Codium fragile reduces adipose tissue expansion and fatty liver incidence by downregulating adipo- and lipogenesis
Hyo-Deok Seo and Eunyoung Lee contributed equally to this work.
Abstract
Codium fragile (C. fragile) is a marine alga with high functional food potential. Recent studies have proven C. fragile extract (CFE) effective against obesity. However, the exact underlying mechanism of CFE's anti-obesity effects remains unclear. Herein, CFE was orally administered to male C57BL/6 mice for 7 weeks, along with a high-fat diet. CFE (100 mg/kg) effectively induced weight loss, lowered serum cholesterol levels, and suppressed adipocyte differentiation in white adipose tissue (WAT). Furthermore, CFE effectively reduced hepatic total triglyceride, cholesterol, and lipid levels, while significantly improving liver size and color. mRNA expression analysis in WAT and liver tissue revealed that CFE significantly suppressed the expression of PPARγ and aP-2 in adipocyte differentiation, and SREBP-1c and FAS in de novo lipogenesis, suggesting that CFE's anti-obesity effect is exerted by gene inhibition.
Practical applications
Research on marine plants with anti-obesity effects has been increasing recently. This study demonstrated that C. fragile extract (CFE) is effective in reducing body weight and suppressing adipocyte differentiation, along with the improvement of fatty liver in mice fed with a high-fat diet (HFD). The anti-obesity effect of CFE was exhibited by the down-regulation of adipogenesis and lipogenesis, respectively. Based on these results, C. fragile could be useful, not only to effectively combat obesity but also in improving obesity-induced liver dysfunction.
1 INTRODUCTION
Overweight and obesity are characterized by the excessive and abnormal accumulation of body fat. The main causes are excessive food intake and decreased energy expenditure owing to insufficient physical activity (Markwald et al., 2013). Frequent intake of high-calorie (typically fatty) foods has been identified as a cause of health-impairing obesity that resultantly increases the risk of hypertension, dyslipidemia, diabetes, and non-alcoholic fatty liver disease (NAFLD) (Cho et al., 2020; De Lorenzo et al., 2019; Jung & Choi, 2014). Natural products have long contributed significantly to health promotion. Plants and microbes are the most widely used, as rich sources of bioactive compounds with structural diversity and complexity, compared with compounds derived from combinatorial chemistry. These advantages have been utilized in drug discovery (Atanasov et al., 2021).
In the last decade, opportunities for applying marine natural products (MNPs) as functional foods have increased (Carroll et al., 2022), as the analysis of massive biological data has identified promising bioactive compounds in these products (Lei & Zhou, 2002). Among the numerous types of MNPs, marine algae, such as red, brown, and green algae have been reported to have antioxidant, anti-obesity, and anti-cancer effects (Alves et al., 2018; Han et al., 2015). Codium fragile (C. fragile) classified as dark green algae of the Codiaceae family mainly inhabits the coastal regions of Oceania, North Europe, and East Asia (Kim, Monmai, et al., 2019). Recently, many studies have been conducted on C. fragile using in vitro and in vivo mouse models. C. fragile extract (CFE) could reduce inflammation in macrophage cells and edema animal models, and a polysaccharide from C. fragile was useful in cancer treatment by immune enhancement (Lee et al., 2017; Park et al., 2020). We previously reported that C. fragile promotes protein synthesis and oxidative remodeling in adult mice, resulting in enhanced muscle weight and exercise endurance (Ahn et al., 2021).
C. fragile has been suggested for its anti-obesity potential in a few studies, but its exact underlying mechanism has not been reported. Herein, we determined whether C. fragile could prevent an increase in body weight and adipose tissue, and ameliorate fatty liver in mice fed with a high-fat diet (HFD). We also investigated whether these effects were ascribable to the downregulation of adipo- and lipogenesis in the adipose tissue and liver.
2 MATERIALS AND METHODS
2.1 C. fragile extract (CFE) preparation
The green algae, C. fragile, was obtained from the Sockcho coast of Gangwon-do (South Korea) and air-dried at 60°C after washing with water. The dried materials ground using a blender is sieved through a 0.5 mm mesh. After collecting the materials, it was filtered through Whatman filter paper (No 6, Whatman plc), concentrated in a vacuum evaporator, and then lyophilized (Ahn et al., 2021).
