion, patients with solid cancer-associated splanchnic vein thrombosis appear to have a risk of recurrent thrombosis and major bleeding that are similar to those of cancer patients with usual site VTE. While awaiting further studies to confirm these results in a larger and more homogenous population, current findings may inform treatment decisions in daily clinical practice and provide the basis for future research studies.
ACKNOWLEDGMENTS
We acknowledge the contribution of M. Bazzan, C. Becattini, S. Betti, E. Bucherini, E. Ciantar, E. D'Amico, M.T. De Sancho, V. De Stefano, D. Di Minno, E. Grandone, B. Nardo, S. Pasca, S.M. Passamonti, D. Poli, M. Senzolo, A. Vaccarino, P. Verhamme, and A. Winder.
CONFLICT OF INTEREST
Dr. Di Nisio reports personal fees from Bayer, Daiichi Sankyo, BMS-Pfizer, Leo Pharma, Sanofi, and Aspen, outside the submitted work; Dr. Sartori reports personal fees from Bayer and Daiichi Sankyo outside the submitted work; Dr. Beyer-Westendorf received honoraria and institutional research support from Bayer HealthCare, Boehringer Ingelheim, BMS/Pfizer, CSL Behring, Daiichi Sankyo, and LEO Pharma; Dr. Ageno has received a research grant from Bayer to support a clinical study in patients with splanchnic vein thrombosis, received honoraria for participation at advisory boards from Bayer, Boehringer Inghelheim, Daiichi Sankyo, BMS/Pfizer, Sanofi, and Portola, and reports grants and personal fees from Bayer, and personal fees from BMS/Pfizer, Daiichi Sankyo, Sanofi, Aspen, Janssen, and Portola, outside the submitted work. Drs. Valeriani, Riva, Caiano, Porreca, Bang, Barillari, Santoro, Kamphuisen, Alatri, Malato, Vidili, Oh, and Schulman have nothing to disclose.
AUTHOR CONTRIBUTION
Study conception and design: Marcello Di Nisio, Emanuele Valeriani, Walter Ageno; data acquisition: Emanuele Valeriani, Nicoletta Riva, Lucia Maria Caiano; statistical analysis: Nicoletta Riva, Emanuele Valeriani, Marcello Di Nisio; interpretation of the data: all authors; drafting of the manuscript: Emanuele Valeriani, Marcello Di Nisio, Nicoletta Riva, Walter Ageno; critical revision of the manuscript for important intellectual content: all authors; final approval of the manuscript: all authors.