Lipid profile in women of different ethnicity with gestational diabetes: Relationship with fetal growth
Abbreviations: AUC, area under the curve; BMI, body mass index; FPG, fasting plasma glucose; GDM, gestational diabetes mellitus; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; OGTT, 75-g oral glucose tolerance test; VLDL, very-low-density lipoprotein.
Abstract
Aims/Introduction
Pregnancy complicated by gestational diabetes mellitus (GDM) is characterized by excessive insulin resistance that impairs the metabolism of glucose and lipids. the aim of the study was to examine lipid profiles during pregnancy of women with GDM, and its impact on fetal growth in a multiethnic population.
Materials and Methods
The study included 322 pregnant women of different ethnicity with GDM attending a clinical unit specializing in metabolic diseases.
Results
The area under the curve for the 75-g oral glucose tolerance test and glycated hemoglobin were significantly different among all groups. At the time of being diagnosed with GDM, Asian and African mothers had significantly lower levels of total and low-density liprotein cholesterol than European mothers (P < 0.001). The trend for high-density liprotein cholesterol was similar. Triglycerides levels in the Asian group (193.6 ± 65.5 mg/dL) were higher than in the African group (133.2 ± 49.6 mg/dL, P < 0.001), whereas the European group presented intermediate values (175.8 ± 58.8 mg/dL), which differed significantly only versus the African group (P < 0.001). Pre-partum lipid profiles showed a trend quite similar to that observed at diagnosis. The newborn's birthweight was significantly different, with that of African women (3,437 ± 503 g) being the highest, followed by that of European women (3,294 ± 455 g) and of Asian women (3,006 ± 513 g). The rates of macrosomia showed a trend with higher values in the African group (13.5%), followed by the European group (5.7%, P = 0.1162), whereas that of the Asian group was zero (P = 0.0023 vs African).
Conclusions
Our data show that lipid profiles in women with GDM differ by ethnicity. The impact of lipid profile on fetal growth is limited and uninfluenced by ethnicity.
Introduction
Pregnancy is characterized by a physiological hyperlipidemia, a mechanism to provide nutrients for the developing fetus1, 2. The mother's levels of total cholesterol, low-density lipoproteins (LDL), high-density lipoproteins (HDL) and triglycerides gradually increase during pregnancy, peaking in the third trimester1-3. There is a linear relationship between lipid levels and fetal weight gain4-7, and under- and overnutrition are both associated with impaired lipid profiles during normal pregnancy, and with newborns that are small for their gestational age (SGA), suffer from intrauterine growth restriction or are large for their gestational age (LGA).
Gestational diabetes mellitus (GDM) is an altered glucose tolerance diagnosed during pregnancy. It is the most common metabolic disease in pregnancy8, 9, associated with short- and long-term maternal and fetal complications, so it must be diagnosed as soon as possible and properly treated to avoid these complications8.
An abnormal insulin resistance, a key feature of GDM, impairs lipid metabolism as well as glucose metabolism9, 10, and lipid levels predict fetal growth in mothers with well-controlled GDM11. Ethnicity-related differences have been shown in people's lipid profiles, with Indians showing a less favorable, and Africans showing a more favorable lipid profile than Western populations12. Studies on the possible impact on maternal and fetal outcomes of the lipid profiles of pregnant women of different ethnicities are scarce, and only refer to women without diabetes13, 14. Hence, our interest in examining the lipid profiles of women with GDM and their impact on fetal growth in a multi-ethnic population.
MATERIALS AND METHODS
Participants
The present retrospective study involved 322 pregnant women diagnosed with GDM whose lipid profile data during pregnancy were recorded while they were attending the ULSS 6 Diabetology Unit in Padova, Italy, between 2016 and 2018. Pregnant women with type 1 or 2 diabetes mellitus, overt diabetes, or twin pregnancies were excluded. GDM was diagnosed with a 75-g oral glucose tolerance test (OGTT) according to the International Association of the Diabetes and Pregnancy Study Groups criteria and Italian guidelines15, 16. A diagnostic OGTT was carried out between the 16th and 18th or between the 24th and 28th weeks of gestation, depending on the women's risk factors, as required by Italian guidelines16.
