Feasibility of the MELD score as a screening tool for pharmacists to identify patients with impaired hepatic function at hospital admission
Funding information
This work was supported by Stiftung Patient & Klinische Pharmazie, Munich, Bavaria, Germany.
Abstract
What is known and Objective
Hepatic impairment (HI) is a known risk factor for drug safety. The MELD score (Model-for-endstage-liver-disease), calculated from serum creatinine, bilirubin and International Normalized Ratio (INR), is a promising screening tool corresponding to Child-Pugh Score (CPS) for drug adjustment. We tested the feasibility of MELD as an automatic screening tool accounting for correct calculation, interfering factors (IF) and detection of patients corresponding to CPS-B/C potentially requiring drug adjustment.
Methods
We retrospectively calculated MELD for a 3-month cohort of surgical patients and assessed need for adjustment of MELD parameters to standard values. IF for INR (oral anticoagulants) and serum creatinine (renal insufficiency (RI; eGFR<60 ml/min/1.73m²); as well as drugs elevating creatinine levels (DECL)) and the number of patients with MELD scores corresponding to CPS-B/C were analysed. For MELD ≥7.5, liver and bile diagnoses were recorded.
Results and discussion
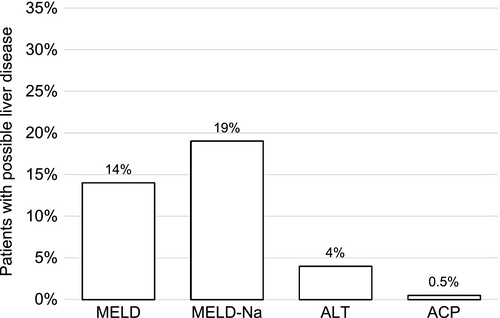
Of 1183 patients, MELD was calculable for 761 (64%; median 7.5, range 6.4–36.8). Parameters had to be adjusted for 690 (91%) patients. IF of parameters were RI in 172 (23%), INR-elevating drugs in 105 (14%) and DECL in 33 (4%) patients. Of 335 (44%) patients with MELD ≥7.5, 122 (36%) had documented liver or bile diagnoses. MELD 10-<15 (corresponding to CPS-B) was found for 105 (14%), MELD ≥15 (corresponding to CPS-C) for 66 (9%) of the 761 patients with a calculated MELD. Referred to all patients, drug adjustments due to possible HI were recommendable for 14% of patients with suspected CPS-B/C.
What is new and Conclusion
MELD is a feasible screening tool for HI as a risk factor for drug safety at hospital admission when appropriately considering correct parameter adjustment and RI and INR-elevating drugs as IF. Further evaluation of sensitivity and specificity is needed.
1 INTRODUCTION
In liver disease, important pathophysiological changes affecting the pharmacokinetics and pharmacodynamics of drugs occur. Drug choice and dosage have to be adjusted to avoid patient harm.1, 2 Hepatic impairment (HI) has been characterized as a risk factor for drug safety, increasing the number of adverse drug reactions (ADR) and hospital admissions.3-6 In hospitalized patients, HI has been reported for 6%–54%, depending on the medical speciality.7-9 Screening for drug safety risk factors is an important task at hospital admission as part of medication reconciliation. However, a validated screening tool for HI in patients admitted to hospital has not yet been established.