2.2 Animal study
Male C57BL/6 (6-week-old) mice provided by Orient Bio, Inc. were purchased. After being allowed to acclimate for 1 week, a total of 40 mice were divided into a control group fed with a normal diet (N + veh) and three groups fed with a high-fat diet (HFD + veh, HFD + CFE 50 mg/kg, and HFD + CFE 100 mg/kg). Diet composition is shown in Table 1, and the HFD was based on the normal diet but contained 45 kcal% fat. In addition to the diet, 0.9% NaCl—acting as a vehicle (veh)—was orally administered to the N + veh and HFD + veh groups. Food intake and body weight were measured three times a week and once a week for 7 weeks, respectively (n = 10/group). After commencing with the group-specific diet regimes, the mice were euthanized and serum, fat tissues, and livers were obtained. Tissues and organs required for RNA extraction were stored at −80 °C. All animal experiments were performed according to relevant institutional and national guidelines and approved by the Institutional Animal Care and Use Committee (IACUC) of the Korea Food Research Institute (approval number: KFRI-M-21006).
| Ingredient (g/kg) | N | HFD |
|---|---|---|
| Casein | 200 | 200 |
| Corn oil | 50 | 50 |
| Corn starch | 350 | 125 |
| Lard | 0 | 220 |
| Cholesterol | 0 | 5 |
| Cellulose | 50 | 50 |
| Sucrose | 300 | 300 |
| Vitamin mix | 10 | 10 |
| Mineral mix | 35 | 35 |
| Methionine | 3 | 3 |
| Choline bitartrate | 2 | 2 |
- Abbreviations: HFD, high-fat diet; N, normal diet.
2.3 Measurement of serum and hepatic lipids
Total triglyceride (TG), total cholesterol (TC), and high-density lipoprotein (HDL) levels in serum and liver were evaluated using assay kits (Shinyang Chemical Co.) and spectrophotometric methods. All extraction steps were carried out according to the product information and procedures. Lipid extraction from the liver was performed based on the Folch method (Folch et al., 1957). Liver samples were homogenized in 0.9% NaCl and incubated overnight at 4 °C in a 20-fold volume of the chloroform-methanol mixture (2:1, v/v) (Choi et al., 2020). Chloroform-soaked filter paper (No. 3, Whatman plc) was placed in the funnel, and the lipid extract was filtered. After evaporation, the lipid extract was redissolved in chloroform and stored at −40 °C until analysis.
2.4 Histologic analysis
Adipose and liver tissues were fixed in a 10% buffered formalin solution. After 24 h, the tissues were covered with paraffin and sliced into 5 μm thick sections before staining with H&E (Hematoxylin and Eosin). Morphologies were evaluated using a microscope (Olympus) at a magnification of 200× (Lee et al., 2020).
2.5 ATP assay
The liver tissue was extracted with RIPA buffer, and ATP content was measured by the manufacturer's method using an ATP luminescence assay kit (PerkinElmer).
2.6 RT-qPCR
We extracted RNA from adipose and liver tissues using the RNeasy mini kit (Qiagen). All extraction steps were carried out according to the product information and procedures. After RNA extraction, ReverTra Ace™ qPCR RT Kit (Toyobo) and 400 ng of RNA were used for the cDNA synthesis. RT-qPCR was conducted using SYBR® Green Master Mix (Toyobo) (Narimatsu et al., 2022) and the ViiA7™ (Applied Biosystems). Synthesized cDNA was pre-denatured at 95 °C for 1 min, then 40 cycles of 95 °C for 15 s, 60 °C for 15 s, and 75 °C for 45 s (Lee et al., 2021). The quantity of the target genes was calibrated using GAPDH as a control gene and calculated using the 2−△△Ct method. The ΔΔCt value for each sample was determined by calculating the difference between the Ct values of the target and reference gene (GAPDH), respectively. The specific primer sequences are shown in Table 2.