Participants were divided into three ethnic groups based on their geographical area of origin: 158 Europeans (from Italy, Romania, Moldova, Albania, Ukraine, Kosovo, Serbia); 68 Asians (South Asians: from Bangladesh, Sri Lanka; Southeast Asians: from Thailand); and 96 Africans (from Nigeria, Cameroon, Congo, Ivory Coast, Togo, Somalia, Morocco, Syria, Tunisia). Figures S1 and S2 show the distribution of patients. The considered ethnic groups represent the distribution of patients attending the Diabetology Unit in Padova, and therefore are not inclusive of all the populations in the three main geographical areas. In particular, the Asian group is representative of South and Southeast populations only.
A priori power analysis (GPower_3.1.9.717) on the basis of a provisional effect size (Cohen's f) of 0.25 (corresponding by convention to a medium effect), with an α-error probability of 0.05, a power of 0.95 and three groups, had yielded a sample size estimation of at least 252 participants. As the frequency of non-European groups at the Diabetology Unit was expected to be lower, a provisional 25% increase of the total number of observations was set, giving the final number of 322 participants.
The women were followed up at the Diabetology Unit by a multidisciplinary team consisting of a diabetologist, a dietician, and a nurse specializing in diabetes and pregnancy. At their first appointment, all the women's anthropometric and clinical data were recorded, including: age, pre-pregnancy bodyweight and body mass index (BMI), height, chronic diseases, family history of diabetes and obstetric history.
All the women were trained to self-monitor their glucose levels and were given a personalized diet, in accordance with the guidelines of the Institute of Medicine18, and the Italian Guidelines for the treatment of diabetes mellitus19. As for diet composition, we referred to the Italian guidelines20: carbohydrates account for 45–60% of caloric needs and lipids account for the 20–35% (saturated fatty acids <10% of caloric needs, polyunsaturated fatty acids 5–10% of caloric needs, total cholesterol <300 mg/day); remaining calories are accounted for by protein. Patients' dietary compliance was assessed using the 24-h recall method and an analysis of the food diaries completed weekly by the patient21. Insulin therapy was started if women did not meet the most recently established glycemic goals (fasting plasma glucose <90 mg/dL, 1 h postprandial plasma glucose <130 mg/dL, 2 h postprandial plasma glucose <120 mg/dL) after 2 weeks of dietary treatment19.
Patients attended follow-up visits every 2–4 weeks, based on individual needs. During these visits, patients' weight, blood pressure, glycemic levels, lipid profile (total cholesterol, HDL, LDL and triglycerides), glycated hemoglobin (HbA1c) levels and any data regarding potential maternal complications (gestational hypertension, preeclampsia, eclampsia) were recorded.
Then, 6–8 weeks after delivery, the maternal (mode and time of delivery, complications during labor and delivery) and neonatal outcomes (weight, length at birth, any shoulder dystocia, hypoglycemia, hyperbilirubinemia, neonatal asphyxia or congenital malformations) were recorded.
Analytical determinations
Plasma glucose levels were measured using the glucose oxidase method23. HbA1c was measured using standard high-performance liquid chromatography24. Plasma lipids were measured as proposed by Dastych et al.25
Statistical analysis
The data were processed with JMP®Pro17 software (SAS Institute Inc., Cary, NC, USA), JASP_0.16.4 (JASP Team, 2022, Computer software) and R_4.2.226. Continuous quantitative variables are expressed as means ± standard deviations. Normality of distribution was assessed by the Shapiro–Wilk and Anderson–Darling test. In case of normal distribution, differences among groups were evaluated by analysis of variance followed by Tukey–Kramer honestly significant difference or Holm–Bonferroni post-hoc tests. Analysis of covariance was carried out on lipid profile to correct differences among ethnicity also according to possible covariates and factors; missing records were imputed using the low-rank matrix approximation method. In case of not-normally distributed data, differences were evaluated by the non-parametric Kruskal–Wallis test followed by the Steel–Dwass post-hoc test. Comparison of lipid profile at GDM diagnosis versus pre-partum within each ethnic group was carried out by Student's t-test for paired data. HDL cholesterol was evaluated between two different gestational groups by the Welch test, due to unequal variances and sample sizes. Qualitative variables were compared using Pearson's χ2-test; Yates' correction was applied when expected frequencies were <5. Bonferroni's correction was used to account for inflation due to multiple comparisons. The presence of a correlation between parameters was evaluated with nominal logistic regression, and odds ratios (OR) obtained with 95% confidence intervals (95% CI). Statistical significance for all the differences was set for P < 0.05.
