Point-of-care nerve conduction device predicts the severity of diabetic polyneuropathy: A quantitative, but easy-to-use, prediction model
Clinical Trial Registry
University Hospital Medical Information Network
UMIN000021916
Abstract
Aims/Introduction
A gold standard in the diagnosis of diabetic polyneuropathy (DPN) is a nerve conduction study. However, as a nerve conduction study requires expensive equipment and well-trained technicians, it is largely avoided when diagnosing DPN in clinical settings. Here, we validated a novel diagnostic method for DPN using a point-of-care nerve conduction device as an alternative way of diagnosis using a standard electromyography system.
Materials and Methods
We used a multiple regression analysis to examine associations of nerve conduction parameters obtained from the device, DPNCheck™, with the severity of DPN categorized by the Baba classification among 375 participants with type 2 diabetes. A nerve conduction study using a conventional electromyography system was implemented to differentiate the severity in the Baba classification. The diagnostic properties of the device were evaluated using a receiver operating characteristic curve.
Results
A multiple regression model to predict the severity of DPN was generated using sural nerve conduction data obtained from the device as follows: the severity of DPN = 2.046 + 0.509 × ln(age [years]) − 0.033 × (nerve conduction velocity [m/s]) − 0.622 × ln(amplitude of sensory nerve action potential [µV]), r = 0.649. Using a cut-off value of 1.3065 in the model, moderate-to-severe DPN was effectively diagnosed (area under the receiver operating characteristic curve 0.871, sensitivity 70.1%, specificity 87.7%, positive predictive value 83.0%, negative predictive value 77.3%, positive likelihood ratio 5.67, negative likelihood ratio 0.34).
Conclusions
Nerve conduction parameters in the sural nerve acquired by the handheld device successfully predict the severity of DPN.
Introduction
Diabetic polyneuropathy (DPN) develops at the earliest stage of diabetes among chronic diabetic complications1, 2. Additionally, DPN often progresses asymptomatically3, and severe DPN increases the risk of gangrene and amputation of the foot, which greatly affects the quality of life and the prognosis of patients with diabetes. Thus, early detection and the severity assessment of DPN are important to improve the prognosis in patients1, 4, 5.
At present, subjective symptoms, physical findings, electrophysiological tests and pathological examinations are used to diagnose DPN6. Although the diagnosis of DPN using physical signs and symptoms has low reproducibility and accuracy, the reliability of quantitative pathological and physiological tests is well established7. However, pathological tests, such as a nerve or skin biopsy, are less versatile, because they are highly invasive and require advanced techniques8. On the contrary, a nerve conduction study (NCS) using electromyography system (EMGS), one of the electrophysiological tests, is less invasive, and excellent in reproducibility and objectivity, and hence has been established as a gold standard for the diagnosis of DPN9; whereas, unfortunately, NCS has not been widely implemented in the world due to requirements of expensive equipment and skilled inspectors. To overcome the non-universality of NCS, a point-of-care nerve conduction device, DPNCheck™ (NeuroMetrix Inc., Waltham, MA, USA), which only examines the sural nerve, has been developed and its reliability verified, including a good correlation with a standard EMGS10-12. Even though the handheld device is very simple, having only one button and requiring no skilled inspector, it has been reported that the device provides NCS data that correlates well with the authorized diagnostic scores of DPN; that is, the Neuropathy Disability Score13 and the Toronto Neuropathic Clinical Score14, 15. In addition, this device is being applied as an alternative to a conventional EMGS, a gold standard for the diagnosis of peripheral neuropathies, to evaluate the validity of newly developed neurological tests, such as LDIflare™16.