| Gene | Primer type | Sequence (5′ → 3′) |
|---|---|---|
| PPARγ | Forward | TCGCTGATGCACTGCCTATG |
| Reverse | GAGAGGTCCACAGAGCTGATT | |
| SREBP-1c | Forward | TGGATTGCACATTTGAAGACAT |
| Reverse | GCCAGAGAAGCAGAAGAG | |
| FAS | Forward | GGAGGTGGTGATAGCCGGTAT |
| Reverse | TGGGTAATCCATAGAGCCCAG | |
| aP-2 | Forward | AAGGTGAAGAGCATCATAACCCT |
| Reverse | TCACGCCTTTCATAACACATTCC | |
| C/EBPα | Forward | CAAGAACAGCAACGAGTACCG |
| Reverse | GTCACTGGTCAACTCCAGCAC | |
| GAPDH | Forward | AGACAGCCGCATCTTCTTGT |
| Reverse | CTTGCCGTGGGTAGAGTCAT |
- Abbreviations: aP-2, adipose protein 2; C/EBPα, CCAAT-enhancer-binding protein α; FAS, fatty acid synthase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; PPARγ, peroxisome proliferator-activated receptor γ; SREBP-1c, sterol regulatory element-binding protein.
2.7 Western blot
Proteins were obtained from adipose and liver tissues using RIPA lysis buffer (Thermo Fisher Scientific-Pierce) contained protease phosphatase inhibitors. The protein concentration was quantified using a BCA protein assay. Proteins of the same concentration loaded on the gel were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane. After transfer, the membranes were incubated with 5% skim milk. After blocking and washing, the membranes were incubated with the primary antibodies at 4 °C overnight. The membranes were incubated with the secondary antibodies at room temperature for 1 h. After washing, the membranes were detected using a chemiluminescence reagent and performed with G:BOX Chemi XX6 from Syngene (Syngene Ltd.).
2.8 Statistical analysis
The data were described using mean ± standard error of mean (SEM). Experimental results were analyzed based on Prism 9.3.1 software (GraphPad Software Inc.) with p < .05 set to statistical significance. One-way analysis of variance (ANOVA) followed by Tukey's post hoc test was used to compare each group.
3 RESULTS
3.1 CFE improved body weight and serum lipids in mice fed with a high-fat diet (HFD)
To evaluate the anti-obesity effects of CFE, male C57BL/6 (6-week-old) mice were orally administered 50 or 100 mg/kg CFE, respectively, while being fed the HFD, for 7 weeks. Changes in body weight (p < .05) differed between the HFD + veh and HFD + CFE 100 mg/kg groups, from the 6th week (Figure 1a), and a significant difference in final body weight (p < .05) was observed (Figure 1b). Monitoring during the experiment showed similar food intakes between groups (Data not shown).
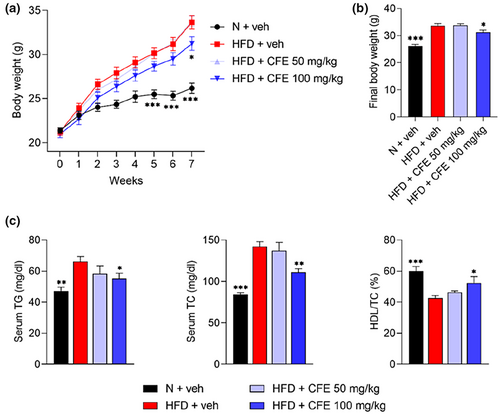
As high lipid levels in the blood represent typical characteristics of obesity, TG, TC, and HDL in serum between each group were measured. Serum TG (p < .05) and TC (p < .01) levels in the HFD-fed group were increased, but these increased levels were notably lowered by the administration of 100 mg/kg CFE (Figure 1c). Moreover, 100 mg/kg CFE was shown to increase HDL levels (p < .05) in relation to TC.