The assessment of liver function is complex and cannot rely on one single parameter. In clinical practice, multiple laboratory liver parameters (LLP) are considered. However, this approach has been questioned, since LLP are not necessarily elevated in all kinds of liver diseases, can be altered for extrahepatic reasons and do not, in fact, reflect liver function and severity of HI.10 In particular, it has been proposed to test for elevated alanine aminotransferase (ALT) as an index parameter to identify patients with liver disease.11, 12
Scores developed to determine the severity of liver disease are another screening tool discussed. The Child-Pugh Score (CPS) categorizes patients into classes A, B and C based on clinical symptoms (ascites and hepatic encephalopathy) and the laboratory parameters bilirubin, albumin and International Normalized Ratio (INR).13 Most recommendations for drug adjustment to liver function refer to CPS, since the Food and Drug Administration and the European Medicines Agency both recommend it.14, 15 The inclusion of clinical symptoms, to be assessed repeatedly by a physician, is a key limitation because judgement is subjective and precludes use of CPS as an automated screening tool.16-19 An analytical CPS (ACP), calculated only from the laboratory parameters, has been investigated for screening by pharmacists.20 However, the number of patients identified with possible HI was very low (0.34%) compared to a prevalence of at least 6–7% for all hospitalized patients, suggesting a low sensitivity.7-9, 20
Another important score, the Model-for-endstage-liver-disease (MELD), is calculated from the laboratory parameters serum creatinine (SCrea), bilirubin and INR. MELD was developed to determine mortality after implementation of transjugular intrahepatic portosystemic shunts.19 Of interest, MELD ranges have been correlated to CPS classes,16, 17 thereby linking CPS-based drug adjustment to MELD calculation. Several MELD variations have been developed. The more sensitive MELD-Na score includes serum sodium as a marker of hyponatremia reflecting severity of cirrhosis and is used to allocate organs for liver transplantation.19, 21 For patients taking vitamin-K antagonists (VKA), elevating INR, MELD-Xi is available excluding INR from calculation.22
Although MELD was not meant for use in patients without or with only mild hepatic disease, MELD and MELD variations have been assessed as general predicting tools, for example for perioperative risks, postoperative complications, length of hospital stay and mortality.16, 18, 23-25 When discussing MELD as a screening tool for HI, interfering factors (IF) of MELD parameters have to be considered. SCrea can be altered by many factors, most importantly renal insufficiency (RI). Also, drugs elevating creatinine levels (DECL), for example due to inhibition of renal creatinine transporters, represent IF.26 Dialysis will decrease SCrea, thus, for MELD calculation the level is corrected to 4 mg/dl.19 INR is elevated by VKA or direct-acting oral anticoagulants (DOAC).27 Many factors like diuretics or intravenous fluids will alter serum sodium.19, 28 Bilirubin can be elevated due to Gilbert-Meulengracht syndrome affecting 4–16% of the population.29 Previous studies regarding the use of MELD or MELD variations as a prognostic or screening tool frequently neglected IF.18, 23-25, 30, 31 Moreover, to avoid negative values of MELD, parameters have to be set to standard levels when they are outside defined ranges.19, 32 No data exist investigating how often these corrections are necessary.
As there is currently no established tool for screening for patients with HI at hospital admission, we aimed to examine the feasibility of MELD for automatic HI screening and associated drug-related problems. To this end, we retrospectively evaluated surgical patients at hospital admission and identified patients with MELD scores corresponding to CPS-B and C, for whom drug adjustment to liver function might be requested. In order to assess feasibility, we considered (1) availability of MELD parameters at hospital admission, (2) the necessity to correctly use standard values for calculation, (3) the importance of IF of MELD parameters, (4) the distribution of MELD scores reflecting the percentage of patients with CPS-B/C potentially needing drug adjustment to liver function, and (5) compared results to MELD-Na, MELD-Xi, elevated ALT and ACP as alternative screening tools.
2 METHODS
2.1 Study design
We retrospectively evaluated patients aged over 18 years who were admitted to the surgical department of a large teaching hospital in Bavaria, Germany, January to March 2019 and had received pharmacist-led medication reconciliation (PhMR). PhMR is routinely performed for all admitted patients Monday to Friday assessing a detailed drug history and generating a medication list including all prescribed and over-the-counter drugs taken. Clinical data were extracted from the electronic patient information system (SAP-i.s.h.med, Cerner Corporation, North Kansas City, USA). Laboratory data from the day of admission were documented for SCrea, bilirubin, INR, sodium, ALT and albumin. Information on drugs was taken from the medication list of PhMR. The study was conducted in accordance with the Declaration of Helsinki. Approval was obtained by the ethics committee of the University Hospital Munich (17–499).
