Protective effects of Dendrobium huoshanense polysaccharide on D-gal induced PC12 cells and aging mice, in vitro and in vivo studies
Mengjuan Ye and Junlin Liu contributed equally to this work.
Abstract
Dendrobium huoshanense C. Z. Tang et S. J. Cheng polysaccharide (DHP) is the essential active ingredient of D.huoshanense and has high medicinal value. A high dose of D-galactose (D-gal) is commonly utilized in the aging model establishment. In this study, we explored whether DHP shields PC12 cells and aging mice from D-gal caused damage and the possible mechanism. In vitro experiments, D-gal induced PC12 cells were used to investigate, and then DHP was used for treatment. In vivo experiments, 72 SPF ICR male mice were randomly divided into six groups (control: normal saline; model: D-gal (400 mg/kg); VE group: VE (50 μg/ml); DHP groups: D-gal + DHP (15.6 mg/ml; 31.2 mg/ml; 62.4 mg/ml)). The results showed that DHP could enhance the viability of D-gal injured PC12 cells and prevent cell apoptosis. DHP effectively promoted the transition from phase G0/G1 to phase S and inhibited cell cycle arrest. DHP has a potential neuroprotective effect on D-gal caused cognitive and memory disorders in mice. On the one hand, DHP protects the antioxidant enzymes SOD, GSH-PX, and CAT from excessive ROS buildup. On the other hand, DHP was demonstrated to block the expression of the P53/P21 signaling pathway-related proteins P53, P21, and P16. These results imply that DHP could be a potential neuroprotective agent against aging.
Practical applications
Cognitive and memory decline caused by aging problems has become a problem in recent years. There are many theories about aging, among which oxidative stress is considered to be one of the important pathophysiological parts of various diseases in the aging process. In this study, DHP could not only improve the damage of D-Gal to PC12 cells, but also improve the cognitive and memory impairment caused by D-Gal in mice. In conclusion, this study verified the anti-aging effect of DHP from in vitro and in vivo experiments, and its mechanism may involve the P53/P21 pathway. Therefore, this study indicated that polysaccharides from Dendrobium huoshanense, a traditional Chinese medicine of homologous medicine and food, had potential and industrial value as potential anti-aging drugs.
1 INTRODUCTION
D.huoshanense, a rare perennial herb, is a species of the Dendrobium genus (Orchidaceae), which is only distributed in China (Wang et al., 2012). DHP is the main active ingredient in D.huoshanense (Fan et al., 2020). In recent years, DHP has attracted much attention with its outstanding and comprehensive biological activities, such as regulating immunity, alleviating diabetes and its complications, anti-tumor, anti-oxidation and other pharmacological effects (Hsu et al., 2022; Yeow et al., 2020). Aging is a natural and gradual process that is characterized by a recession in the bodily organs. And cognitive and memory abilities, like Alzheimer's disease (AD) (Ashiqur Rahman et al., 2021; Chen et al., 2019). With the improvement of living standards, it has become a new research hotspot to explore potential drugs with antioxidant and antiaging effects. However, there are few studies on the role of DHP in aging. As a traditional Chinese medicine of homologous medicine and food, D.huoshanense has high research values and development prospects (X. Liu et al., 2022; Zhou et al., 2020). Therefore, it is critical to research the effects of DHP on aging as well as its mechanism for the development and usage of D.huoshanense resources and associated products. This study took a D-gal induced senescence PC12 cell (from a pheochromocytoma of the rat adrenal medulla) as one of the research subjects, then analyzed the cell viability, cytotoxicity, apoptosis, and cell cycle of PC12 cells after DHP treatment (X. Liu et al., 2022; Y. Liu et al., 2021). In addition, D-gal mice were used as the animal model and the protective effects of DHP were verified by testing the antioxidant, pathological indicator, behavioral indicators, and related gene expression. In brief, this study used in vitro and in vivo experiments to investigate DHP's anti-aging impact and potential mechanism.
