Comparison of transseptal puncture using a dedicated RF wire versus a mechanical needle with and without electrification in an animal model
Disclosures: None.
Abstract
Introduction
Mechanical force to achieve transseptal puncture (TSP) using a standard needle may lead to overshooting and injury, and can potentially be avoided using a radiofrequency (RF)-powered needle or wire. Applying electrocautery to needles and guidewires as an alternative to purpose-built RF systems has been associated with safety risks, such as tissue coring and thermal damage. The commercially available AcQCross needle-dilator system (Medtronic) features a sharp open-ended needle for mechanical puncture, as well as a built-in connector to enable energy delivery for RF puncture. This investigation compares the safety and efficacy of the AcQCross needle to the dedicated VersaCross RF wire system and generator (Baylis Medical/Boston Scientific).
Methods
In an ex vivo porcine model, VersaCross wire punctures were performed using 1 s, constant mode (approx. 10 W) with maximum two attempts. AcQCross punctures were performed by applying energy for 2 s using a standard electrosurgical generator at 10 W (max. five attempts), 20 W (max. two attempts), and 30 W (max. two attempts). Efficacy was assessed in terms of puncture success and a number of energy applications required for TSP. Safety was assessed quantitatively as force required for TSP, energy required to puncture, and incidence of tissue coring, as well as by qualitative assessment of puncture sites. Additional qualitative observation of tissue cores and debris were obtained from TSP performed in live swine.
Results
RF TSP was 100% successful using the VersaCross wire with 1.0 ± 0.0 attempts. When power was used with the AcQCross needle, it failed to puncture at low (10 and 20 W) power settings; TSP was achieved with 30 W of energy with 91% success using 1.53 ± 0.51 attempts (p < .05 vs. VC) with greater variability (F1,33 = 9223.5, p < .0001). Compared to RF puncture using the VersaCross system, mechanical puncture, alone, using the AcQcross needle required six times more force (8 mm additional forward device displacement) to perforate the septum. Qualitative assessment of puncture sites revealed larger defects and more tissue charring with the AcQCross needle at 30 W compared to punctures with VersaCross wire. Tissue coring with the open-ended AcQCross needle was observed in vivo and measured to occur in 57% of punctures using the ex vivo model; no coring was observed with the closed-tip VersaCross wire.
Conclusions
The AcQCross needle frequently required higher energy of 30 W to achieve RF TSP and was associated with tissue coring and charring, which have been, previously, reported when electrifying a standard open-ended mechanical needle or guidewire. These findings may limit safety and effectiveness compared to the VersaCross system.
1 INTRODUCTION
Standard approaches to perform transseptal puncture (TSP) for left atrial (LA) procedures involve either mechanical puncture or radiofrequency (RF) energy-assisted puncture.1 Mechanical needles have been associated with greater risk of tissue perforation and cardiac tamponade, and can be ineffective for septa with challenging anatomies (i.e., fibrotic, aneurysmal, prior transseptal cannulation).2-4 In contrast, dedicated RF TSP platforms, such as the NRG transseptal needle and VersaCross RF transseptal solution (Baylis Medical/Boston Scientific), have been shown to improve procedural efficacy, safety, and efficiency compared to the conventional mechanical needle.3, 5, 6 Ex vivo studies have shown that attempts to perform RF TSP by applying electrocautery to a mechanical transseptal needle or guidewire can result in reduced efficacy and safety, with increased risk for tissue coring and excessive thermal damage.7, 8
The mechanisms underlying RF energy delivery directly influence the pattern of tissue heating, which can affect overall TSP characteristics and patient outcomes.1 Standard electrosurgical energy delivery results in slow tissue heating, which can be similar to ablation; however, RF energy waveforms optimized for tissue perforation ensure rapid local temperature rises with minimal collateral damage and similar levels of tissue healing to nonthermal, mechanical puncture.9 Application of energy to standard metal needles and guidewires, which lack a dedicated electrode or optimized current density, results in more power required, more extensive areas of tissue damage, and variability in outcomes related to TSP safety and efficacy.8-10
A new commercially available transseptal needle-dilator system (AcQCross Transseptal Access System, Medtronic) features a sharp open-ended needle for mechanical puncture, as well as an electrosurgical connector for RF TSP. This system does not, however, have a defined electrode tip or dedicated RF puncture generator to optimize energy delivery for TSP. This study aims to assess the safety and efficacy of applying electrocautery to the AcQCross needle for TSP in comparison to the use of the VersaCross RF wire and its dedicated puncture generator.
2 METHODS
2.1 Ethics approval
Animal testing was performed under the supervision of veterinary staff and in compliance with institutional protocols at the Research and Technology Center (Boston Scientific). Bench testing was performed on ex vivo tissues acquired from a third-party supplier (Sofina Foods).