RESULTS
The clinical and metabolic parameters of the women with GDM are shown in Table 1, grouped by ethnicity. European and African women were significantly older than Asian women (P < 0.0001), and African women had a higher pre-pregnancy BMI than European and Asian women (P < 0.0001). Weight gain during pregnancy, as well as the time of GDM diagnosis, was similar in all three ethnic groups. The first fasting plasma glucose (FPG) in early pregnancy in Asian women (94.7 ± 14.2 mg/dL) was higher than the other two groups (90.5 ± 13.2 mg/dL in African women and 87.0 ± 10.4 mg/dL in European women), but the difference resulted significant only versus European women (P = 0.0001). The area under the curve (AUC) for the OGTT test was significantly different among all groups (Asian women 322.5 ± 48.7 mg h/dL; European women 289.6 ± 41.6 mg h/dL; African women 267.1 ± 55.8 mg h/dL). HbA1c levels at diagnosis and at the end of pregnancy, despite being very similar in the three groups, showed a significant difference for European women versus African women (P < 0.0001) and versus Asian women (P < 0.0001; (Table 1). Insulin therapy was prescribed in a similar percentage of African and European women (20%), but for Asian women, the use of insulin was twice than that of the other groups (40%).
| Parameters | European (n = 158) | African (n = 96) | Asian (n = 68) | P | P | P |
|---|---|---|---|---|---|---|
| European vs African | European vs Asian | African vs Asian | ||||
| Age (years) | 34.0 ± 4.1 | 33.0 ± 5.1 | 29.0 ± 4.8 | 0.1902 | <0.0001 | <0.0001 |
| Pre-pregnancy BMI (kg/m2) | 25.2 ± 5.5 | 29.8 ± 5.6 | 25.7 ± 4.6 | <0.0001 | 0.1121 | <0.0001 |
| Weight gain (kg) | 10.0 ± 5.1 | 9.5 ± 9.4 | 8.5 ± 5.5 | 0.1813 | 0.0777 | 0.9666 |
| GDM diagnosis (weeks) | 23.7 ± 4.4 | 22.3 ± 5.7 | 22.4 ± 5.1 | 0.1934 | 0.2324 | 0.9955 |
| FPG in early pregnancy (mg/dL) | 87.0 ± 10.4 | 90.5 ± 13.2 | 94.7 ± 14.2 | 0.2350 | 0.0001 | 0.0562 |
| OGTT AUC (mg h/dL) | 289.6 ± 41.6 | 267.1 ± 55.8 | 322.5 ± 48.7 | 0.0003 | <0.0001 | <0.0001 |
| HbA1c at diagnosis (%) | 5.1 ± 0.4 | 5.5 ± 0.4 | 5.5 ± 0.5 | <0.0001 | <0.0001 | 0.9747 |
| HbA1c at 3rd trimester (%) | 5.3 ± 0.4 | 5.5 ± 0.5 | 5.6 ± 0.5 | <0.0001 | <0.0001 | 0.9526 |
| Insulin therapy, n (%) | 32; 20% | 19; 20% | 27; 40% | 1.0000 | 0.0090 | 0.0234 |
- Continuous variables are presented as mean ± standard deviation; qualitative variables are expressed as absolute frequencies and percentages. Bold values indicate statistically significant differences.
