Long-term effects of sirolimus treatment for slow-flow vascular malformations: Real-world evidence from the French observational multicentre SIROLO study
This article relates to:
-
Long-term sirolimus in SFVMs: A critical appraisal of the SIROLO study
- Journal of the European Academy of Dermatology and Venereology
- First Published online: May 30, 2025
Cécilia Maillet
Unit of Pediatric Dermatology, Department of Dermatology, University Hospital Center (CHRU) of Tours, Tours, France
Reference Center for Genodermatoses and Rare Skin Diseases (MAGEC-Tours), Tours, France
Search for more papers by this authorOlivia Boccara
Department of Dermatology and Reference Center for Genodermatoses and Rare Skin Diseases (MAGEC), AP-HP, Paris University, Necker-Enfants Malades Hospital, Paris Centre University, Imagine Institute, Paris, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorStéphanie Mallet
Department of Dermatology, Assistance Publique-Hôpitaux de Marseille (AP-HM), Marseille, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorDidier Bessis
Department of Dermatology, University Hospital Center (CHU) of Montpellier, Montpellier, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorChristine Labrèze
Department of Dermatology, University Hospital Center (CHU) of Bordeaux, Bordeaux, France
BRIC (BoRdeaux Institute of onCology), UMR1312, INSERM, University of Bordeaux, Bordeaux, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorSorilla Mary-Prey
Department of Dermatology, University Hospital Center (CHU) of Bordeaux, Bordeaux, France
BRIC (BoRdeaux Institute of onCology), UMR1312, INSERM, University of Bordeaux, Bordeaux, France
Search for more papers by this authorLaurent Guibaud
University Hospital Center (CHU) Lyon, Mère-Enfant Hospital, Reference Center for Superficial Vascular Anomalies, Lyon Bron, France
Search for more papers by this authorAnnouk Bisdorff
Department of Neuroradiology/Vascular Anomalies Clinic, AP-HP, Lariboisière Hospital, Paris, France
Search for more papers by this authorAnne Dompmartin
Department of Dermatology, University Hospital Center (CHU) of Caen, University Caen-Normandie, Caen, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorJuliette Mazereeuw-Hautier
Department of Dermatology, University Hospital Center (CHU) of Toulouse, Reference Center for Rare Skin Diseases, Paul Sabatier University, Toulouse, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorChristine Chiaverini
Department of Dermatology, Reference Center for Genodermatoses and Rare Skin Diseases (MAGEC-Sud), University Hospital Center (CHU) of Nice, Nice, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorThomas Hubiche
Department of Dermatology, Reference Center for Genodermatoses and Rare Skin Diseases (MAGEC-Sud), University Hospital Center (CHU) of Nice, Nice, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorBertille Bonniaud
Department of Dermatology, University Hospital Center (CHU) of Dijon, Dijon, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorCaroline Degrugillier-Chopinet
Department of Cardiovascular Functional Explorations and Pediatric Cardiology, Coeur-Poumons Institute, University Hospital Center (CHRU) of Lille, Lille, France
Search for more papers by this authorAnne-Claire Bursztejn
Department of Dermatology and Allergology, University Hospital Center (CHU) of Nancy, Brabois Hospital, Nancy, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorHélène Aubert
Department of Dermatology, University Hospital Center (CHU) of Nantes, Nantes, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorMaella Severino
Department of Dermatology, University Hospital Center (CHU) of Toulouse, Reference Center for Rare Skin Diseases, Paul Sabatier University, Toulouse, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorSophie Leducq
Unit of Pediatric Dermatology, Department of Dermatology, University Hospital Center (CHRU) of Tours, Tours, France
Reference Center for Genodermatoses and Rare Skin Diseases (MAGEC-Tours), Tours, France
INSERM 1246-SPHERE, University of Tours & University of Nantes, Tours, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorMathilde Tardieu
Department of Dermatology, University Hospital Center (CHU) of Grenoble-Alpes, Couple Enfant Hospital, Grenoble, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorAline Joly
Unit of Pediatric Dermatology, Department of Dermatology, University Hospital Center (CHRU) of Tours, Tours, France
Department of Maxillofacial Surgery, University Hospital Center (CHRU) of Tours, Tours, France
Search for more papers by this authorGrégoire Boulouis
Unit of Pediatric Dermatology, Department of Dermatology, University Hospital Center (CHRU) of Tours, Tours, France