2 MATERIALS AND METHODS
2.1 Animals
Pizhou Oriental Breeding Co. LTD provided male ICR mice (20 ± 2 g) (SPF grade). The experimental animal quality certificate number is No. 0020351. The license number is SCXK (SU) 2017-001. For 7 days before the experiment, the mice were placed in a clean cage in a comfortable environment, the temperature was controlled at 22°C and the humidity was 40%~70% (Kim et al., 2014), and the mice were allocated at random following adaptive feeding.
2.2 Preparation of DHP
Dendrobium huoshanense plants were purchased from the Anhui Huoshan Changchong Chinese Herbal Medicine Co. Ltd. The botanical origins of D.huoshanense were identified by Nianjun Yu. Voucher specimens have been deposited in the Chinese Medicine Resource Centre, Anhui University of Chinese Medicine (Anhui, China) with voucher No.20211201. Freeze-dried D.huoshanense powder was refluxed three times with ultrapure water (material/liquid ratio = 1:100; g:ml) for 1 h each time. All the filtrate was collected and decompressed to concentrate. They were added with five times the volume of 95% ethanol. After centrifugation, the crude extract of D.huoshanense polysaccharide precipitation was obtained. Then it was dissolved in ultrapure water, and sevage reagent was added at a 1:1(V/V) ratio to remove proteins. The extracts were stored at − 20°C for 24 h and before being lyophilized. Finally, the refined DHP was obtained.
2.3 Experiments in vitro
2.3.1 Cell culture and treatment and cell viability assay
The PC12 cells were purchased from Pu-nuo-sai Life Technology (Wuhan, China). They were maintained in 44.5 ml 1640 (HyClone, USA), 5 ml FBS medium (BI, Israel) containing 0.5 ml penicillin/streptomycin. Using containing 5% CO2 incubator culture cell at room temperature for 24 h. Thereafter, they were cultured for 24 h with or without different doses of DHP (25, 50, 100, 200, 400, and 800 μg/ml). To evaluate the cytotoxic effect of DHP on PC12 cells. PC12 cells were exposed to D-gal (1.25, 2.50, 5.00, 10.00, and 20.00 mg/ml) to determine the appropriate D-gal concentration for aging induction. Then, DHP was added to protect PC12 cells stimulated by D-gal (Sigma, USA), and the protective effect was compared with that of the positive VE group (Vitamin E, 50 μg/ml)(Zhong et al., 2009). Finally, the CCK-8 kit (Biyuntian Biotechnology, China) was used to determine cell viability.
2.3.2 SA-β-Gal staining analysis
The PC12 cells with good growth conditions were seeded in a 96-well plate (1 × 104/well). According to the results of 1.3.1 experiments, 100, 200, and 400 μg/ml were determined as low, medium, and high concentrations of DHP. D-gal (10 mg/ml) was used as the modeling concentration. PC12 cells were routinely cultured in a clean incubator for 24 h after dosing. The activity of β-galactosidase was analyzed by SA-β-Gal staining kit (Biyuntian Biotechnology, China). The staining results were obtained under an inverted microscope (Olympus, Japan). β-galactosidase positive cells were observed and counted at 8 randomly selected fields in each well.
2.3.3 ROS detection
After modeling and dosing, the original medium was discarded. The cells were collected after they were cleaned with sterile and digested by pancreatin. The cells were resuspended and the density was reduced to 1 × 106/ml. And left in an incubator at 37°C for 20 min. The cell suspension was mixed every 3 min to promote integrating the probe with the cells. To sufficiently remove the DCFH-DA working solution, a serum-free 1640 medium was used to resuspend and wash the cells 3 times. Finally, ROS was detected by a flow cytometer (Beckman, USA) (parameters: excitation wavelength at 488nm, emission wavelength at 525 nm).