Table 2 and Figure 1 present the differences by ethnic group in the lipid profiles of the women at the time of their diagnosis of GDM and in the pre-partum period. When their GDM was diagnosed, the Asian and African mothers had significantly lower levels of total cholesterol than European mothers (199.5 ± 38.9, 201.8 ± 36.2 and 245.6 ± 47.3 mg/dL, respectively; P < 0.001) as well as LDL cholesterol (99.7 ± 34.4, 107.8 ± 33.3 and 138.6 ± 43.0 mg/dL, respectively; P < 0.001 and P = 0.011). The trend for HDL cholesterol was similar (61.0 ± 13.7, 67.2 ± 13.7 and 71.8 ± 16.4 mg/dL, respectively), but the difference was significant for European women versus Asian women (P = 0.010). Triglycerides levels in Asian women (193.6 ± 65.5 mg/dL) were higher than in African women (133.2 ± 49.6, P < 0.001), whereas European women presented intermediate values (175.8 ± 58.8 mg/dL), which differed significantly only versus African women (P < 0.001).
| Parameters | European (n = 158) | African (n = 96) | Asian (n = 68) | P | P | P | |
|---|---|---|---|---|---|---|---|
| European vs African | European vs Asian | African vs Asian | |||||
| At diagnosis | Total cholesterol (mg/dL) | 245.6 ± 47.3 | 201.8 ± 36.2 | 199.5 ± 38.9 | <0.001 | <0.001 | 0.999 |
| HDL cholesterol (mg/dL) | 71.8 ± 16.4 | 67.2 ± 13.7 | 61.0 ± 13.7 | 0.095 | 0.010 | 0.739 | |
| LDL cholesterol (mg/dL) | 138.6 ± 43.0 | 107.8 ± 33.3 | 99.7 ± 34.4 | 0.011 | <0.001 | 0.591 | |
| Triglycerides (mg/dL) | 175.8 ± 58.8 | 133.2 ± 49.6 | 193.6 ± 65.5 | <0.001 | 0.345 | <0.001 | |
| Pre-partum | Total cholesterol (mg/dL) | 268.0 ± 48.5*** | 202.7 ± 39.5 | 210.6 ± 42.6*** | <0.001 | <0.001 | 0.915 |
| HDL cholesterol (mg/dL) | 70.9 ± 14.6 | 65.8 ± 14.7 | 60.4 ± 16.1 | 0.176 | 0.043 | 0.840 | |
| LDL cholesterol (mg/dL) | 153.9 ± 46.0*** | 106.1 ± 36.7 | 109.2 ± 39.0** | 0.008 | 0.003 | 0.915 | |
| Triglycerides (mg/dL) | 212.2 ± 63.0*** | 154.2 ± 44.5*** | 205.1 ± 69.8** | <0.001 | 0.789 | 0.020 |
- Data are presented as mean ± standard deviation. ***P < 0.0001, **P < 0.005, comparison of parameters at diagnosis versus pre-partum, within each ethnic group, Student's t-test for paired data. P-values at diagnosis and pre-partum were adjusted for the following covariates: age, body mass index, fasting plasma glucose, glycated hemoglobin, 75-g oral glucose tolerance test, area under the curve, gestation week and insulin therapy. Bold values indicate statistically significant differences.
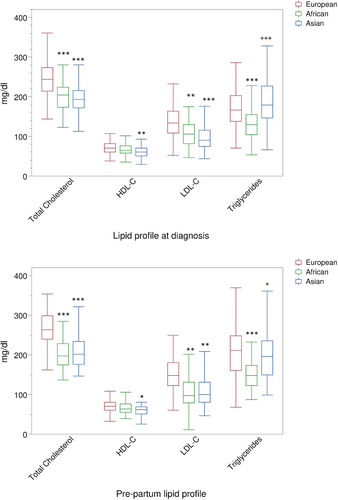
As for their pre-partum lipid profiles (Table 2 and Figure 1), the values showed a trend quite similar to that observed at diagnosis, comparing the three different groups. However, a paired comparison between values at diagnosis versus those observed pre-partum showed a significant increase of total and LDL cholesterol, as well as of triglycerides, in both European (P < 0.0001) and Asian (P < 0.0001/P < 0.005) women; no variation occurred for HDL cholesterol. In African women, only triglycerides at pre-partum presented an increase compared with values at GDM diagnosis (P < 0.0001).