Department of Interventional Neuroradiology, University Hospital Center (CHRU) of Tours, Tours, France
Search for more papers by this authorAnne Le Touze
Unit of Pediatric Dermatology, Department of Dermatology, University Hospital Center (CHRU) of Tours, Tours, France
Department of Pediatric Surgery, University Hospital Center (CHRU) of Tours, Clocheville Hospital, Tours, France
Search for more papers by this authorArnaud Paré
Unit of Pediatric Dermatology, Department of Dermatology, University Hospital Center (CHRU) of Tours, Tours, France
Department of Maxillofacial Surgery, University Hospital Center (CHRU) of Tours, Tours, France
Search for more papers by this authorElsa Tavernier
INSERM 1246-SPHERE, University of Tours & University of Nantes, Tours, France
Clinical Investigation Center (CIC) INSERM 1415, University Hospital Center (CHRU) of Tours, Tours, France
Search for more papers by this authorCorresponding Author
Annabel Maruani
Unit of Pediatric Dermatology, Department of Dermatology, University Hospital Center (CHRU) of Tours, Tours, France
Reference Center for Genodermatoses and Rare Skin Diseases (MAGEC-Tours), Tours, France
INSERM 1246-SPHERE, University of Tours & University of Nantes, Tours, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Correspondence
Annabel Maruani, Unit of Pediatric Dermatology, Department of Dermatology, Center of Reference of Vascular Anomalies MAGEC-Tours, CHRU Tours, 37044 Tours Cedex 9, France.
Email: [email protected]
Search for more papers by this authorCécilia Maillet
Unit of Pediatric Dermatology, Department of Dermatology, University Hospital Center (CHRU) of Tours, Tours, France
Reference Center for Genodermatoses and Rare Skin Diseases (MAGEC-Tours), Tours, France
Search for more papers by this authorOlivia Boccara
Department of Dermatology and Reference Center for Genodermatoses and Rare Skin Diseases (MAGEC), AP-HP, Paris University, Necker-Enfants Malades Hospital, Paris Centre University, Imagine Institute, Paris, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorStéphanie Mallet
Department of Dermatology, Assistance Publique-Hôpitaux de Marseille (AP-HM), Marseille, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorDidier Bessis
Department of Dermatology, University Hospital Center (CHU) of Montpellier, Montpellier, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorChristine Labrèze
Department of Dermatology, University Hospital Center (CHU) of Bordeaux, Bordeaux, France
BRIC (BoRdeaux Institute of onCology), UMR1312, INSERM, University of Bordeaux, Bordeaux, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorSorilla Mary-Prey
Department of Dermatology, University Hospital Center (CHU) of Bordeaux, Bordeaux, France
BRIC (BoRdeaux Institute of onCology), UMR1312, INSERM, University of Bordeaux, Bordeaux, France
Search for more papers by this authorLaurent Guibaud
University Hospital Center (CHU) Lyon, Mère-Enfant Hospital, Reference Center for Superficial Vascular Anomalies, Lyon Bron, France
Search for more papers by this authorAnnouk Bisdorff
Department of Neuroradiology/Vascular Anomalies Clinic, AP-HP, Lariboisière Hospital, Paris, France
Search for more papers by this authorAnne Dompmartin
Department of Dermatology, University Hospital Center (CHU) of Caen, University Caen-Normandie, Caen, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorJuliette Mazereeuw-Hautier
Department of Dermatology, University Hospital Center (CHU) of Toulouse, Reference Center for Rare Skin Diseases, Paul Sabatier University, Toulouse, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorChristine Chiaverini
Department of Dermatology, Reference Center for Genodermatoses and Rare Skin Diseases (MAGEC-Sud), University Hospital Center (CHU) of Nice, Nice, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorThomas Hubiche
Department of Dermatology, Reference Center for Genodermatoses and Rare Skin Diseases (MAGEC-Sud), University Hospital Center (CHU) of Nice, Nice, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorBertille Bonniaud
Department of Dermatology, University Hospital Center (CHU) of Dijon, Dijon, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorCaroline Degrugillier-Chopinet
Department of Cardiovascular Functional Explorations and Pediatric Cardiology, Coeur-Poumons Institute, University Hospital Center (CHRU) of Lille, Lille, France
Search for more papers by this authorAnne-Claire Bursztejn
Department of Dermatology and Allergology, University Hospital Center (CHU) of Nancy, Brabois Hospital, Nancy, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorHélène Aubert