3.2 CFE reduced the weight and size of white adipose tissue (WAT) in the HFD-fed mice
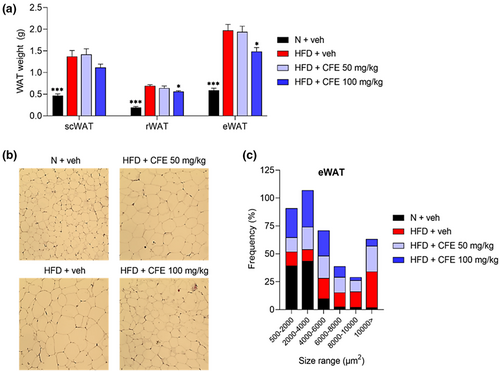
Adipose tissue expansion is associated with obesity, both through increased cell size (hypertrophy) and greater proliferation (hyperplasia) (Vishvanath & Gupta, 2019). To confirm the weight loss effect of CFE, we first evaluated the weight of three types of white adipose tissue (WAT) isolated from HFD-fed mice: subcutaneous (scWAT), retroperitoneal (rWAT) (p < .05), and epididymal (eWAT) (p < .05). Weight gain was observed in all three types of WAT in the HFD-fed groups, whereas the WAT weight was notably lower in the HFD + 100 mg/kg CFE group when compared with the HFD + veh group (Figure 2a). The number and size of lipid droplets in the eWAT were quantified using histologic analysis. As shown in Figure 2b, 100 mg/kg CFE administration reduced the size of the lipid droplets and effectively suppressed surface area size in excess of 8000 μm2 (Figure 2c).
3.3 CFE ameliorated fatty liver in the HFD-fed mice
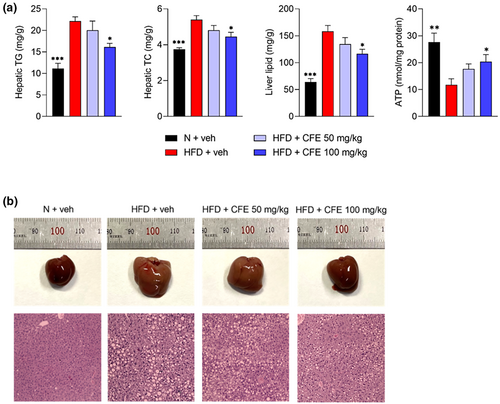
Excessive and frequent intake of the HFD and cholesterol is closely associated with the accumulation of lipids in the liver and increases the risk of chronic liver-related diseases, such as high blood pressure, insulin resistance, and fatty liver disease (Ore et al., 2021; Shao et al., 2020). Herein, a significant increase in hepatic TG (p < .05) and TC (p < .05) levels was measured in the HFD groups, whereas 100 mg/kg CFE administration notably reduced these levels, compared with the HFD alone (Figure 3a). Moreover, 100 mg/kg CFE was shown to increase ATP levels that were lowered by a high-fat diet (p < .01). According to the results of hepatic lipid weight (p < .05) measurement using the Folch method, significantly lower numbers were observed in the HFD + 100 mg/kg CFE group compared with the HFD + veh group. Compared to the N + veh group, the HFD + veh group had an enlarged liver, of which the color had changed from dark red to brown, whereas the livers of mice in the CFE-treated groups were smaller and of a dark red color, similar to those from the N + veh group (Figure 3b). Lipid droplet accumulation in the liver was clearly visible in the HFD group, but the size of the lipid droplets was relatively decreased after CFE administration.
3.4 CFE downregulated expression of adipo- and lipogenic genes
To explain the basic mechanism of anti-obesity effects of CFE, we analyzed the mRNA expression levels of adipo- and lipogenesis-related genes using eWAT and liver. PPARγ is a major adipogenic transcription factor, mainly expressed in adipose tissue and the liver. As a result, the PPARγ gene was significantly upregulated in the eWAT of the HFD group, but its mRNA expression was suppressed (p < .05) by 100 mg/kg CFE treatment. aP-2, a carrier protein for fatty acids, showed significantly lower mRNA expression levels (p < .05) with the administration of 100 mg/kg CFE, compared with the HFD alone (Figure 4a). FAS, which regulates the de novo biosynthesis of fatty acids, also tended to be suppressed by 100 mg/kg CFE treatment. As a result of protein expression analysis through western blot, PPARγ, C/EBPα, and aP-2 were significantly decreased (p < .05, p < .001) by 100 mg/kg CFE treatment.