Overall, only 15 (4.7%) women had a preterm delivery (<37 gestational weeks); these women (Figure 2) had lower HDL cholesterol levels (measured at diagnosis) in comparison with women who had physiological delivery time (≥37 gestational weeks; 59.8 ± 10.0 mg/dL, n = 10, vs 68.1 ± 15.6 mg/dL, n = 260, P = 0.029 at Welch test). HDL cholesterol before delivery was similar in the two groups (69.7 ± 12.0 mg/dL, n = 9, vs 67.1 ± 15.6 mg/dL, n = 225, P = 0.542 at Welch test).
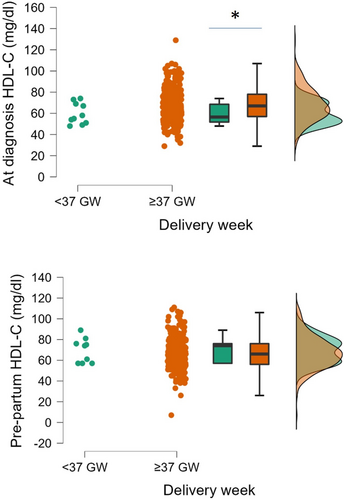
Table 3 shows the neonatal outcomes for the different ethnic groups of women with GDM. Among the three groups, the newborn's birthweight was significantly different, except for European versus African women, with that of African women being the highest (3,437 ± 503 g), followed by that of European women (3,294 ± 455 g) and of Asian women (3,006 ± 513 g). The ponderal index was the same (2.8 ± 0.3 g/cm3) in African and Asian newborns, and slightly lower in European newborns (2.7 ± 0.3 g/cm3), although not significantly different. The rates of macrosomia showed a trend, with higher values in African women (13.5%), followed by European women (5.7%, P = 0.1162 vs African women), whereas that of Asian women was zero (P = 0.0023 vs African women). In all groups, the newborn's gestational age findings were found to be appropriate for gestational age (AGA) for most cases (80.4%, 69.8% and 72.1% for European, African and Asian women, not significant). The rate of LGA cases was higher for African women (26.0%), followed by European (11.8%) and Asian (4.4%) women, showing a significant difference between European versus African women (P = 0.0164) and African versus Asian women (P = 0.0007). SGA outcome was higher in Asian women (23.5%), followed by European (7.8%) and African women (4.2%), leading to a significant difference for Asian women versus European women (P = 0.0060) and African women (P = 0.0012).
| Parameters | European (n = 158) | African (n = 96) | Asian (n = 68) | P | P | P |
|---|---|---|---|---|---|---|
| European vs African | European vs Asian | African vs Asian | ||||
| Birthweight (g) | 3,294 ± 455 | 3,437 ± 503 | 3,006 ± 513 | 0.169 | 0.004 | <0.001 |
| Ponderal index (g/cm3) | 2.7 ± 0.3 | 2.8 ± 0.3 | 2.8 ± 0.3 | 0.150 | 0.532 | 0.837 |
| Macrosomia (>4,000 g), n (%) | 9; 5.7% | 13; 13.5% | 0; 0% | 0.1162 | 0.1812 | 0.0023 |
| SGA, n (%) | 12; 7.8% | 4; 4.2% | 16; 23.5% | 0.8947 | 0.0060 | 0.0012 |
| AGA, n (%) | 123; 80.4% | 67; 69.8% | 49; 72.1% | 0.1991 | 0.6567 | 1.0000 |
| LGA, n (%) | 18; 11.8% | 25; 26.0% | 3; 4.4% | 0.0164 | 0.4006 | 0.0007 |
- Continuous variables are presented as mean ± standard deviation; qualitative variables are expressed as absolute frequencies and percentages. P-values for birthweight and ponderal index were adjusted for mother's age, prepartum glycated hemoglobin, delivery week, and body mass index. Bold values indicate statistically significant differences. AGA, appropriate for gestational age; LGA, large for gestational age; SGA, small for gestational age.