Department of Dermatology, University Hospital Center (CHU) of Nantes, Nantes, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorMaella Severino
Department of Dermatology, University Hospital Center (CHU) of Toulouse, Reference Center for Rare Skin Diseases, Paul Sabatier University, Toulouse, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorSophie Leducq
Unit of Pediatric Dermatology, Department of Dermatology, University Hospital Center (CHRU) of Tours, Tours, France
Reference Center for Genodermatoses and Rare Skin Diseases (MAGEC-Tours), Tours, France
INSERM 1246-SPHERE, University of Tours & University of Nantes, Tours, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorMathilde Tardieu
Department of Dermatology, University Hospital Center (CHU) of Grenoble-Alpes, Couple Enfant Hospital, Grenoble, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Search for more papers by this authorAline Joly
Unit of Pediatric Dermatology, Department of Dermatology, University Hospital Center (CHRU) of Tours, Tours, France
Department of Maxillofacial Surgery, University Hospital Center (CHRU) of Tours, Tours, France
Search for more papers by this authorGrégoire Boulouis
Unit of Pediatric Dermatology, Department of Dermatology, University Hospital Center (CHRU) of Tours, Tours, France
Department of Interventional Neuroradiology, University Hospital Center (CHRU) of Tours, Tours, France
Search for more papers by this authorAnne Le Touze
Unit of Pediatric Dermatology, Department of Dermatology, University Hospital Center (CHRU) of Tours, Tours, France
Department of Pediatric Surgery, University Hospital Center (CHRU) of Tours, Clocheville Hospital, Tours, France
Search for more papers by this authorArnaud Paré
Unit of Pediatric Dermatology, Department of Dermatology, University Hospital Center (CHRU) of Tours, Tours, France
Department of Maxillofacial Surgery, University Hospital Center (CHRU) of Tours, Tours, France
Search for more papers by this authorElsa Tavernier
INSERM 1246-SPHERE, University of Tours & University of Nantes, Tours, France
Clinical Investigation Center (CIC) INSERM 1415, University Hospital Center (CHRU) of Tours, Tours, France
Search for more papers by this authorCorresponding Author
Annabel Maruani
Unit of Pediatric Dermatology, Department of Dermatology, University Hospital Center (CHRU) of Tours, Tours, France
Reference Center for Genodermatoses and Rare Skin Diseases (MAGEC-Tours), Tours, France
INSERM 1246-SPHERE, University of Tours & University of Nantes, Tours, France
On behalf of the Groupe de Recherche de la Société Française de Dermatologie Pédiatrique (Research Group of the French Society for Pediatric Dermatology)
Correspondence
Annabel Maruani, Unit of Pediatric Dermatology, Department of Dermatology, Center of Reference of Vascular Anomalies MAGEC-Tours, CHRU Tours, 37044 Tours Cedex 9, France.
Email: [email protected]
Search for more papers by this authorCécilia Maillet and Olivia Boccara equal contributions.
Abstract
Rationale
Sirolimus is a treatment for slow-flow vascular malformations (SFVMs). However, the long-term management remains challenging.
Objectives
The SIROLO study assessed the long-term effects and real-life management of oral sirolimus for SFVMs by investigating data from 15 French tertiary centres for vascular anomalies.
Methods
Participants were retrospectively included if they had a SFVM that was being/had been treated with sirolimus for at least 3 years in total. Data were collected on treatment goals when initiating sirolimus, investigator-reported efficacy, safety, dosages and treatment withdrawal.
Results
The cohort involved 67 patients with various SFVM entities (mean [±SD] age 19.6 ± 12.5 years, 35 children, 52.2%). We found a heterogeneity of predefined treatment goals, the most frequent being cessation of pain. The investigators considered that sirolimus had persistent efficacy for bleeding, ulceration and pain but only slight efficacy for reducing volume. It was reported to be well-tolerated, although serious adverse events (mainly infections and also two ovarian cysts) were reported in 6 patients (9.0%) and required definitive sirolimus discontinuation for one. Overall, 11 patients (16.4%) had at least one temporary withdrawal period, leading to symptom recurrence and sirolimus resumption at a mean of 6.4 ± 9.6 months. The mean sirolimus concentration was 6.4 ± 3.7 ng/mL during the first 6 months and decreased over time (mean concentration during the last 6 months: 4.2 ± 3.2 ng/mL), probably to target the minimal efficient dosage. Eight patients (11.9%) switched to alpelisib because of insufficient efficacy of sirolimus.