2.2 Features of transseptal devices studied
The VersaCross RF transseptal solution (VC) is comprised of a 0.035-inch RF wire with an atraumatic RF electrode at the distal pigtail or J-tip curl, and a dedicated shapeable dilator (Figure 1A). The VersaCross wire can be used as an initial guidewire, TSP device, and as an exchange wire to deliver therapies into the LA. RF energy is delivered using a dedicated RF generator (RFP-100A, Baylis Medical/Boston Scientific). As per the manufacturer's instructions for use (IFU), septal punctures were attempted up to two times for VC (1 s constant).

The AcQCross transseptal access system (AQ) consists of a dilator with an integrated deployable metal needle that has a sharp, open-ended tip (0.032-inch inner diameter; Figure 1B). A separate 0.032-inch guidewire is required for initial access and for device exchange into the LA. TSP can be achieved using mechanical force or by applying energy using an electrocautery pen (Bovie pen, Aspen Surgical) and a standard electrosurgical generator (ValleyLab Force FXc Electrosurgical Generator; Medtronic). Energy is applied manually for approx. 2 s using 10 W in cut mode up to five times, per manufacturer IFU recommendations; however, if this fails, generator power is, generally, increased in 10 W increments (20 and 30 W).
2.3 Ex vivo septal puncture
Ex vivo experimental set-up is outlined in Figure 2. Interatrial septa were freshly excised from swine (approximate animal weight of 100 kg) and immersed in saline at room temperature until experiments were performed within 10 h of animal sacrifice. Porcine septa were fixed onto a custom cork ring (inner diameter: 3.5 cm, outer diameter: 7 cm). The buoyancy of the cork sample holder in solution normalizes the manually applied tenting force to ensure consistency between punctures using both setups. An Instron mechanical tester (Instron) was also used as an alternative method to advance the assembly and tent the septum. Samples were placed in 100% egg white to mimic the electrical conductivity of human blood.11, 12 Transseptal devices were flushed with saline before each puncture. Multiple punctures were performed using both devices on the same excised septum to control for tissue variability. An oscilloscope (MDO4000C, Tektronix) was used to measure voltage and current during energy application for TSP with both transseptal devices and, subsequently, used to calculate the cumulative energy delivered during RF application. After successful punctures (i.e., if the wire or needle tip passed through the septum), transseptal devices were inspected for tissue coring. The closed tip of the VersaCross wire was visually inspected and the VersaCross dilator was flushed with saline to observe any debris. In the case of the open-ended AcQCross needle, the lumen was flushed with saline and a guidewire was passed through the needle to release any tissue debris. Using the Instron machine, mechanical force, and needle displacement were also compared between VersaCross RF puncture and mechanical puncture alone (i.e., no RF) with AcQCross. Individual particulates were fixed in 10% formalin and stained using Masson's trichrome to differentiate between tissue cores and coagulated egg from the solution; staining and analyses were performed by blinded board-certified veterinary pathologists (Pathology Core at The Center for Phenogenomics, University Health Network, Toronto, Canada) for presence of cardiomyocytes and connective tissues. Septal puncture sites were visually inspected using an optical microscope (VHX-5000, Keyence) for tissue charring.
2.4 In vivo septal puncture
TSP was performed in a live swine, as per institutional protocol. The femoral vein was used to access the right atrium to perform TSP under intracardiac echocardiography and fluoroscopy guidance. Heparin was administered to maintain activated clotting time (ACT) of >300 s. AcQCross device was positioned on interatrial septum and TSP was achieved using 30 W. The device was retracted and the presence of particulates at the needle tip was assessed by inserting a guidewire through the device lumen and flushing it with saline into a petri dish. The animal was euthanized at the end of experiments.
2.5 Endpoints
The efficacy endpoints were defined as TSP success and number of energy applications to perforate the atrial septum (Figure 2). The safety endpoints were assessed in terms of the percentage of punctures resulting in tissue cores, applied force and forward displacement, and qualitative assessment of puncture size and tissue charring.
2.6 Statistical analysis
Success rate and tissue coring were compared using the χ2 test. The Shapiro–Wilk test was used to assess normality of data sets. Number of energy applications, applied force, and forward displacement were compared using a paired t-test (normally distributed data) or Wilcoxon rank-sum test (non-normally distributed data). Variance in RF puncture applications was compared using Levene's test. A probability value of p < .05 was used as a threshold for statistical significance.