2.2 Calculation of MELD and hepatic diagnoses
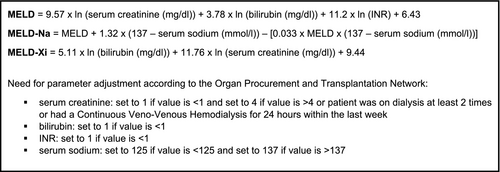
For all patients, MELD, MELD-Na, and MELD-Xi were calculated (Figure 1). The minimum MELD achievable is six when appropriately adjusting parameters.19 We set MELD ≥7.5 as potentially indicating liver disease since this will exclude all cases with maximal normal values of the parameters. Adjustment of drug therapy is mainly necessary for CPS classes B and C, corresponding to MELD 10-<15 and ≥15 respectively.17 Therefore, patients were grouped into the following MELD ranges: 6 to <7.5 (normal); 7.5 to <10 (elevated); and 10 to <15 (CPS-B); ≥15 (CPS-C). For patients with a MELD ≥7.5, electronic records were searched for diagnoses and symptoms suggesting liver/bile disease.
2.3 Assessment of interfering factors of MELD parameters
For patients with calculated MELD score, IF of MELD parameters were assessed. For SCrea, chronic kidney disease (CKD) and intake of DECL were considered. Patients were classified into CKD stages (Kidney Disease Improving Global Outcome Initiative) based on the estimated glomerular filtration rate (eGFR) calculated by the CKD-EPI formula.33 Renal impairment (RI) was defined as eGFR <60 ml/min/1.73 m². Electronic records were searched for dialysis. DECL included tyrosine kinase inhibitors (TKI; imatinib, bosutinib, sorafenib, sunitinib, crizotinib, gefitinib and pazopanib), poly-ADP-ribose-polymerase inhibitors olaparib and rucaparib, cobicistat, dronedarone, amiodarone, cimetidine, trimethoprime, pyrimethamine, rilpivirine, dolutegravir and fibrates (fenofibrate, bezafibrate).26, 34-37 For INR, intake of VKA and DOAC was considered as IF. Electronic charts were searched for Gilbert-Meulengracht syndrome as an IF for bilirubin. Since serum sodium is affected by a broad variety of factors, no IF were considered because of the large number of possible interferences.19, 28
2.4 Alternative screening tools ALT and ACP
ALT and ACP were assessed as alternative screening tools. For ALT, a threefold elevation of the upper limit of normal (ULN) was rated as clinically relevant in accordance with local hepatologists and literature recommendations.38, 39 ACP was calculated for all patients with albumin, bilirubin and INR values available from the day of admission. ACP ≥7 was defined as indicating potential HI.20
2.5 Statistical analyses
Categorical variables were expressed with their frequency distribution and continuous variables as median and range. For comparison of MELD scores concerning intake of DECL, VKA or DOAC, Mann-Whitney U test was applied for independent samples (continuous variables, nonnormal distribution). Friedman test and following post hoc test (Bonferroni) were applied for comparison of the MELD variants (continuous variables, nonnormal distribution and dependent samples). Kruskal-Wallis test with subsequent post hoc test (Bonferroni) was used to compare eGFR within predefined MELD ranges (continuous variables, nonnormal distribution and independent samples). Cohen's kappa was calculated to determine interrater reliability between MELD/MELD-Na, MELD/ALT, and MELD/ACP and interpreted according to Landis&Koch.40, 41 Statistical significance was accepted as p < 0.05. Data documentation and statistical analysis were performed with Microsoft Excel® 2016 (Seattle, WA, USA) and IBM SPSS Statistics® version 25.0 (Armonk).
3 RESULTS
3.1 Calculation of MELD and MELD-Na for surgical patients at hospital admission
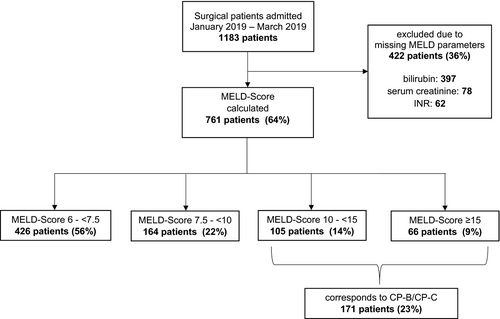
Of 1183 patients, 817 (69%) were male and the median age was 63 years (18–99). For 761 patients (64%), all MELD parameters at admission were electronically available (Figure 2). The most often missing parameter was bilirubin (397, 34%). The median MELD for the 761 patients was 7.5 (6.4–36.8) and 335 (44%) presented with MELD ≥7.5, potentially indicating liver disease. Figure 2 and Table 1 show details on distribution in MELD ranges and patient characteristics. When setting MELD scores to corresponding CPS classes, for 171 (23%) patients assessment of drug therapy regarding liver function would be necessary.