Conclusions
This real-life study gives answers to frequent questions patients and parents ask before sirolimus initiation for SFVMs, such as persistence of efficacy over time, long-term side effects and time to recurrence in case of withdrawal.
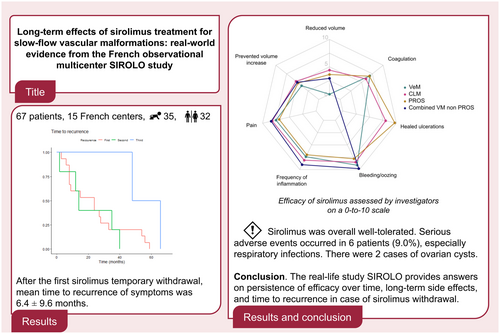
Graphical Abstract
Overall, 67 patients were included from 15 French centres (35 children and 32 adults). After the first sirolimus temporary withdrawal, mean time to recurrence of symptoms was 6.4 ± 9.6 months (survival curve). Sirolimus was overall well-tolerated (radar chart). Serious adverse events occurred in six patients (9.0%), especially respiratory infections. There were two cases of ovarian cysts. Conclusion. The real-life study SIROLO provides answers on persistence of efficacy over time, long-term side effects and time to recurrence in case of sirolimus withdrawal.
CONFLICT OF INTEREST STATEMENT
None declared.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
| Filename | Description |
|---|---|
| jdv20385-sup-0001-FileS1.docxWord 2007 document , 17.1 KB |
File S1 |
| jdv20385-sup-0002-FileS2.docxWord 2007 document , 19.8 KB |
File S2 |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1Wassef M, Blei F, Adams DM, Alomari A, Baselga E, Berenstein A, et al. Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics. 2015; 136(1): e203–e214.
- 2Keppler-Noreuil KM, Rios JJ, Parker VE, Semple RK, Lindhurst MJ, Sapp JC, et al. PIK3CA-related overgrowth spectrum (PROS): diagnostic and testing eligibility criteria, differential diagnosis, and evaluation. Am J Med Genet A. 2015; 167A(2): 287–295.
- 3Martinez-Lopez A, Blasco-Morente G, Perez-Lopez I, Herrera-Garcia JD, Luque-Valenzuela M, Sanchez-Cano D, et al. CLOVES syndrome: review of a PIK3CA-related overgrowth spectrum (PROS). Clin Genet. 2017; 91(1): 14–21.
- 4Ten Broek RW, Eijkelenboom A, van der Vleuten CJM, Kamping EJ, Kets M, Verhoeven BH, et al. Comprehensive molecular and clinicopathological analysis of vascular malformations: a study of 319 cases. Genes Chromosomes Cancer. 2019; 58(8): 541–550.
- 5Arleo TL, Swerdlin RF, Gill AE, Goudy SL, Meisel JA, Briones MA, et al. Baseline quality of life in pediatric patients with low-flow vascular malformations. J Pediatr Hematol Oncol. 2023; 45(7): e847–e856.
- 6Pang C, Gibson M, Nisbet R, Evans N, Khalifa M, Papadopoulou A, et al. Quality of life and mental health of patients with vascular malformations in a single specialist center in the United Kingdom. J Vasc Surg Venous Lymphat Disord. 2022; 10(1): 159–169.
- 7Maruani A, Tavernier E, Boccara O, Mazereeuw-Hautier J, Leducq S, Bessis D, et al. Sirolimus (rapamycin) for slow-flow malformations in children: the observational-phase randomized clinical PERFORMUS trial. JAMA Dermatol. 2021; 157(11): 1289–1298.
- 8Bertino FJ, Hawkins CM. Contemporary management of extracranial vascular malformations. Pediatr Radiol. 2023; 53(8): 1600–1617.