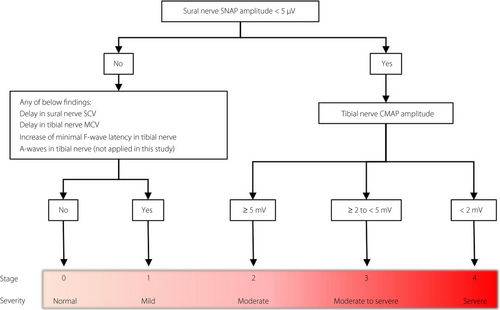
Despite the increase in opportunities for application, as described above, no proper interpretation of NCS data obtained from the device has been established. Here, we investigated whether the NCS data from the device could predict the severity of DPN. At the same time, we introduced the widely used diagnostic classification in Japan, the Baba classification17, by which objective and reproducible staging of DPN using NCS parameters acquired by a standard EMGS has been substantialized (Figure 1). A recent 5-year prospective study (reported in Japanese) showed that the severity of DPN differentiated by the Baba classification corelated with cardiovascular events and prognosis. Thus, we verified the predictive performance of the device using the Baba classification as a diagnostic reference in addition to the authorized diagnostic criterion that consisted of nerve conduction abnormalities in two or more nerves.
Methods
Eligibility criteria
From 2014 to 2019, 422 participants in total, who were previously diagnosed with diabetes mellitus and were hospitalized at Aichi Medical University Hospital (Nagakute, Japan) to improve their hyperglycemia, were invited. All participants signed a document of consent for the study. Patients were excluded if they had been previously diagnosed with type 1 diabetes; had a history of other causes of peripheral neuropathy; failed to evoke sural nerve action potential in DPNCheck™; or had diabetic ketoacidosis, severe infection or severe injuries. Study procedures were approved by the ethics committee of Aichi Medical University Hospital (No. 14-019).
NCS
The NCS in bilateral sural nerves was carried out to evaluate the sensory nerve conduction velocity (SNCV) and amplitude of sensory nerve action potential (SNAP) in the sural nerves utilizing DPNCheck™ and a standard EMGS (Neuropack X1, MEB-2312; Nihon Kohden, Tokyo, Japan). The NCS in bilateral tibial nerves was also carried out using the standard EMGS. The NCS using the standard electromyography system was carried out in an air-conditioned electrically shielded room by trained technicians. For tibial NCS, surface recording electrodes were placed on the abductor hallucis according to the belly-tendon technique. Ankle stimulation was carried out with the cathode 80-mm proximal to the active electrode slightly posterior to the medial malleolus. F-waves were evaluated using a series of 16 supramaximal stimuli. For sural NCS, the stimulation electrodes were placed 140-mm proximal to the recording electrodes placed on the midpoint of the lateral malleolus and ankle.
DPNCheck™ needs to be attached to a disposable biosensor that detects surface temperature, facilitates electrical stimulation and integrates the nerve conduction data. The NCS can start only under a surface temperature of 28 ± 5°C, and the acquired values are automatically corrected by the temperature. The skin temperature was measured at the ankle; the foot was warmed with a hot towel before testing when the temperature was <32°C. Clinical information for each participant was withheld from all examiners. If compound muscle action potential or sensory nerve action potential was undetectable, no value of conduction velocity or amplitude was included in the statistical analyses.
The tibial compound muscle action potential (CMAP) and SNAP of the sural nerves were measured with peak-to-peak amplitudes. Well-trained technicians evaluated each participant with the EMGS followed by DPNCheck™.
To determine abnormalities of NCS parameters in the EMGS, cut-off values were used as follows: <40 m/s of tibial motor nerve conduction velocity, <5 mV of tibial CMAP, >(12.8 + 0.22 × height [cm]) ms of tibial minimal F-wave latency, <40 m/s of sural SNCV and <5 µV of sural SNAP amplitude. A nerve, whose parameters in NCS showed one or more abnormal values, was included in the number of nerves with abnormal value(s) (NNAV).