| Categories | Overall | MELD 6-< 7.5 | MELD 7.5-< 10 | MELD 10-<15 | MELD ≥15 |
|---|---|---|---|---|---|
| No. of patients | 761 | 426 | 164 | 105 | 66 |
| Male (n, %) | 536 (70%) | 262 (62%) | 135 (82%) | 83 (79%) | 56 (85%) |
| Age [years] | 64 (18–98) | 62 (18–98) | 66 (20–91) | 72 (25–90) | 63 (21–92) |
| Hepatic/biliary diagnoses and symptomsa | n.a.b | n.a.b | n (%) | n (%) | n (%) |
| Patients with at least one diagnosis | – | – | 63 (38%) | 40 (38%) | 19 (29%) |
| Diseases of the gall bladder or bile duct | – | – | 36 (22%) | 40 (38%) | 25 (38%) |
| Neoplasm of the liver | – | – | 36 (22%) | 18 (17%) | 2 (3%) |
| Liver cirrhosis | – | – | 15 (9%) | 7 (7%) | 8 (12%) |
| Hepatitides | – | – | 14 (9%) | 7 (7%) | 7 (11%) |
| Liver resection | – | – | 11 (7%) | 7 (7%) | 0 |
| Post liver transplantation | – | – | 6 (4%) | 2 (2%) | 1 (2%) |
| Oesophageal varices | – | – | 4 (2%) | 6 (6%) | 5 (8%) |
| Portal vein thrombosis | – | – | 2 (1%) | 0 | 3 (5%) |
| Fatty liver | – | – | 2 (1%) | 4 (4%) | 0 |
| Hepatic encephalopathy | – | – | 2 (1%) | 1 (1%) | 0 |
| Ascites | – | – | 0 | 0 | 5 (8%) |
| Portal hypertensive gastropathy | – | – | 0 | 3 (3%) | 2 (3%) |
| Otherc | – | – | 4 (2%) | 3 (3%) | 8 (12%) |
| ALT 3 times > ULNd (n, %) | 42 (6%) | 19 (4%) | 12 (7%) | 8 (8%) | 3 (5%) |
| Renal function (classified by KDIGO)e | n (%) | n (%) | n (%)f | n (%)f | n (%) |
| G1 | 345 (45%) | 279 (65%) | 51 (31%) | 12 (11%) | 3 (5%) |
| G2 | 242 (32%) | 135 (32%) | 79 (48%) | 25 (24%) | 3 (5%) |
| G3a | 76 (10%) | 12 (3%) | 30 (18%) | 29 (28%) | 5 (7%) |
| G3b | 42 (6%) | 0 | 3 (2%) | 32 (30%) | 7 (11%) |
| G4 | 22 (3%) | 0 | 0 | 6 (6%) | 16 (24%) |
| G5 | 32 (4%) | 0 | 0 | 0 | 32 (48%) |
| Interfering factors | n (%) | n (%) | n (%) | n (%) | n (%) |
| Renal insufficiency (≤ G3a)* | 172 (23%) | 12 (3%) | 33 (20%) | 67 (64%) | 60 (91%) |
| DECLg,* | 33 (4%) | 10 (2%) | 5 (3%) | 11 (10%) | 7 (11%) |
| Drugs elevating INRh,* | 105 (14%) | 28 (7%) | 33 (20%) | 27 (26%) | 17 (26%) |
- aonly assessed for patients with MELD ≥ 7.5 at admission; more than one diagnosis per patient possible.
- bn.a.: not applicable.
- cother diagnoses/symptoms were: Echinococcus multilocularis, liver fibrosis, hepatic cavernoma, portal hypertension, liver cyst, hepatopathy, icterus, liver failure, portal vein closure, hepatorenal syndrome, hepatomegaly, liver atrophy.