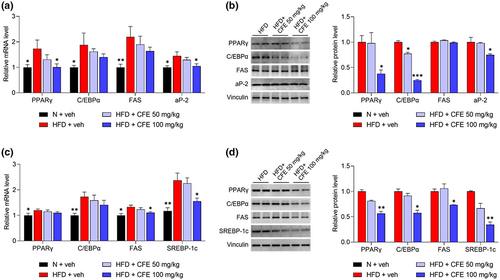
The expression of the same genes in the liver was also investigated. Although the mRNA expression levels of PPARγ and C/EBPα in the HFD + 100 mg/kg CFE group were not significantly lower than in the HFD + veh group, a decreasing trend was observed, as shown in Figure 4b. SREBP-1c, a hepatic transcription factor, is generally elevated in obesity-induced fatty liver (Kim, Lee, et al., 2019). With the administration of 100 mg/kg CFE, the mRNA expression levels of FAS and SREBP-1c, which are target signaling enzymes, were significantly suppressed (p < .05). As a result of mRNA levels, protein expression of FAS and SREBP-1c was significantly reduced (p < .05, p < .01), and expression of PPARγ and C/EBPα is also reduced in the 100 mg/kg administration group (p < .05).
Taken together, these results suggested that oral administration of CFE could effectively suppress the expression of adipo- and lipogenesis-related genes in adipose tissue and the liver of mice fed with a HFD.
4 DISCUSSION
Genetic defects, excessive intake of high-calorie foods, lack of exercise, and unhealthy eating habits are the main causes of obesity. Moreover, such an unhealthy lifestyle is known as a major cause of increased incidence of various diseases such as coronary heart disease, type 2 diabetes, and NAFLD (Ades & Savage, 2017; Shao et al., 2020). Conversely, it is well known that intake of functional foods has a significant effect on improving obesity and related diseases (Cao et al., 2019).
To date, several marine foods, such as fish, shellfish, crustaceans, and seaweeds, have been identified as excellent sources of essential nutrients, such as minerals and vitamins, and are actively utilized in the fields of health, and food science and medicine (Lordan et al., 2011). Recently, studies on the functionality of CFE, derived from seaweed C. fragile, have been reported. Component analysis revealed that CFE contains canthaxanthin, siphonaxanthin, retinoic acid, and α-tocopherol, of which canthaxanthin successfully strengthens muscle, and siphonaxanthin is effective against obesity (Ahn et al., 2021; Zheng et al., 2020). Polysaccharides isolated from C. fragile have also reduced body weight in rodents, and CFE has suppressed weight gain and adipocyte differentiation, by regulating the gut microbiota (Kim et al., 2020; Kolsi et al., 2017). CFE's weight loss and adipocyte differentiation inhibition effects have been reported, but analysis of fatty liver caused by obesity is excluded. It is thus expected that the anti-obesity effect of CFE can be confirmed more accurately by analyzing adipose tissue and liver tissue together.
Herein, we confirmed that CFE has anti-obesity effects in HFD-fed mice. Along with the observed weight loss, TG and TC levels were significantly decreased and that of HDL—which serves as a cholesterol transporter from peripheral tissue to the liver—increased, with CFE administration. Compared to previous studies that evaluated only eWAT, we analyzed three different types of WAT and quantitatively confirmed the suppression of adipocyte differentiation by CFE.
Obesity causes fat accumulation in the liver, high levels of which increase the risk of serious health problems. NAFLD is considered the most common cause of long-term liver disease and is a serious problem for public health, worldwide (Younossi, 2019). Our results demonstrated that CFE administration significantly lowered hepatic lipid weight and reduced the number and size of lipid droplets in the liver. We further observed that the liver's size and color improved, resembling features of those from the N + veh group when CFE was administered. These results indicated that CFE could effectively suppress fatty liver induced by HFD and may be utilized to alleviate serious liver damage caused by obesity.