During interpretation, NCS data using the EMGS were categorized from stage 0 to stage 4 based on the Baba classification on the severity of DPN17. In brief, participants were divided into five stages: stage 0, normal without any NCS abnormalities; stage 1, mild neuropathy with the presence of any delay in tibial motor nerve conduction velocity (<40 m/s), sural SNCV (<40 m/s), tibial minimal F-wave latency (>[12.8 + 0.22 × height (cm)] ms) or the presence of A wave; stage 2,: moderate neuropathy with a decrease in sural SNAP amplitude <5 µV; stage 3, between moderate-to-severe neuropathy with a decrease in sural SNAP amplitude <5 µV and a decrease in tibial CMAP amplitude ≥2 to <5 mV; stage 4, severe neuropathy with a decrease in sural SNAP amplitude <5 µV and a decrease in tibial CMAP amplitude <2 mV. For the current study, the item, “presence of A wave”, was discounted because of the low interrater agreement.
Coefficient of variation of RR intervals
The coefficient of variation of RR intervals (CVR-R) was measured based on previously reported methods18. To analyze the CVR-R, electrocardiogram recordings were collected in the supine position with normal or deep breathing for 1 min after 5 min of bed rest. Normal resting electrocardiogram was recorded for 1 min, followed by another 1-min recording during deep breathing at six breaths per minute. The CVR-R was calculated as follows: CVR-R(%) = (standard deviation of RR intervals) / (mean RR intervals) × 100.
Statistical analysis
Lower values of SNAP, CMAP and NCVs, or higher values of minimal F-wave latency in the bilateral nerve responses, were used during data analysis.
SPSS Statistics version 20 for Windows (IBM SPSS, Chicago, IL, USA) was utilized for data analyses. All analyses were carried out by personnel unaware of the participants’ identities. Student’s t-tests and χ2-tests with Yates’ correction were used for analyses of differences in continuous and categorical variables, respectively. For multiple comparisons, Welch’s anova and Games–Howell post-hoc tests were used. Correlations were analyzed using Spearman's correlation coefficients. The sural NCV and SNAP amplitude retrieved by DPNCheck™ were incorporated into the multiple regression models to create an effective prediction model of DPN. Diagnostic validity was analyzed using a receiver operating characteristic (ROC) curve and the area under the ROC curve (AUROC).
Results
Clinical information
The clinical characteristics of the participants are presented in Table 1. In total, 375 individuals were included in the analyses (216 men, 159 women; aged 61.9 ± 14.6 years). A total of 14 individuals whose SNAP in bilateral sural nerves was evoked by neither EMGS nor DPNCheck™ were excluded. The mean duration of diabetes was 11.0 ± 11.9 years. Participants had a mean glycosylated hemoglobin of 9.6 ± 2.1% and a mean body mass index of 25.6 ± 6.1 kg/m2. Based on the Baba classification, 24.5% of the participants (n = 92) were classified as mild DPN (stage 1), and 46.7% of the participants (n = 175) were classified as moderate-to-severe DPN (stage 2–4). Although the mean age and duration of diabetes significantly increased as the DPN stage progressed, glycosylated hemoglobin and body mass index showed no significant change with the progression of DPN stages. Between DPN stages 0 and 1, the parameters of DPN – that is, tibial NCV, F-wave minimal latency, and CMAP and sural NCV using EMGS, and sural NCV and SNAP using DPNCheck™ – showed significant differences, but the parameters of diabetic nephropathy, estimated glomerular filtration rate and urine albumin-to-creatinine ratio showed no significant difference. NCS showed that tibial NCV, minimal F-wave latency, and CMAP, sural NCV and SNAP decreased as the DPN stage progressed. CVR-R, especially deep breathing CVR-R, decreased in the advanced stages of DPN, or stages 2 and 3.