- 9Leboulanger N, Bisdorff A, Boccara O, Dompmartin A, Guibaud L, Labrèze C, et al. French national diagnosis and care protocol (PNDS, protocole national de diagnostic et de soins): cystic lymphatic malformations. Orphanet J Rare Dis. 2023; 18(1): 10. https://doi.org/10.1186/s13023-022-02608-y
- 10Nguyen JT, Koerper MA, Hess CP, Dowd CF, Hoffman WY, Dickman M, et al. Aspirin therapy in venous malformation: a retrospective cohort study of benefits, side effects, and patient experiences. Pediatr Dermatol. 2014; 31(5): 556–560.
- 11Budge EJ, Khalil Allam MA, Mechie I, Scully M, Agu O, Lim CS. Venous malformations: coagulopathy control and treatment methods. Phlebology. 2021; 36(5): 361–374.
- 12Van Es J, Kappelhof NA, Douma RA, Meijers JCM, Gerdes VEA, van der Horst CMAM. Venous thrombosis and coagulation parameters in patients with pure venous malformations. Neth J Med. 2017; 75(8): 328–334.
- 13Dompmartin A, Acher A, Thibon P, Tourbach S, Hermans C, Deneys V, et al. Association of localized intravascular coagulopathy with venous malformations. Arch Dermatol. 2008; 144(7): 873–877.
- 14Wagner KM, Lokmic Z, Penington AJ. Prolonged antibiotic treatment for infected low flow vascular malformations. J Pediatr Surg. 2018; 53(4): 798–801.
- 15Venot Q, Blanc T, Rabia SH, Berteloot L, Ladraa S, Duong JP, et al. Targeted therapy in patients with PIK3CA-related overgrowth syndrome. Nature. 2018; 558: 540–546.
- 16Queisser A, Seront E, Boon LM, Vikkula M. Genetic basis and therapies for vascular anomalies. Circ Res. 2021; 129(1): 155–173.
- 17Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009; 122(Pt 20): 3589–3594.
- 18Knoll GA, Kokolo MB, Mallick R, Beck A, Buenaventura CD, Ducharme R, et al. Effect of sirolimus on malignancy and survival after kidney transplantation: systematic review and meta-analysis of individual patient data. BMJ. 2014; 349:g6679.
- 19Hammill AM, Wentzel M, Gupta A, Nelson S, Lucky A, Elluru R, et al. Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr Blood Cancer. 2011; 57(6): 1018–1024.
- 20Adams DM, Trenor CC 3rd, Hammill AM, Vinks AA, Patel MN, Chaudry G, et al. Efficacy and safety of sirolimus in the treatment of complicated vascular anomalies. Pediatrics. 2016; 137(2):e20153257.
- 21Parker VER, Keppler-Noreuil KM, Faivre L, Luu M, Oden NL, De Silva L, et al. Safety and efficacy of low-dose sirolimus in the PIK3CA-related overgrowth spectrum. Genet Med. 2019; 21(5): 1189–1198.
- 22Nadal M, Giraudeau B, Tavernier E, Jonville-Bera AP, Lorette G, Maruani A. Efficacy and safety of mammalian target of rapamycin inhibitors in vascular anomalies: a systematic review. Acta Derm Venereol. 2016; 96(4): 448–452.
- 23Hammer J, Seront E, Duez S, Dupont S, Van Damme A, Schmitz S, et al. Sirolimus is efficacious in treatment for extensive and/or complex slow-flow vascular malformations: a monocentric prospective phase II study. Orphanet J Rare Dis. 2018; 13(1): 191. https://doi.org/10.1186/s13023-018-0934-z
- 24Ji Y, Chen S, Yang K, Zhou J, Zhang X, Jiang X, et al. A prospective multicenter study of sirolimus for complicated vascular anomalies. J Vasc Surg. 2021; 74(5): 1673–1681.
- 25Harbers VEM, Rongen GAPJM, van der Vleuten CJM, Verhoeven BH, de Laat PCJ, van der Horst CMAM, et al. Patients with congenital low-flow vascular malformation treated with low dose sirolimus. Adv Ther. 2021; 38(6): 3465–3482.
- 26Tongruang C, Wananukul S, Chatproedprai S, Narkbunnam N, Nitiyarom R, Sirachainan N, et al. Cost and effectiveness comparison of sirolimus versus standard treatment in Kasabach-Merritt phenomenon: a real-world evidence study in Thailand. Pediatr Hematol Oncol. 2024; 18: 1–11.