2.3.4 Apoptosis assay
The remaining medium was transferred to the centrifuge tube after modeling and dosing. Using Trypsin Solution Without EDTA (Beyotime, China) to digest the cells. Collected and counted the cells after resuspended in PBS. Then the cells were gently resuspended by adding Annexin V-FITC binding solution (Biyuntian Biotechnology, China) after the supernatant was discarded. Next, in the dark, 5 μl Annexin V-FITC solution was added and cultivated for 15 min, followed by 10 μl Propidium Iodide staining solution and incubated for 20 min. Finally, flow cytometry was used to analyze apoptosis.
2.3.5 Cell cycle assay
The cells were digested and harvested. After which, they were fixed in 70% ethanol for 12 h at 4°C. The next day, the cells were washed 2 times in pre-cooled PBS (Kilton organism, China). The cells were incubated for 30 min after being stained with propidium iodide (PI). The cell cycle distribution was examined by flow cytometry. (Parameter: excitation wavelength: 488 nm).
2.3.6 Quantitative real-time PCR analysis
RNA extractions were added to break the cells completely. After adding chloroform, the samples were centrifuged for 15 min at 12,000 rmp, 4°C. Retain the supernatant and an equal volume of isopropanol was added. After centrifugation, we gently purged precipitates with 75% ethanol. The precipitate was dissolved with ultrapure water. Subsequently, an ultra-micro-nucleic acid detector (Analytik Jena AG, Germany) was adopted to analyze the total RNA. The first-strand cDNA was got by reverse transcription kit (Servicebio, China). P53, P21, and P16 related gene mRNA transcription levels in PC12 cells were evaluated using quantitative real-time qRT-PCR kit (Abcam Inc, Canada). β-Actin was used as an internal reference among them. Using the 2−ΔΔCt method to assay the relative mRNA expression levels. The primer sequences were as follows in Table 1.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| P53 | CCCTCTGAGCCAGGAGACATT | CCCAGGTGGAAGCCATAGTTG |
| P21 | CAGCCATGACGAGCTGTTCT | CTTTCGGTACCTTCGCCCTC |
| P16 | CTACTCTCCTCCGCTGGGAA | GGCCTAACTTAGCGCTGCTT |
| β-actin | GTGACGTTGACATCCGTAAAGA | GTAACAGTCCGCCTAGAAGCAC |
2.4 Experiments in vivo
2.4.1 Animal model and treatment
We randomly divided 72 SPF ICR male mice into the following 6 groups (12 mice per group) after adaptive feeding for one week: Control: normal saline; Model: D-gal (400 mg/kg); VE group: Vitamin E (50 μg/ml); DHP groups: D-gal + DHP1(15.6 mg/ml); D-gal + DHP2(31.2 mg/ml); D-gal + DHP3(62.4 mg/ml). Mice in the control group were received the same volume of normal saline, while other mice were injected with D-gal (400 mg/kg, 0.2 ml) orally once daily for 8 weeks continuously. In addition, the DHP groups were given DHP orally once daily for 8 weeks, while the other groups were given the equal volume of normal saline.
2.4.2 Survival quality and weight monitoring
After adaptive feeding for a week, mice were randomly grouped and weighed. The mental state, diet, drinking water, activity, and hair color of mice per group were observed daily during the process of body-weight modeling and dosing. The animals have weighed every 7 days and the curve of weight changes.
2.4.3 Morris water maze
The MWM test was constructed by referring to the previous study (Sudeep et al., 2021; Temido-Ferreira et al., 2020). The water surface of the circular water maze (1.2 m in width and 0.45 m in height) was divided into four quadrants clockwise, and the platform was hidden in the center of the third quadrant, 1 cm below the water surface. Then the mice were put into the water from different quadrants in turn and asked to search for the hidden platform within 60 s. The time for the mice to search the platform was defined as latency. If the platform did not be found within 60 s, the mice were directed to the platform and asked to stay on the platform for 10 s to familiarize themselves with the environment, and we noted down the latency was 60 s. All mice were trained for 5 consecutive days, three trials per day. The average value of three training sessions was used as the latency of the day to evaluate the spatial learning capacity of mice per group. On the sixth day, the submerged platform was removed. The trajectories of mice were recorded by the EthoVision XT animal motion tracking system (Norstar, China). The memory ability of mice in each experimental group was evaluated by the times of passing the original platform and movement track within 60 s.