3 RESULTS
3.1 Puncture efficacy
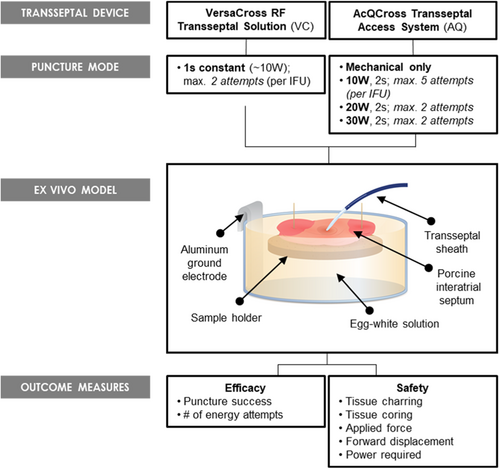
An ex vivo bench model for TSP was used to further interrogate the transseptal devices. Septal puncture of the fossa with VC was achieved in 100% of samples, requiring 1.0 ± 0.0 attempts. All AQ attempts failed to achieve TSP of the fossa at 10 W (AQ-10W) and achieved 5.9% puncture success at 20 W (AQ-20W), after maximum number of attempts. At 30 W (AQ-30W), TSP was achieved with 91% success (Figure 3A). Furthermore, TSP with AQ-30W required an average of 1.53 ± 0.51 attempts (VC vs. AQ, p < .05; Figure 3B), increasing variability in number of RF applications to achieve a successful TSP compared to VC (F1,33 = 9223.5, p < .0001).
3.2 Tissue assessment
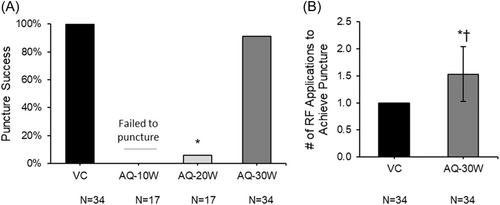
Qualitative visual inspection revealed larger septal defects for punctures with AQ at 30 W (AQ-30W) and exhibited larger areas of tissue charring compared to punctures with VC (Figure 4A). Small indentation of the septum was noted where AQ failed to puncture at 10 and 20 W (AQ-10W and AQ-20W).
3.3 Tissue coring
No tissue cores were observed with punctures made by the closed-tipped VC wire (Figure 4B). Coring of the septum was observed in 57% of successful AQ punctures at 30 W; no cores were observed at 10 or 20 W, as the device failed to puncture at these power settings. Masson's trichrome staining was used to delineate between cored tissues and other artifacts or debris (Figure 4D). The resulting tissue cores ranged in length between 0.5 and 3.2 mm (mean: 1.82 ± 0.88 mm; Figure 4C).
3.4 Energy delivery
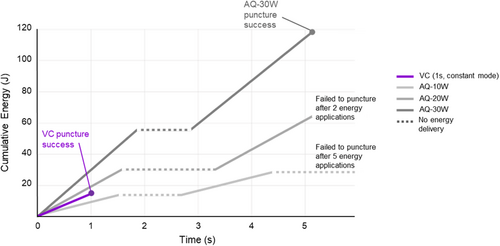
Oscilloscope measurements during energy application for tissue puncture demonstrated higher cumulative energy usage at all generator settings applied to AQ compared to the VC system and RF generator (Figure 5). During VC puncture, energy accumulates throughout 1 s constant RF delivery, during which puncture is achieved. In contrast, with AQ puncture, energy accumulates during each 2 s energy application, leading to greater total energy accumulation, despite failure to puncture at lower generator settings.
3.5 Mechanical versus RF puncture
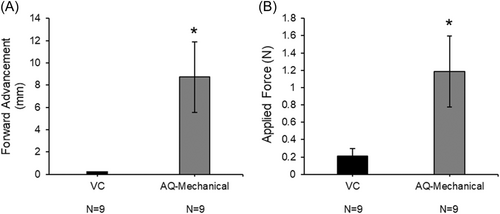
Using the Instron mechanical tester to achieve baseline tenting of the tissue, mechanical-only puncture with the AcQCross needle required additional 8.72 ± 3.16 mm of forward device advancement compared to VC (p < .05; Figure 6A). This correlated to an overall crossing force of 1.19 ± 0.41 N using AQ compared to 0.21 ± 0.09 N for RF puncture using VC (Figure 6B; p < .05).
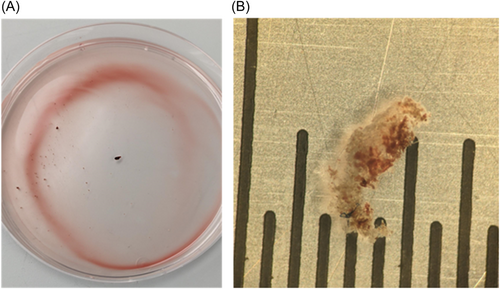
3.6 In vivo TSP
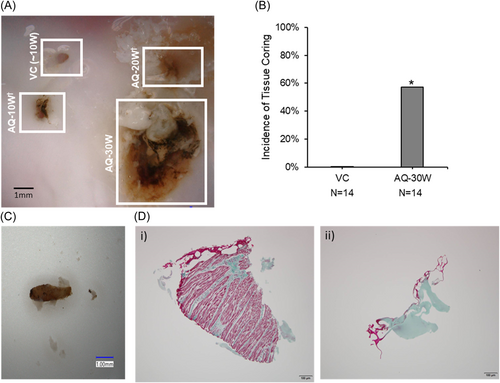
TSP was successfully performed using the AcQCross needle using 30 W of power in a live swine. Investigation of the needle after removal from the animal, flushing with saline and inserting a guidewire through the lumen demonstrated the presence of several particulates that may represent tissue cores and/or thrombus (Figure 7A). Visualization under microscope revealed an approximate particle length of 3 mm and diameter of 0.7 mm (Figure 7B).