| Stage of DPN | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| n | 108 | 92 | 147 | 21 | 7 |
| Age (years) | 57.0 ± 15.9 | 60.0 ± 13.0 | 65.1 ± 13.9***,† | 71.2 ± 10.0***,†† | 69.4 ± 5.4**,†† |
| Male (%) | 51.9 | 64.1 | 59.2 | 47.6 | 57.1 |
| Duration of diabetes (years) | 7.5 ± 9.8 | 9.4 ± 9.0 | 13.7 ± 11.3***,† | 13.0 ± 11.5 | 22.1 ± 13.6 |
| Height (cm) | 160.7 ± 9.0 | 162.6 ± 9.5 | 161.9 ± 9.5 | 157.7 ± 9.6 | 159.3 ± 12.4 |
| Bodyweight (kg) | 68.4 ± 20.3 | 66.8 ± 16.4 | 65.7 ± 17.6 | 66.1 ± 19.0 | 76.0 ± 20.8 |
| BMI (kg/m2) | 26.5 ± 7.5 | 25.2 ± 5.5 | 24.8 ± 5.1 | 26.4 ± 5.8 | 30.4 ± 6.9 |
| HbA1c (mmol/mL) | 80.3 ± 24.9 | 85.8 ± 23.0 | 79.2 ± 21.9 | 89.1 ± 20.8 | 81.4 ± 20.0 |
| HbA1c (%) | 9.5 ± 2.2 | 10.0 ± 2.1 | 9.4 ± 2.0 | 10.3 ± 1.9 | 9.6 ± 1.8 |
| Glycoalbumin (%) | 24.7 ± 9.4 | 27.8 ± 8.2 | 25.6 ± 6.5 | 30.2 ± 8.0 | 30.2 ± 9.3 |
| CVR-R, resting (%) | 2.9 ± 1.6 | 2.4 ± 1.2 | 2.2 ± 1.3* | 1.9 ± 0.9* | 2.6 ± 1.2 |
| CVR-R, deep breathing (%) | 5.0 ± 2.8 | 4.1 ± 2.0 | 3.5 ± 2.0** | 3.0 ± 1.5* | 3.3 ± 1.4 |
| eGFR (mL/min/1.73 m2) | 86.8 ± 25.3 | 87.0 ± 27.1 | 73.6 ± 30.2**,†† | 70.5 ± 25.5 | 64.3 ± 25.6 |
| uACR (mg/g) | 40.1 ± 130.5 | 32.2 ± 78.8 | 245.6 ± 839.9*,† | 192.7 ± 477.8 | 364.1 ± 647.1 |
| In(uACR) | 2.4 ± 1.5 | 2.4 ± 1.4 | 3.4 ± 1.9***,†† | 3.9 ± 1.5**,†† | 4.1 ± 2.4 |
| Tibial nerve | |||||
| NCV (m/s) | 43.9 ± 2.3 | 40.7 ± 2.9*** | 40.3 ± 3.0*** | 38.2 ± 3.5***,† | 36.4 ± 2.6***,†,§ |
| Amplitude (mV) | 16.7 ± 6.6 | 14.2 ± 5.7* | 11.8 ± 4.3***,†† | 3.5 ± 0.8***,†††,§§§ | 1.1 ± 0.6***,†††,§§§,¶¶¶ |
| F-wave latency (ms) | 45.8 ± 2.9 | 51.0 ± 3.4*** | 50.6 ± 4.9*** | 51.3 ± 4.5*** | 57.6 ± 4.8** |
| Sural nerve (standard electromyography system) | |||||
| NCV (m/s) | 47.9 ± 4.2 | 45.2 ± 4.4*** | 42.9 ± 5.3***,†† | 42.9 ± 6.1** | 39.6 ± 3.6*,† |
| Amplitude (µV) | 10.0 ± 4.0 | 8.6 ± 3.7 | 2.7 ± 1.3***,††† | 1.9 ± 1.5***,††† | 0.8 ± 0.8***,†††,§§ |
| Sural nerve (DPNCheck™) | |||||
| NCV (m/s) | 52.1 ± 4.2 | 48.7 ± 4.6*** | 46.6 ± 5.9***,† | 47.0 ± 5.6** | 46.1 ± 4.1* |
| Amplitude (µV) | 15.2 ± 6.9 | 12.5 ± 5.9* | 6.9 ± 6.9***,††† | 5.7 ± 4.2***,††† | 3.3 ± 1.7***,†††,§§ |
- Categorical variables are given as the number (percentage), whereas continuous variables are reported as mean ± standard deviation. *P < 0.05; **P < 0.01; ***P < 0.001 versus subgroup stage 0. †P < 0.05; ††P < 0.01; †††P < 0.001 versus subgroup stage 1. §P < 0.05; §§P < 0.01; §§§P < 0.001 versus subgroup stage 2. ¶P < 0.05 versus subgroup stage 3. CVR-R, coefficient of variation of R-R intervals; DPN, diabetic polyneuropathy; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; In, natural logarithm; NCV, nerve conduction velocity; uACR, urine albumin-to-creatinine ratio.