- dULN: upper limit of normal.
- erenal function (classified by KDIGO), eGFR: estimated glomerular filtration rate.
- G1: eGFR ≥ 90 ml/min/1.73m²
- G2: eGFR 60-89 ml/min/1.73m²
- G3a: eGFR 45-59 ml/min/1.73m²
- G3b: eGFR 30-44 ml/min/1.73m²
- G4: eGFR 15-29 ml/min/1.73m²
- G5: eGFR < 15 ml/min/1.73m²
- feGFR missing for one patient.
- gDECL: drugs elevating creatinine levels.
- hINR: International Normalized Ratio.
- *Presence of all interfering factors significantly elevated MELD (RI p < 0.001; DECL p < 0.001; drugs elevating INR p < 0.001).
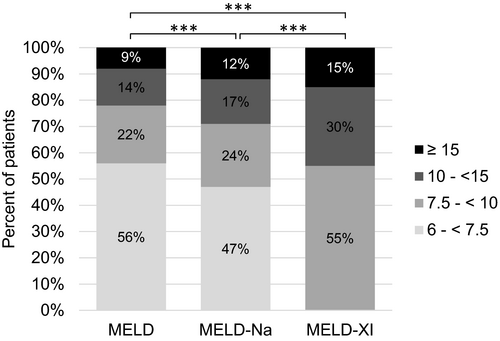
MELD-Na was calculable for 757 (64%) patients. Compared to MELD, this achieved slightly higher results with a median score of 7.9 (6.4–36.8). Classification in the same ranges as MELD and corresponding CPS classes leads to 220 (29%) of the 757 patients with calculated MELD-Na with CPS-B or C and potentially requiring drug adjustment to liver function (Figure 3).
3.2 Adjustment of MELD parameters
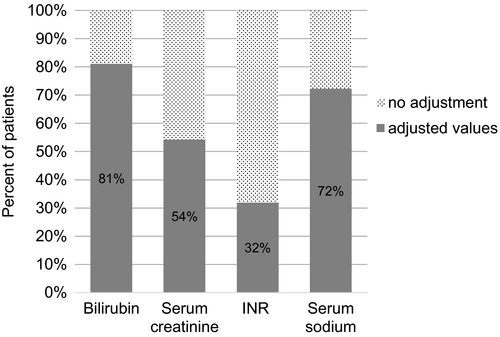
To characterize the feasibility of MELD as an automatic screening tool, we determined how often MELD parameters had to be set to standard values. Of 761 patients with calculated MELD, one or more parameter had to be adjusted for 690 (91%). Bilirubin, INR and SCrea were <1 and had to be set to one in 617, 242 and 382 cases respectively. For 32 patients (4%), adjustment of SCrea to 4 mg/dl was necessary (30 cases >4 mg/dl, two haemodialysis). For MELD-Na, sodium levels had to be adjusted for 548 patients (545 >137 mmol/l, three <125 mmol/l) (Figure 4).
3.3 Hepatic diagnoses in patients with MELD ≥7.5 at hospital admission
Of 335 patients with MELD ≥7.5, liver/bile diagnoses or symptoms were found for 122 (36%; Table 1). Of note, for 65 patients with MELD 10-<15 (corresponding to CPS-B) and 47 with MELD ≥15 (corresponding to CPS-C), no documentation of liver or biliary tract disease was found.
3.4 Interfering factors of MELD parameters
Renal impairment was present in 160 (48%) of the 335 patients with MELD ≥7.5 The number of patients with RI as IF increases in parallel to MELD scores (Figure 5, Table 1).
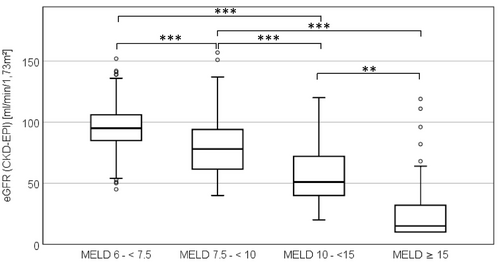
DECL were taken by 23 (7%) of the 335 patients with MELD ≥7.5. These patients had significant higher MELD scores (p = 0.01), and 17 (74%) had an eGFR <60 ml/min/1.73m² reflecting true RI and/or drug effect on creatinine excretion. Of the ten drugs studied, trimethoprim (frequently as pneumocystic-carinii-pneumonia prophylaxis) occurred 13, amiodarone four, dolutegravir three, fibrates two times and TKI once.