The presence of possible correlations between the neonatal outcomes and mother's lipid profile, as well as mother's glycemic control parameters, was evaluated considering the participants altogether, to have a global view of the phenomena. Table S1 shows all the tested correlations; significant inverse correlations were detected for macrosomia versus AUC under OGTT (OR 0.9887, 95% CI 0.9797–0.9979, P = 0.0142) and versus triglyceride levels at diagnosis (OR 0.9869, 95% CI 0.9765–0.9975, P = 0.0065). Analysis was also carried out by separate groups, and reported in Table S2. The group “Asian” did not present any case of macrosomia, so the logistic regression analysis was not possible, and consequently excluding for the Asian group any influence on macrosomia of the parameters evaluated.
When considering SGA/LGA/AGA outcome (Table S3), a significant correlation was found versus FPG at diagnosis of GDM (P = 0.0092), versus AUC under OGTT (P = 0.0001) and versus pre-partum HbA1c (P = 0.0234). In particular, considering FPG at diagnosis of GDM, the OR was slightly, although significantly, increased for SGA (OR 1.03583, 95% CI 1.00694–1.0656, P = 0.015) and LGA (OR 1.02933, 95% CI 1.00397–1.0553, P = 0.023) compared with AGA condition. Also, the OR for AUC under OGTT was slightly, but significantly, increased for SGA compared with AGA (OR 1.01358, 95% CI 1.0060–1.0213, P = 0.001), whereas OR was not significant for LGA-AGA comparison. Finally, the OR for pre-partum HbA1c showed a significantly increased value for the comparison LGA-AGA (OR 2.680, 95% CI 1.3201–5.4405, P = 0.006). No parameter linked to lipid profile presented a significant correlation with neonatal SGA/LGA/AGA outcome (Table S2).
DISCUSSION
The present data show that, among women with GDM, Asian women have significantly lower levels of total cholesterol, HDL and LDL cholesterol than European women, and African women also have significantly lower levels of total cholesterol, LDL and triglycerides than European women, both at diagnosis and before delivery. These data agree with previous findings of lipid profile in different ethnic groups during early gestation in the general population13, 14.
Various factors have been suggested to explain ethnicity-related differences in lipid profiles, including genetic differences, smoking, diet, alcohol abuse and type of fat deposition. Visceral fat is deposited differently in different ethnic groups, and it has been strongly associated with lipid profiles in the general population27. The metabolic action of abdominal fats is also strictly linked to certain differences in insulin resistance between African and European women, with subsequent effects on their lipid levels28. Schreuder et al.13 showed that, after adjusting for age, physical activity and smoking, only pre-pregnancy BMI could partially explain ethnicity-related differences identified in 30,125 healthy pregnant women of different ethnic origin (mainly Turkish and Suriname Hindustani). Unfortunately, BMI gives no indication regarding the preferential accumulation of fat (i.e., visceral or subcutaneous). Despite higher pre-pregnancy BMI observed in our cohort of African women, the lipid profile appears reduced in comparison with European women, leading to exclude a causal link between the BMI and lipid turnover in women with GDM.