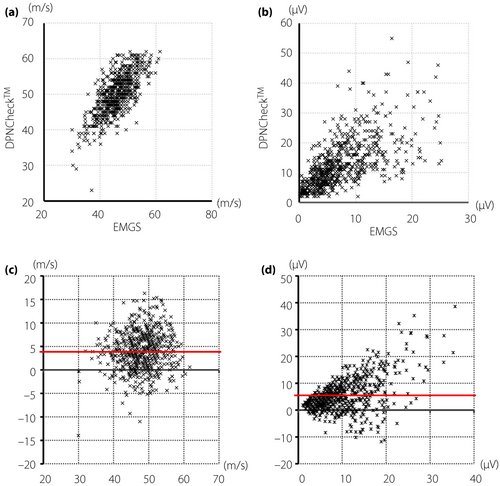
Correlations between the values acquired by a standard EMGS and DPNCheck™
Correlation analysis between the values measured by the standard EMGS and DPNCheck™ showed moderate positive linear relationships both in the SNCV (r = 0.683) and in the amplitude of SNAP (r = 0.687). The comparisons were depicted in scatterplots and Bland–Altman plots (Figure 2). The scatterplots showed good correlations of conduction velocities or amplitudes between the two methods (Figure 2a,b). Although these analyses revealed good correlations between standard EMGS and DPNCheck™ in SNCV and amplitude of SNAP, DPNCheck™ produced higher values than EMGS (mean difference of SNCV: +4.00 m/s, SNAP: +5.50 µV). The Bland–Altman plots evaluated the agreement of values (Figure 2c,d). The difference between the two methods appears to become larger in the range of high values in amplitudes, but not in velocities.
Estimation of the severity of DPN using DPNCheck™ data
To examine whether DPNCheck™ replaces a standard EMGS, which is difficult to implement widely in clinical practice, we evaluated the correspondence of DPNCheck™ data to the stages diagnosed using the Baba classification. Before starting the analysis, as the tibial CMAP was used in diagnosing stages 2–4 of DPN in the Baba classification, the correlation between the CMAP and the results of DPNCheck™ was examined. As a result, the correlation with the CMAP was r = 0.140, P = 0.241 for SNAP, and r = 0.031, P = 0.795 for SNCV. It seemed not to be useful to distinguish between moderate-to-severe stages of DPN using DPNCheck™. Therefore, we reclassified the participants into three groups: stage 0, no DPN (stage 0 in the Baba classification); stage 1, mild DPN (stage 1 in the Baba classification); and stage 2, moderate-to-severe DPN (stages 2–4 in the Baba classification; Table S1). We tentatively named this staging method the modified Baba classification (MBC). In a multiple regression analysis, using the stage numbers of MBC as the dependent variable, and age, amplitude of SNAP in DPNCheck™ and NCV in DPNCheck™ as independent variables, the estimated severity in MBC (eMBC) was obtained as follows: eMBC = 2.046 + 0.509 × ln(age [years]) − 0.033 × (NCV [m/s]) − 0.622 × ln(SNAP amplitude [µV]), r = 0.649 (Figure 3a, Table S2).