Of 1183 study patients, 154 (13%) took INR-elevating drugs (122 DOAC, 32 VKA) and 40 (26%) of these had an elevated INR at admission. For 105 (68%) patients, MELD-Xi parameters were available with a median score of 11.5 (9.4–25.8). In comparison, the median MELD score was 9.4 (6.4–36.8) and median MELD-Na score 10.5 (6.4–36.8) in this subgroup.
Also, when calculating MELD-Xi for all patients, scores were significantly higher (median 9.4 (9.4–32.7); p<0,001) and more patients in higher MELD ranges compared to MELD or MELD-Na (Figure 3). Of 335 patients with MELD ≥7.5, 77 patients (23%) took INR-elevating drugs (DOAC 56 (17%), VKA 21 (6%)) and had significant higher MELD scores (p = 0.03). No documentation of Gilbert-Meulengracht syndrome as IF of bilirubin was found.
3.5 Comparison of MELD, ALT and ACP as screening tools
ALT was available for 782 (66%) of the 1183 patients and clinically relevant elevation present in 43 (4%). Of these, 23 (53%) had a MELD Score ≥7.5 and a liver or bile diagnosis documented for 19 (83%).
ACP was calculable for 638 (54%) patients, the most often missing parameter was albumin (522, 44%). The median ACP was 3 (3–9). Of six patients (0.9%) with an ACP ≥7, indicating possible liver disease, all had a MELD score ≥7.5 (median 19.2 (13.7–31.2)). Three patients had documented liver or bile diagnoses/symptoms.
Figure 6 compares the percentage of patients identified by the different screening tools who potentially require drug adjustment to liver function. The interrater reliability was almost perfect (κ = 0.83, p < 0.001) for MELD/MELD-Na, but only slight agreement (κ < 0.2) was found for MELD/ACP or MELD/ALT (p < 0.001; p = 0.5 respectively).
4 DISCUSSION
4.1 Summary of findings
To our knowledge, this is the first study systematically evaluating the feasibility of MELD as an automatic screening tool for HI at hospital admission and determining conditions for its use in routine care for drug adjustment. We found (1) MELD parameters were available and MELD calculable for 64% of surgical patients at hospital admission, but, (2) adjustment of MELD parameters to standard values for correct calculation was necessary in over 90%. Further (3), renal insufficiency and intake of INR-elevating drugs were prominent interfering factors for MELD parameters. As clinical consequence (4), for 23% of the patients MELD scores corresponding to CPS-B/C potentially needing drug adjustment to liver function were found. Finally (5), the alternative screening tools ACP and ALT will most likely underestimate HI as risk factor for drug safety, while MELD-Na and MELD-Xi are not suitable because of unknown clinical interpretation of their results.
4.2 Parameter adjustment and interfering factors
Appropriate adjustment of MELD parameters to standard values is necessary to avoid falsified scores.19 Here, adjustment was needed for an unexpected high number (91%) of patients. This is an important finding, since previous studies recommending MELD as a prediction tool did not stress this point.18, 23, 25, 30
Moreover, attention to IF for SCrea and INR is important. Most prominently, RI has to be considered as it concerns about 20% of hospitalized patients.42-44 In automatic calculation, patients with RI will display in higher MELD ranges, and, therefore, renal diagnoses have to be checked. SCrea is not ideal to determine renal function in patients with HI. Cystatin-C was investigated as a more reliable parameter.45, 46 We suggest to first check for renal diagnoses and, if needed, consider cystatin-C measurement when MELD screening identified a patient for further evaluation.
In addition, intake of INR-elevating drugs was identified to be a relevant IF. For patients on DOAC or VKA, MELD-Xi could be considered but resulted in markedly higher scores compared to MELD or MELD-Na. Currently, it is unknown, how these findings should be interpreted for HI screening and, subsequently, for drug adjustment to liver function. Thus, at the moment we consider MELD-Xi as not suitable for screening for patients with HI.