Regarding fetal outcomes, higher birthweights were found for African women with GDM in comparison with the other ethnic groups, although the PI was not significantly higher than other groups. The rate of macrosomia for African newborns was the highest among groups, whereas the occurrence of SGA was reduced and that of LGA increased, in comparison with the other ethnic groups. Despite different lipid profiles in the ethnic groups, only a modest correlation was found for macrosomia with maternal triglycerides at GDM diagnosis, and the occurrence of SGA/LGA/AGA frequencies among groups appeared linked only to glycemic control parameters, such as OGTT AUC, first FPG and HbA1c, at the third trimester. These data appear in part to agree with the literature. Fasting triglyceride levels measured in the third trimester of pregnancy were found to be independently associated with neonatal birthweight in pregnant women with a normal glucose tolerance29. In women with GDM achieving a good metabolic control, the mother's triglyceride and free fatty acid levels were found to be related to the rate of LGA infants11. These studies only examined European women, however.
Chen et al.30 carried out a case–control study on a multiethnic cohort of healthy pregnant women whose offspring were delivered preterm or at term. African American women had significantly higher levels of HDL cholesterol and apolipoprotein (Apo)A1, and lower levels of triglycerides and ApoB than Hispanic and non-Hispanic white women. High HDL cholesterol and ApoA1 concentrations were associated with an increased risk of preterm delivery in all pregnant women. The present data also confirm in GDM women the differences in lipid profile in the different ethnicities, but do not show a clear link between HDL levels and increased risk of preterm delivery, as reported by Niyaty et al.31 in pregnant women affected by metabolic syndrome. Preterm delivery has been associated with inflammation, oxidative stress and endothelial dysfunction32, and HDL can exert anti-inflammatory effects by modulating T-cell activation and protecting ApoB lipoprotein from oxidation, contributing to the maintenance of the endothelial function33. The absence of a change in HDL cholesterol during gestation can explain the absence of a specific relationship with newborn outcome, in agreement with findings in physiological pregnancy34, where no association was found between maternal HDL cholesterol and birthweight or adverse birth outcomes. However, the reduced HDL level found in the early stage of gestation in women with preterm delivery (Figure 2) might have influenced the placental/fetal development, with more pronounced tendency to early delivery, although the level of HDL increased prepartum, possibly as a consequence of dietary standardized intervention.
A previous investigation35 documented how triglycerides in pregnancy remain unchanged up to the 30th week, but without data on late pregnancy. From the present study, for the first time the lipid profiles of different ethnic groups were compared at the diagnosis of GDM and at the end of pregnancy, suggesting that triglycerides increase during late pregnancy in all the different ethnic groups, in line with fetal growth. It should be remembered that the fetus needs VLDL to increase fat mass and for surfactant synthesis36. It has been shown that also in normal pregnancy, maternal hypertriglyceridemia during late pregnancy derives from elevated lipolytic activity and reduced lipoprotein lipase activity in adipose tissue37; however, as shown by a recent meta-analysis, in women with GDM, triglyceride levels, as well as total cholesterol, are higher than in healthy pregnant women38.
The observed results interestingly highlight that, although significant differences were observed among ethnicities, the tendency of lipid profile is similar in the different populations, regardless of the context in which it is observed. Possible interpretations include the hypothesis that the lipid profile during gestational diabetes is genetically affected and, in contrast, that the eating habits of the countries of origin remain consolidated, even if one lives in a new country. Considering other covariates and factors besides ethnicity, such as pre-pregnancy BMI, OGTT AUC, HbA1c and the use of insulin, the data analysis showed that ethnicity remains the main contributing factor for observed differences in the lipid profile. A recent study by Shen et al.39 suggested that in pregnancy, a correlation exists between triglyceride level and glucose metabolism, showing that persistently elevated triglycerides could be an independent risk factor for insulin resistance. The study still did not consider pregnant women's lifestyle, including physical activity and dietary patterns. However, previous observations by Couch et al.40 found that exaggerated hypertriglyceridemia is a feature of GDM, which also occurs if women are diet-treated, suggesting that dietary factors are not major determinants of lipoprotein profile during gestation, even in the case of GDM.