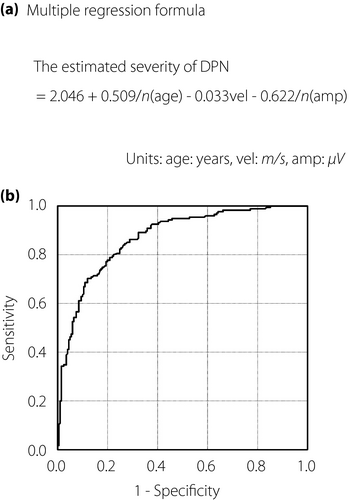
ROC analysis was used to identify the optimal cut-off on the eMBC to categorize participants as having stage 2 versus stage 0 or 1 DPN. The eMBC showed an excellent discriminative power (AUROC 0.871; Figure 3b). The cut-off value with maximum accuracy was 1.3065 of the eMBC (sensitivity 70.1%, specificity 87.7%, positive predictive value 83.0%, negative predictive value 77.3%, positive likelihood ratio 5.67, negative likelihood ratio 0.34; Table 2).
| Definitions of DPN | Stage 2 DPN in MBC (n = 174/375 of participants with diabetes) | DPN defined by abnormal values in two or more NCS parameters (n = 228/375 of participants with diabetes) | |
|---|---|---|---|
| Predicted items | eMBC† | eMBC† | eNNAV‡ |
| AUROC | 0.871 (0.835–0.906) | 0.829 (0.788–0.870) | 0.839 (0.800–0.879) |
| Cut-off value | 1.3065 | 1.0835 | 2.4225 |
| Sensitivity (%) | 70.1 (63.3–76.9) | 76.3 (70.8–81.8) | 73.7 (68.0–79.4) |
| Specificity (%) | 87.6 (82.7–92.5) | 73.0 (67.2–78.7) | 81.1 (76.0–86.2) |
| PPV (%) | 83.0 (77.4–88.6) | 81.3 (76.2–86.4) | 85.7 (81.2–90.3) |
| NPV (%) | 77.3 (71.1–83.5) | 66.7 (60.5–72.8) | 66.7 (60.5–72.8) |
| +LR | 5.67 | 2.82 | 3.89 |
| −LR | 0.34 | 0.32 | 0.32 |
| Accuracy (%) | 79.5 | 75.0 | 76.6 |
- Values in parentheses are 95% confidence intervals.
- AUROC, area under the receiver operating characteristic curve; DPN, diabetic polyneuropathy; eMBC, an estimated severity in the modified Baba classification; eNAV, an estimated number of abnormal values; +LR, positive likelihood ratio; −LR, negative likelihood ratio; MBC, modified Baba classification; NCS, nerve conduction study; NCV, nerve conduction velocity in DPNCheck™; NPV, negative predictive value; PPV, positive predictive value; SNAP, sensory nerve action potential in DPNCheck™.
- † eMBC = 2.046 + 0.509 × ln(age [years]) − 0.033 × (NCV [m/s]) − 0.622 × ln(SNAP amplitude [µV])
- ‡ eNNAV = 12.149 + 0.55 × ln(age [years]) − 0.171 × (NCV [m/s]) − 1.613 × ln(SNAP amplitude [µV]).