For Gilbert-Meulengracht syndrome as possible IF, no documented diagnosis was found, but undiagnosed cases might have occurred and could be revealed by automatic MELD screening. DECL were only taken by 4% of the study patients. Effects of these drugs on SCrea levels will be prominent at start of therapy, and the absolute impact remains unclear.26 Thus, we consider intake of DECL as neglectable for MELD screening.
4.3 Clinical interpretation of MELD screening
The sensitivity and specificity of MELD as a screening tool for HI remain to be determined. This task is challenging since data on the prevalence of HI in hospitalized patients are still sparse and chronic liver disease is judged to be heavily underestimated. It often develops silently, and up to 50% of cirrhosis cases are discovered by chance, for example at hospital admission.11, 47, 48 Autopsy studies indicate that the prevalence of cirrhosis in the general population ranges from 4.5% to 9.5%.49 The need for drug adjustment will start with fibrotic rebuilding of the liver and be necessary in cirrhosis.50 Inappropriate adjustment of drug therapy to liver function has repeatedly been reported in hospitalized patients with HI.3, 20 We found contraindicated drugs and inappropriate dosage adjustment in 2% and 3% of surgical patients admitted to hospital respectively.51
In this study, in patients with MELD ≥7.5 bile or liver diagnoses were documented for 36% which corresponds to 10% referred to all patients. However, the real number might be higher since we did only search for the diagnoses in this subgroup. In addition, we suspect incomplete documentation and/or unknown liver disease to be of importance. Despite doubts concerning the use of CPS for drug adjustment to liver function, this is still the standard.2, 14, 15 The possibility to set MELD ranges to corresponding CPS classes is an advantage for its use as screening tool. In our patient cohort, 23% of those with calculable MELD achieved scores corresponding to CPS classes B and C. Referred to all admitted patients, this concerned 14% who would appear for further drug evaluation by a pharmacist when implementing automatic MELD screening. While MELD-Na identified more patients when using the same correlation of MELD ranges to CPS, this correlation has not been proofed yet.
4.4 Comparison to alternative screening tools
There was low agreement in identification of patients with possible HI and need of drug adjustment between screening by MELD and the alternative tools ACP and ALT. Sensitivity of ACP seems to be low. ALT has been correlated with hepatic inflammation, but not the stage of fibrosis and importance of even slightly elevated values is increasingly discussed.11 In our study, 103 patients with documented bile or liver diagnoses and MELD ≥7.5 would have been missed by ALT screening. However, approximately 20% of all patients have elevated LLP without known liver disease, but only about 1% of those develop a hepatic disease within the next few years.52 In a previous study, we found clinically relevant elevated LLP in 11% of surgical patients at hospital admission.51
4.5 Limitations and future research
Limitations are the retrospective design, incomplete documentation of liver/bile diagnoses and unavailability of laboratory parameters. Especially, availability of bilirubin was a limiting factor, since routine measurement is not requested for preoperative evaluation so far.53 No clinical outcome has been considered in this study, since the focus was on the evaluation of MELD as a screening tool and assessment of IF. Prospective studies are planned to clarify the reliability of MELD screening to identify HI patients and the subsequent need to adjust drug therapy to liver function.
5 CONCLUSION
In summary, MELD can be used as a screening tool for HI as risk of drug safety at hospital admission when appropriately considering correct parameter adjustment for calculation and RI and INR-elevating drugs as interfering factors. More data are needed to evaluate the sensitivity and specificity to identify patients in need of medication adjustment. Since MELD scores can be related to corresponding CPS classes for drug adjustment purposes, use of MELD can be an important tool to increase drug safety.
ACKNOWLEDGEMENT
This work was supported by the interprofessional Doctoral Program Clinical Pharmacy, Ludwig-Maximilians-University Munich, Germany and contains parts of the doctoral thesis of Kathrin Golla. Open access funding enabled and organized by ProjektDEAL.
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
PATIENT CONSENT
Because of the retrospective design, patient informed consent was not requested and not obtained.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.