2.4.4 Histological examination
Histological examination was assessed by using haematoxylin and eosin staining (Quan et al., 2022). After the mice were sacrificed by cervical dislocation, the brain tissues and liver tissues harvested. We cleaned the tissues with pre-cooled normal saline and dried them with filter paper. Finally, the samples were preserved in neutral buffer at −80°C. The tissue was conventionally embedded and fixed in paraffin and then stained with hematoxylin-eosin (HE) after cutting into sections (4~7 μm). Finally, using the inverted microscope (Leica, Germany), pathological changes in brain and liver tissues were observed. The remaining parts were stored in −80°C for subsequent Western Blot detection.
2.4.5 MDA, SOD, GXH-PX, and CAT levels assay
MDA, SOD, GXH-PX, and CAT levels in brain and liver tissues were measured according to the guidance of each kit (Jiancheng Bioengineering, China).
2.4.6 Western Blotting analysis
PC12 cells and fresh mouse liver and brain tissues of different treatment groups were collected. Using PMSF-containing lysis buffer to lyse cells. The liver and brain tissues were transferred to a tissue grinder and the tissues were fully lysed with lysate containing histone. After centrifugation of tissue homogenates, the supernatants were transferred to a new tube and protein concentration was determined. The protein extract was transferred from 12% SDS-PAGE gel to PVDF membrane. Dilute primary antibody at 1:1,000, except for P21, which was used at a 1:2,000 dilution. After that, horseradish peroxidase (HRP) conjugated secondary antibodies (1:10,000) were applied to the membranes for 1 h at 37°C. Subsequently, using ultrasensitive chemiluminescence imaging system to observe the expression of proteins (Dorszewska & Adamczewska-Goncerzewicz, 2004).
2.5 Statistical analysis
SPSS 23.0 software and Graph Pad Prism 5 software were used for statistical analysis. Data were presented as mean ± standard deviation (SD), and statistical results were compared using one-way analysis of variance (ANOVA). p < .05: illustrates a statistical difference and p < .01: illustrates a significant difference.
3 RESULTS
3.1 DHP improves the viability of D-gal - induced PC12 cells
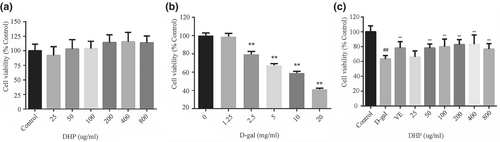
To assess the cytotoxicity of DHP on PC12 cells and the appropriate dose of D-gal for modeling, and to verify the protective effect of DHP on D-gal stimulated PC12 cells, the CCK8 method was used to detect cell viability. In the Figure 1, the results revealed that DHP (50, 100, 200, 400, and 800 μg/ml) has no significant cytotoxicity (p > .05). Although D-gal (1.25 mg/ml) slightly decreased cell viability, but there was no statistical difference. PC12 cells were treated with D-gal (2.5, 5, 10, and 20 mg/ml), and there was a negative correlation between cell viability and D-gal concentration, with statistical differences (p < .01). Therefore, a D-gal concentration of 10 mg/ml (60% cell viability) was selected for aging induction. Compared with the model group, DHP reversed the D-gal induced decline in PC12 cell viability. The protective effect of DHP (100, 200, and 400 μg/ml) on PC12 cells was stronger than that of VE (50 μg/ml). Therefore, the concentration above was selected as the low, medium, and high dose of DHP.