To be more confident about the associations between lipid profile and fetal weight, a logistic regression analysis was carried out. Glucose level at the time of diagnosing GDM, as well as HbA1c at the third trimester, were possible determinants of fetal growth in our multi-ethnic population, as, although marginally, the glycemic parameters appeared linked to the different occurrence of SGA/LGA/AGA rates. These data confirm, in our population of different ethnic origins, that plasma glucose is a major determinant of fetal growth, as suggested by Pedersen's hypothesis41 and the recent HAPO study findings42, 43. Although the mothers' lipid levels differed, both at diagnosis and at term, in the present cohort of pregnant women with GDM of different ethnic origins, this seems to have had no appreciable impact on fetal growth. We did not observe specific correlations between fetal growth and lipid profile parameters, probably because glucose metabolism and hypoglycemic therapy play a predominant role in GDM. In the whole group of women with GDM, despite a significant inverse correlation between macrosomia and the first OGTT test during pregnancy (P = 0.0142), as well as the plasma triglycerides at diagnosis (P = 0.0065), the clinical relevance of these findings were limited, as the OR was very close to one. A separate analysis of the correlation according to each ethnic group (Table S2) does not show any significant correlation between lipid profile and birthweight.
There are various published data regarding the OGTT AUC in pregnancy. In a review on biomarkers for macrosomia in pregnancies affected by diabetes, Nahavandi et al.44 reported that maternal blood glucose is not associated with birthweight, and variations in glucose testing protocols and treatment regimens might provide some explanation for this fact. Zhang et al.45 observed that although OGTT AUC could evaluate the severity of maternal hyperglycemia, an elevated AUC determines more aggressive glucose control, suggesting that this might reduce the correlation with perinatal outcomes. In fact, in the present study, the group with higher OGTT AUC (Asian women) had a significantly higher percentage of insulin treatment (Table 1). Geifman-Holtzman et al.46 found no correlation between true fetal macrosomia and an abnormal OGTT at term. Also, Sahin and Madendag47 recently confirmed that OGTT has a low capacity for predicting macrosomia and LGA in pregnant women without GDM. On the other end, the risk of macrosomia linked to triglycerides levels at diagnosis of GDM confirms the role of hypertriglyceridemia on fetal growth, and the increasing cardiovascular risk later in life1.
Regarding limitations, the present study analyzed an unselected cohort of women with GDM of different ethnic origins, and although the number of patients was relatively small, it was based on a priori power analysis based on a medium effect size. Our findings consequently need to be confirmed in a larger number of cases. No pregnant women without diabetes were available as a control, as the database consisted exclusively of records from patients attending our Diabetology Unit. However, as aforementioned, similar trends have been reported in the literature regarding lipid profile in healthy pregnant women13, 14. Due to the retrospective structure of the study, the data regarding diet derive from database records, and not from a programmed prospective protocol with individual diet diary. Therefore, no individual details were available for additional multivariate analysis taking into account single-patient diet; this issue will be considered in future prospective investigations. We did not have data on other lipid fractions (ApoA1, ApoB etc.), but the aim of our study was to establish the lipid profile vis-à-vis fetal growth, not to analyze the cardiovascular risk profile. The provenance of patients of the Asian group was limited to South and Southeast Asia, due to the characteristics of people attending our Diabetology Unit; therefore, the observations should be intended as referring to this particular ethnic group and might not be extended to other regions of Asia.
The main strength of the present study lies in that it is the first, to our knowledge, to have examined the behavior of plasma lipids in women with GDM of different ethnic origins, as well as the relationship between their lipid profile and fetal growth. Our patients were also closely monitored in terms of their compliance with dietary recommendations and the management of metabolic control.
In conclusion, the present study suggests that lipid profiles differ by ethnic origin in women with GDM, but show a common behavior tendency, resembling what happens in non-diabetic pregnant women, indicating a physiological role of lipoprotein modification occurring during gestation. That said, in women with GDM – whatever their ethnic origin – glucose is a main determinant of fetal growth, so every effort should be made to normalize their glycemia as soon as possible.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: The study was carried out in accordance with the Declaration of Helsinki and approved by the Ethics Committee for Clinical Trials of the Province of Padova (Italy), study protocol No. 14749.
Informed consent: N/A.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.