- 27Labonnelie A, Soupre V, Maruani A, Cisternino S, Hadj-Rabia S, Boccara O. Management of sirolimus treatment for tumours associated with Kasabach-Merritt phenomenon. J Eur Acad Dermatol Venereol. 2022; 36(7): e586–e588.
- 28Maruani A, Moineau AG, Boccara O, Mazereeuw-Hautier J, Leducq S, Bessis D, et al. Vascular endothelial growth factor, tissue factor, coagulation and fibrinolysis markers in slow-flow vascular malformations: a prospective study of treatment with sirolimus. Br J Dermatol. 2023; 188(1): 152–154.
- 29Duong JT, Geddis A, Carlberg K, Rudzinski E, Len M, Zheng HB. Sirolimus for management of GI bleeding in blue rubber bleb nevus syndrome: a case series. Pediatr Blood Cancer. 2022; 69(11):e29970.
- 30Seront E, Van Damme A, Legrand C, Bisdorff-Bresson A, Orcel P, Funck-Brentano T, et al. Preliminary results of the European multicentric phase III trial regarding sirolimus in slow-flow vascular malformations. JCI Insight. 2023; 8(21):e173095.
- 31Triana P, Dore M, Cerezo VN, Cervantes M, Sánchez AV, Ferrero MM, et al. Sirolimus in the treatment of vascular anomalies. Eur J Pediatr Surg. 2017; 27(1): 86–90.
- 32Wu C, Song D, Guo L, Wang L. Refractory head and neck lymphatic malformation in infants treated with sirolimus: a case series. Front Oncol. 2021; 11:616702.
- 33Cho YJ, Kwon H, Kwon YJ, Kim SC, Kim DY, Namgoong JM. Effects of sirolimus in the treatment of unresectable infantile hemangioma and vascular malformations in children: a single-center experience. J Vasc Surg Venous Lymphat Disord. 2021; 9(6): 1488–1494.
- 34Rössler J, Baselga E, Davila V, Celis V, Diociaiuti A, El Hachem M, et al. Severe adverse events during sirolimus “off-label” therapy for vascular anomalies. Pediatr Blood Cancer. 2021; 68(8):e28936.
- 35Kalbfell R, Cohen-Cutler S, Grisham E, Bereitschaft C, Borst AJ, Green AM, et al. Infectious complications of vascular anomalies treated with sirolimus: a systematic review. Pediatr Blood Cancer. 2024; 71(1):e30758.
- 36Navarro M, Allemang-Trivalle A, Leducq S, Jonville-Bera AP, Maurier A, Zejli T, et al. Indication for a pneumocystis prophylaxis therapy in patients with vascular anomalies treated with PIK3/AKT/mTOR pathway inhibitors: experts' opinion and systematic review from the literature. Dermatology. 2023; 239(6): 942–951.
- 37Braun M, Young J, Reiner CS, Poster D, Krauer F, Kistler AD, et al. Low-dose oral sirolimus and the risk of menstrual-cycle disturbances and ovarian cysts: analysis of the randomized controlled SUISSE ADPKD trial. PLoS One. 2012; 7(10):e45868.
- 38Alfadhli E, Koh A, Albaker W, Bhargava R, Ackerman T, McDonald C, et al. High prevalence of ovarian cysts in premenopausal women receiving sirolimus and tacrolimus after clinical islet transplantation. Transpl Int. 2009; 22(6): 622–625.
- 39Sterba M, Pokorna P, Faberova R, Pinkova B, Skotakova J, Seehofnerova A, et al. Targeted treatment of severe vascular malformations harboring PIK3CA and TEK mutations with alpelisib is highly effective with limited toxicity. Sci Rep. 2023; 13:10499.
- 40Remy A, Tran TH, Dubois J, Gavra P, Lapointe C, Winikoff R, et al. Repurposing alpelisib, an anti-cancer drug, for the treatment of severe TIE2-mutated venous malformations: preliminary pharmacokinetics and pharmacodynamic data. Pediatr Blood Cancer. 2022; 69(10):e29897.
- 41Boccara O, Maruani A. Diagnosis of vascular malformations: clinical examination first, then molecular biology. J Eur Acad Dermatol Venereol. 2024; 38(7): 1232–1233.