3.2 SA-β-Gal staining results
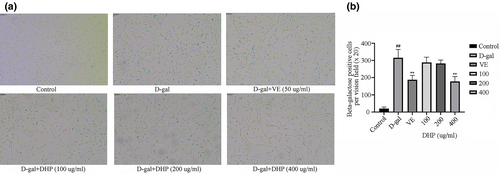
β-galactosidase activity is one of the most widely used a marker of cellular senescence. The staining of different groups was noticed under an inverted microscope, and the percentage of positive staining was analyzed. As demonstrated in Figure 2, 5% of cells were positive in the control group, whereas over 70% were in the model group. The results showed that the model of aging was established successfully. However, the DHP and VE groups had a lower positive rate in comparison to the model group. The resistance of high dose DHP (400 μg/ml) to cellular senescence was close to that of the VE group, and the positive rate was remarkably lower in comparison to the model group. (p < .01).
3.3 DHP decreased the ROS production
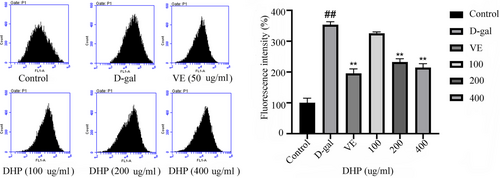
ROS detection results by DCFH-DA are shown in Figure 3 illustrated that the ROS fluorescence in the model group was considerably higher than in the control group. (p < .01). DHP (200, 400 μg/ml) and VE (50 μg/ml) groups markedly decreased the ROS production (green fluorescence) (p < .01).
3.4 DHP alleviated PC12 cell apoptosis induced by D-gal
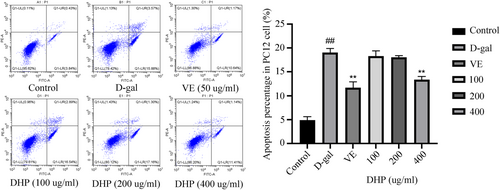
The staining of cells in the upper right and bottom right quadrant was more obvious in the model group than in the control group, as shown in Figure 4. D-gal markedly expanded apoptosis (p < .01) in PC12 cells, whereas the treatment with both DHP (400 μg/ml) and VE markedly decreased (p < .01) the apoptosis rate. These results indicated that DHP could effectively alleviate PC12 Cell apoptosis induced by D-gal.
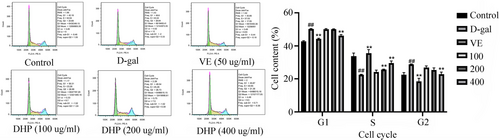
3.5 Effects of DHP on the cell cycle
Figure 5 shows that the model group has a higher G0/G1 fraction than the control group (p < .01), thus suggesting that blockage at the G1/S checkpoint may be induced following D-gal treatment. In contrast to the model group, the VE group and DHP group promoted the transformation from phase G0/G1 to phase S and decreased phase G0/G1 significantly (p < .01). These data revealed that DHP could promote the initiation of cell division, protect cells and delay cellular senescence.
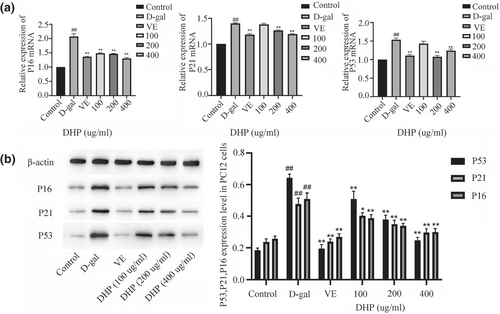
3.6 DHP reduced the mRNA and protein expressions of P53, P21, and P16 genes
We designed to verify the anti-aging effect of DHP through the P53/P21 and P16/PRB signaling pathway. As shown in Figure 6, the observed variations in the transcription level and protein expression of genes P53, P21, and P16, which enhanced in comparison to the control group. (p < .01). However, the medium and high-dose groups and VE group reduced the expression levels of the senescent regulatory genes in damaged PC12 cells, with statistical significance compared with the model group (p < .01). Similarly, in comparison to the model group, the levels of aging-related protein expression in each group were lower, with significant differences. (p < .05).