Obesity generally causes mitochondrial dysfunction and imbalances in energy metabolism and lowers ATP levels in obese mice (de Mello et al., 2018; Mingorance et al., 2012). We measured ATP levels in liver tissues to analyze energy expenditure and observed that CFE increased ATP levels lowered by a high-fat diet. Excessive accumulation of lipids during adipogenesis and adipogenesis leads to decreased number and function of mitochondria, and CFE is expected to ameliorate these problems while promoting lipid metabolism. This provides evidence for how CFE contributes to energy expenditure during high-fat diets, but further analysis is needed.
Subsequently, we attempted to identify CFE's exact mechanism of action by analyzing gene expression. In animal cells, PPARs, C/EBPs, and SREBPs are considered key regulators of adipogenesis (Lee et al., 2019; Oh et al., 2022). PPARγ is essential for adipocyte differentiation and interacts with C/EBPα, resulting in the expression of transcription factors that determine adipocyte phenotypes. Lipoprotein lipase, acyl-CoA synthetase, and aP-2 are expressed during terminal differentiation of adipocytes, and resultantly, lipid droplets of triglycerides appear in the cytoplasm, grow, and merge to complete adipocyte differentiation (Moseti et al., 2016). As a result, CFE decreased the mRNA and protein expression levels of PPARγ, C/EBPα, FAS, and aP-2 in eWAT. Of these, expression of the adipocyte marker proteins, PPARγ, C/EBPα, and aP-2, was most significantly reduced. These results indicated that the downregulation of adipocyte differentiation revealed in the WAT analysis, was due to suppression of PPARγ, C/EBPα, and aP-2 gene expression.
It is widely known that adipogenesis is controlled by transcription factors expressed in the early, middle, and late stages. PPARγ and C/EBPα are known as the early adipogenic transcription factors of adipocyte differentiation and have become targets for various active components aimed at treating obesity (Chang & Kim, 2019). However, in other studies, C/EBPβ and C/EBPδ are the major transcription factors in the early stage, and PPARγ and C/EBPα are known to regulate fat accumulation in the middle stage. In particular, the stages of adipogenesis in human are poorly defined and requires further study (da Silva et al., 2020).
A sustained high-calorie and -fat diet induces fatty liver, which mainly involves the SREBP-1c and FAS signaling pathways (Moslehi & Hamidi-zad, 2018). SREBP-1c, which is a major lipogenic transcription factor, regulates the synthesis of fatty acid and TG and upregulates the FAS gene, which is a key enzyme in lipid metabolism. Using mRNA and protein analysis of liver tissue, we showed that SREBP-1c and FAS expression levels were significantly suppressed by CFE administration, and protein expression of PPARγ and C/EBPα was significantly decreased. It is demonstrated that inhibition of the signaling pathway related to lipogenesis, is effective in improving liver health.
5 CONCLUSIONS
We demonstrated that C. fragile exerts anti-obesity effects in mice fed with a high-fat diet. C. fragile reduced lipid levels in serum and WAT weight and effectively inhibited the accumulation of fat in the liver. Using mRNA expression analysis, we proved that the anti-obesity effect was exhibited by suppressing the expression of PPARγ and aP-2 in adipocyte differentiation, and SREBP-1c and FAS in de novo lipogenesis, respectively. Based on these results, C. fragile is expected to help, not only to effectively ameliorate obesity but also to improve liver function that might have been damaged by obesity.
AUTHOR CONTRIBUTIONS
Hyo-Deok Seo: conceptualization, investigation, writing—original draft, formal analysis, and Software. Eunyoung Lee: investigation, writing—original draft, data curation, and Software. Jiyun Ahn: data curation and methodology. Jeong-Hoon Hahm: visualization and validation. Tae-Youl Ha: resources, visualization. Dae-Hee Lee: methodology and investigation. Chang Hwa Jung: supervision, conceptualization, writing—review and editing, funding acquisition, and project administration.
ACKNOWLEDGMENTS
This study was supported by the Ministry of Trade, Industry, and Energy (MOTIE) Korea, under the “Infrastructure Support Program for Industry Innovation” (P0014714) supervised by the Korea Institute for Advancement of Technology (KIAT).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