Additionally, we determined whether an analysis using the mean values of bilateral sural NCS showed better diagnostic ability compared with the above analysis using lower values. In this analysis using mean values, 22 participants were excluded due to the lack of SNAP response from either sural nerve. Although multiple regression analysis showed a good correlation (r = 0.672) and ROC curve analysis also showed excellent diagnostic ability (AUROC 0.890), there was no significant change in the effectiveness between these two different ways of data processing. Furthermore, we determined whether an analysis using the values of unilateral sural NCS showed inferior diagnostic ability compared with the above analysis using lower values of bilateral NCS. In the analysis using values from a unilateral nerve, multiple regression analysis showed a good correlation (r = 0.647) and ROC analysis also showed excellent diagnostic ability (AUROC 0.871). These results showed that the effectiveness of analysis using unilateral sural NCS was comparable to that using bilateral sural NCS.
eMBC predicted multiple abnormalities in NCS parameters of EMGS
According to the traditional diagnosis of DPN, in which abnormalities of NCS parameters in two or more nerves were used as a diagnostic criterion19, 20, we assessed the correspondence of the eMBC with the diagnostic criterion. In multiple regression analysis using the number of abnormal values in NCS, using the EMGS as a dependent variable, and age, amplitude of SNAP in DPNCheck™ and NCV in DPNCheck™ as independent variables, the estimated NNAV (eNNAV) was obtained as follows: eNNAV = 12.149 + 0.55 × ln(age [years]) − 0.171 × (NCV [m/s]) − 1.613 × ln(SNAP amplitude [µV]), r = 0.694. ROC analysis showed that the optimized cut-off value of eNNAV was 2.4225 to predict the abnormalities in two or more nerves (AUROC 0.839, sensitivity 73.7%, specificity 81.1%, positive predictive value 85.7%, negative predictive value 66.7%, positive likelihood ratio 3.89, negative likelihood ratio 0.32; Table 2). In contrast, ROC analysis using the eMBC to predict multiple abnormalities resulted in a comparable diagnostic performance with an analysis using the eNNAV (AUROC 0.829, cut-off value 1.0835, sensitivity 76.3%, specificity 73.0%, positive predictive value 81.3%, negative predictive value 66.7%, positive likelihood ratio 2.82, negative likelihood ratio 0.32).
Discussion
We examined a cohort of 422 participants with diabetes with or without DPN to assess the prediction ability of a point-of-care nerve conduction device for DPN. The prediction ability of the device was expressed by the estimated severity value eMBC obtained from multiple regression analysis. Combined with ROC analysis, the optimized cut-off value of the eMBC achieved nearly 80% diagnostic accuracy and a positive likelihood ratio >5 for the diagnosis of moderate-to-severe DPN. Furthermore, the eMBC was able to moderately predict the early stage of nerve conduction dysfunction; that is, abnormalities of NCS parameters in two or more nerves. The eNNAV, the regression equation using the traditional quantitative diagnostic approach; that is, one or more nerves shows nerve conduction dysfunctions, had similar diagnostic ability with eMBC in ROC analyses. Many quantitative examinations for assessment of DPN have been suggested, and some of them have been established; for example, NCS using a standard EMGS, intraepidermal nerve fiber density, quantitative sensory tests using specialized equipment, and scoring systems of physical findings and symptoms. As it has been verified that the abnormalities of NCS parameters using a standard EMGS strongly correlate with the pathological changes in the peripheral nervous system, the NCS has been used as the gold standard to verify new examinations. However, none of those aforementioned quantitative examinations is yet employed in clinically important large-scale trials due to the lack of usability, reproducibility or objectivity. In contrast, the DPNCheck™ is usable and objective, and its reproducibility has been verified in several papers11, 13. The current study will strengthen the versatility of the device through its high diagnostic ability, and might help the device to be widely applied to clinical practice.