3.7 DHP alters the survival quality and weight in aging mice
During the experiment, the mice in the model group began to develop loose and dull skin, yellowish hair, a flagging spirit and decreased appetite after 2 weeks of modeling. In the positive drug group and DHP group, the above conditions were improved to a certain extent, especially alleviated decreased appetite and yellowish hair. The weight before and after modeling per group of mice is also one of the key indicators to judge the success of the aging model. The weight of mice per group was determined on Monday. The results revealed that the weight of mice in the control group increased normally, but in the model group declined substantially. The weight loss of mice in the VE and DHP groups was alleviated to some extent in comparison to the model group, and the weight recovered well (Figure 7).

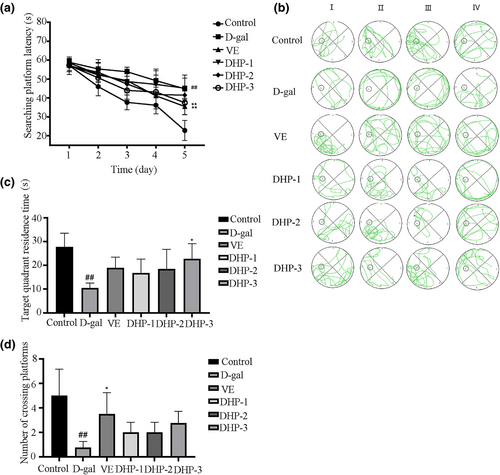
3.8 Effect of DHP on D-gal-induced aging mice behavior
The mice's learning ability were assessed using a positioning and navigation test, it was found that during the 5-day training period, the latency of the mice per group decreased as the training time increased. For the model group, its reduction was small in magnitude, indicating that the learning ability of mice reduced considerably (p < .01). However, the high dose DHP and VE group were markedly better than the model group (p < .01), as illustrated in Figure 8a. The model group had short trajectories, slow motility, disorder path, and without purpose, as shown in Figure 8b, whereas the movement trajectory of the VE and DHP group almost mapped onto the optimal path (the third quadrant). Moreover, the target quadrant residence time in each group was longer than that in the model group, and the high-dose DHP group differs markedly from the model group (p < .05), as illustrated in Figure 8c. Furthermore, Figure 8d shows that the treatment with both DHP and VE increased the number of crossing the platform within 60 s on day 6. Taken together, these results suggest that brain injury was induced in mice via an intraperitoneal injection of D-gal, leading to memory decay. In contrast, mice given DHP or VE showed a good mental state and learning and memory ability.
3.9 DHP affects the histopathology in aging mice
As evident in Figure 9, the control group's liver tissue revealed no pathological changes, the hepatocytes were well-differentiated, and the central vein was also clearly visible. The D-gal group's liver tissue had disarranged hepatic cords, chromatin is rough, and the sizes of the cells were uneven. Moreover, the model group's pyramidal cells in the hippocampus were loosely arranged and a large number of inflammatory cells were found infiltrating the area. However, there were complete cell morphology, intact and closely arranged, and no obvious cell necrosis in DHP administration groups. These results illustrate the use of DHP could reduce liver, brain damage caused by D-gal.

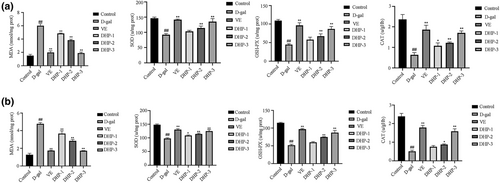
3.10 DHP improves oxidative stress levels in aging mice
As indicated in Figure 10, in comparison to the control group, the content of brain and liver MDA in the model group was increased, while the activities of SOD, GSH-Px, and CAT were lower (p < .01). Our findings indicate that D-gal induced the MDA products and affect the activity of antioxidant enzymes. Moreover, we found that decreased content of MDA was caused by VE or DHP treatment, accompanied by evident elevated activities of anti-oxidative enzymes such as SOD, GSH-Px, and CAT.
3.11 DHP affects protein levels in brain and liver tissue
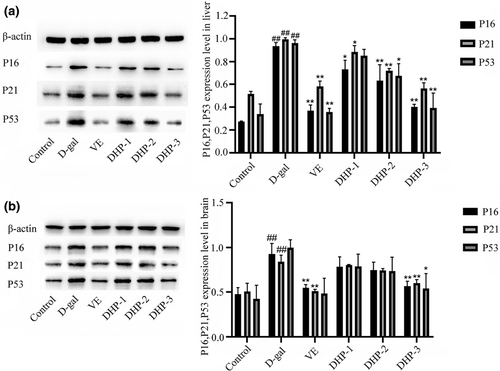
The results showed the same trend as in vitro experiments. We further analyzed protein expression of P16, P21and P53 in brain and liver tissue and found a significant upregulation in aged mice. Western blot analysis showed that the protein expression levels were obviously downregulated after DHP treatment. This effect appeared to be dose-dependent with the effect being significant (p < .05) at a dose of 62.4 mg/ml (Figure 11).
4 DISCUSSION
To investigate DHP's possible anti-aging mechanism, we designed in vitro and in vivo model, which we are here sharing for the first time.
In vitro experiments, D-gal induced PC12 cells were used to investigate, then DHP was used for treatment. The results indicated that DHP has no significant cytotoxicity toward the PC12 cells. DHP-treated PC12 cells had increased cell viability with decreased apoptosis. In addition, we found that both DHP and VE could improve the SA-β -Gal staining characteristics of D-gal induced PC12 cells, which showed similar effects on antagonistic cell senescence.
There are many theories about aging, among which oxidative stress is recognized as one of the important pathophysiological processes that usually accompany aging-related diseases (Mykhailenko et al., 2020; Zhu et al., 2019). Growing evidence in recent years suggests that the connection between ROS and health (Suzuki et al., 2020). On the one hand, low levels of ROS are one of the key signal molecules. On the other hand, excessive production of ROS results in oxidative damage to intracellular biomacromolecules, which in turn leads to aging, cancer, and many other diseases (Chanmanee et al., 2022; Momchilova et al., 2014). This study showed that DHP can significantly reduce ROS production compared with the model group. In this paper, we propose that DHP is the key to balancing the oxidative and antioxidant systems of cells by reducing the production of ROS.
Recent studies have shown that the increasing pressure of cell replication causes DNA damage and cell cycle arrest, and replicative senescence is the main cause of body aging (Go et al., 2020; Nechiporuk et al., 2016). Cyclin-dependent kinases (CDKs) are critical regulators of replicative senescence procession (Cui et al., 2021; Gao et al., 2016; Misono et al., 2019). Furthermore, P21 and P16 are key genes involved in the regulation of CDKs, and their expression levels have been used as important reference indexes in related anti-aging studies (Chu et al., 2017; Rasool et al., 2017). P53 is a tumor suppressor gene and its expression level varies in various pathways of cell senescence induced by oxidative stress (Inao et al., 2019; Kim et al., 2021; Kumar et al., 2021). Accumulated evidences have established that the P53 and P53/P21 pathways play a significant effect during the aging process (Priyanga et al., 2020; Xin et al., 2019). The possible involvement of P53/P21pathways in progressive aging diseases has been the focus of a lot of studies in recent years (Bai et al., 2011; Niu et al., 2021). Our study also confirmed that DHP may have an anti-aging role by negatively regulating mRNA and protein expressions of P53, P21, and P16 related genes by participating in the regulation of P53-P21 pathways, and protecting D-gal induced senescence cells.