Although the current study showed a good correlation between DPNCheck™ and a standard EMGS, we should consider three differences between them. First, electric stimuli to the sural nerve are orthodromic in DPNCheck™, but antidromic in a standard EMGS. Although it is known that the SNAP evoked by orthodromic stimulus would be principally smaller than that by antidromic stimulus, in the current study, orthodromic stimuli by DPNCheck™ acquired higher SNAPs compared with those by antidromic stimuli using the standard EMGS. A difference of stimulation intensity might explain the unexpected results; DPNCheck™ uses up to 70 mA of electric stimuli, but the standard EMGS uses up to approximately 20 mA. The stronger stimulus in DPNCheck™ might overcome technical obstacles; for example, leg edema or thickened skin in obese persons. Second, unlike a standard EMGS, <5 µV values of SNAP amplitudes in DPNCheck™ are not certified by the manufacturer. In the present study, among 86 legs showing amplitudes of <5 µV, 80 legs belonged to the participants who were classified into stage 2 DPN of MBC (data not shown). Given the consistency of the low values with higher severity of DPN, although we should consider the limited guarantee of values <5 µV, these low values might be used to predict moderate-to-severe DPN. Third, DPNCheck™ automatically excludes values with <2 µV of SNAP amplitudes. As a result, DPNCheck™ lacked NCS data in 4.7% of legs, which was higher than the ratio in 1.7% of legs using a standard EMGS. In the current cohort, 13 participants lacked bilateral NCS data using DPNCheck™, but data from physical assessments were available for nine of those 13 participants. In all of those nine participants, physical assessments showed one or more abnormalities of vibratory threshold of bilateral medial malleoli or bilateral Achilles tendon reflexes (data not shown); therefore, persons whose SNAP of bilateral sural nerves are absent should be carefully assessed for neurological symptoms and physical signs.
Although the eMBC successfully predicted moderate-to-severe DPN, we should address three issues before applying the item widely to clinical practice. First, it has been pointed out that the distributions of values obtained from DPNCheck™ were different between Westerners and Japanese people21. Therefore, further data need to be accumulated to determine the cut-off value of the eMBC for each ethnicity. Second, the amount of information obtained from DPNCheck™ is smaller than that by a standard EMGS. Although the Baba classification uses information acquired from motor and sensory nerves, DPNCheck™ uses information only from sensory nerves. Given that stage 1 mild DPN is diagnosed depending on abnormalities of motor nerve conduction velocity, F-wave latency and SNCV in the Baba classification, it would be difficult to distinguish mild DPN from no DPN using only sural SNCV. In particular, the elongation of minimal F-wave latency has been verified as an appropriate marker for the early stage of DPN22, 23. Indeed, minimal F-wave latency diagnosed 93.5% of stage 1 DPN cases (86/92) in the present study. In contrast to F-wave latency, a decrease in sural SNCVs detected just 10.9% of participants in stage 1 DPN (10/92). To differentiate mild DPN from no DPN, additional item(s) might be combined with the NCS data of DPNCheck™. For example, CVR-R obtained during resting or deep breathing showed a significant difference between stage 0–1 or 2 DPN in MBC (resting: mean CVR-R: 2.9 ± 1.6% in stage 0, 2.3 ± 1.3% in stage 1 or 2, P = 0.0012 by Student’s t-test; deep breathing: mean CVR-R: 5.0 ± 2.8% in stage 0, 3.7 ± 2.0% in stage 1 or 2, P < 0.001). We will evaluate the usefulness of CVR-R for diagnosis of DPN in future research. Third, eMBC is currently unable to distinguish moderate from moderate-to-severe or severe DPN. As described in the Results, NCS parameters of DPNCheck™ had no significant correlations with progress of the severity in stage 2–4 of DPN in the Baba classification. Therefore, we regrouped the participants with stage 2–4 as stage 2 participants in MBC for the current analysis. However, participants with moderate-to-severe or severe DPN might be exposed to an increased risk of diabetic foot including ulcerations and amputations. We should accumulate further data of participants with developed DPN, and also consider other candidate parameters to better predict developed DPN.
The point-of-care nerve conduction device predicts DPN well, and could provide comprehensive and sequential management of diabetic complications in the future.
Acknowledgments
The authors thank the nurses and staff from the Department of Clinical Laboratory at Aichi Medical University Hospital. The authors are particularly grateful to Carson Maynard (Department of Philosophy, University of Michigan, Ann Arbor, MI, USA) for his editorial assistance.
Disclosure
The authors declare no conflict of interest.