Based on the in vitro experimental results, to further verify the anti-aging activity of DHP, the effect of DHP was investigated in vivo D-gal mice experiments. Physical function decline, which includes learning and spatial memory impairment, is a major sign of aging (Albeely et al., 2022; Qi et al., 2019). Originally designed by psychologists and applied to rats, the Morris water maze test has been continuously improved to become the best experimental method to evaluate spatial learning and memory ability in rodents, which has been extensively recognized at home and abroad (Luo et al., 2021; Tsering et al., 2022). The results of the Morris water maze indicated that DHP can enhance the learning and memory ability of aging mice, that is, it can shorten the latency of platform searching and increase the retention time of the target quadrant.
Excessive D-gal has been shown to produce free radicals, decrease the activities of antioxidant enzymes and ultimately induce senescence in cells (Zheng, 2020). In vitro studies have shown that DHP has a strong ROS scavenging ability. In vivo animal experiment results showed that DHP protects brain and liver tissues from oxidative damage by restoring the activities of SOD, GSH-Px, and CAT antioxidant enzymes against excessive accumulation of ROS.
Aging is associated with a variety of morphological and functional changes in the liver (Azman et al., 2021). Recent studies have found that D-gal treatment induced noticeably aging-related changes such as increasing the pathological injury and cellular senescence of liver, and hippocampus (Sun et al., 2018). In this study, this protective effect can be directly reflected by HE staining, which reflected by improving the arrangement disorder of hepatic cord and hepatic sinusoid caused by D-gal, decrease in inflammatory cell infiltration, edema.
In addition, consistent with the findings of in vitro experiments. The underlying mechanism by which DHP delays the aging process may be correlated with significantly reducing the overexpression of P16, P21, and P53 proteins, reducing oxidative stress and affecting the cell cycle by regulating the P53/P21 signaling pathway.
5 CONCLUSIONS
In general, our study verified the anti-aging effect of DHP from in vitro and in vivo experiments, and its mechanism may involve the P53/P21 pathway, including inhibiting the expression of aging-related protein, promoting the transition from phase G0/G1 to phase S, reduce oxidative stress. The specific molecular mechanism of how DHP regulates P53/P21 remains unclear. However, its strong antioxidant potential provides a new direction for the search for anti-oxidation and anti-aging drugs.
AUTHOR CONTRIBUTIONS
Mengjuan Ye: Writing - original draft; Conceptualization; Data curation; Formal analysis; Validation. Junlin Liu: Formal analysis; Conceptualization; Data curation; Methodology; Validation; Writing - original draft. Guanghui Deng: Investigation; Conceptualization. Xiao Cai: Investigation; Conceptualization. Xiaoqian Zhang: Investigation; Conceptualization. Liang Yao: Investigation; Conceptualization. Jing Wu: Investigation; Conceptualization. Xianglin He: Supervision; Resources. Daiyin Peng: Investigation; Conceptualization. Nianjun Yu: Conceptualization; Project administration; Supervision; Writing - review & editing.
FUNDING INFORMATION
This study financially was founded by the National Natural Science Foundation of China (U19A2009); Collaborative Innovation Project of Anhui Universities (GXXT-2019-043); MOE-Anhui Joint Collaborative Innovation Center for Quality Improvement of Anhui Genuine Chinese Medicinal Materials ([2022](4)); Anhui University of Traditional Chinese Medicine—Qianshan Pharmaceutical health development industry scientific research project (2021QSHZ02); 2021 university-level exploratory research project of Anhui University of Traditional Chinese Medicine (2021hxts14).
CONFLICT OF INTEREST
The authors declared that they have no conflict of interest.
ETHICS STATEMENT
All animal handling procedures were per formed strictly in accordance with the P.R. China Legislation on the Use and Care of Laboratory Animals and were approved by the Animal Care Review Committee, Anhui University of Traditional Chinese Medicine (approval identification: AHTCM-mice-005).
Open Research
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.