4 DISCUSSION
This investigation evaluated the safety and efficacy of two commercially available systems for atrial septal puncture: (1) the AcQCross needle, which can be used for mechanical puncture or as an alternate method for RF puncture and when connected to a standard electrosurgical generator, and (2) the VersaCross wire and dedicated generator, which are specifically designed for RF TSP. A comparison of mechanical puncture using the AcQCross needle to RF puncture using the VersaCross wire system revealed that mechanical puncture, alone, required six times more force than RF puncture with the dedicated RF wire system. This was, also, evidenced by greater displacement of the AcQCross transseptal assembly, which, especially in hyperelastic septa, can present an increased risk of inadvertent perforation of the left atrial wall and cardiac tamponade.2, 3
The current density at the tissue interface and the energy waveform characteristics produced by the electrosurgical generator1, 10 impacts the efficacy and safety outcomes of energy-assisted procedures, such as TSP. While 100% puncture success was achieved using the VersaCross system with one energy application at manufacturer settings (1 s constant mode; approx. 10 W), this study failed to demonstrate successful RF puncture with the AcQCross device at IFU settings (10 W) after five attempts and was, only, able to achieve puncture at a higher power (30 W) and with multiple energy applications. Puncture efficacy was, also, more consistent across different septal tissues with the VersaCross system compared to the AcQCross needle. In clinical use, this can translate to greater consistency between patients despite anatomic differences that could encumber atrial septal puncture. These findings are consistent with previously published data showing greater transseptal efficacy and consistency using a dedicated RF wire system than by applying energy to mechanical guidewires.8
The use of higher generator power settings (30 W) to achieve effective puncture using the AcQCross needle led to larger septal defects and evidence of tissue charring, suggesting greater extent of thermal injury, compared to punctures made using the VersaCross wire. The energy waveforms delivered to the VersaCross wire by its dedicated RF puncture generator enable a rapid increase in current to facilitate local tissue desiccation (i.e., tissue puncture)9, 13 followed by a rapid drop to near zero current after TSP has been achieved, and complete energy shut-off after 1 s. Combined with the discrete RF electrode tip on the wire for optimized current density, the system minimizes excess current delivery and collateral damage. In contrast, conventional electrosurgical generators are not optimized for rapid tissue desiccation and metal needles (e.g., AcQCross) do not have a defined electrode or current density; a high level of current is delivered to the needle to maintain a manually preset power setting for the entire energy application, resulting in slow tissue heating over an extended period and greater cumulative energy output. This can lead to excess heating of the tissue and surrounding blood, resulting in thermal injury, as evidenced by tissue charring, and thrombus formation.14
No coring was observed using the closed-tip VersaCross RF wire; however, application of electrocautery to the open-ended AcQCross needle led to coring in 57% of successful punctures using the ex vivo model. This is consistent with the qualitative observation of particulates in the AcQCross needle lumen following RF puncture in a live animal, and with previous ex vivo findings using electrified open-ended Brockenbrough needles.7 It is important to note that transseptal needle manipulation and/or guidewire insertion can cause the tissue core to be released into the left atrium, thereby, presenting an embolic stroke risk. It has been reported that particulates as small as 15–50 µm can obstruct the microvascular bed, causing microinfarcts and left ventricular dysfunction.15, 16 The AcQCross needle has a larger diameter than standard Brockenbrough needles, which may present a greater stroke risk if the tissue core embolizes.
In addition to minimizing power delivery, dedicated RF puncture generators employ local impedance measurement to interrupt energy delivery if the electrode tip of the transseptal device comes into unintended contact with a metal object, such as the struts of a septal occluder. There have been reports of unintended atrial fibrillation, ventricular fibrillation, and disturbance of pacemakers17, 18 due to the use of low-frequency alternating current, which has a lower threshold for muscle and nerve cell stimulation,19 in standard electrosurgical generators. This can be mitigated using optimized RF puncture generators by selectively delivering high-frequency alternating current (~500 kHz) that does not reach the threshold for nerve or muscle cells stimulation.13
5 STUDY LIMITATIONS
These findings are based on an ex vivo porcine model that attempts to mimic clinical use and anatomic conditions11, 12 to understand device performance noninvasively but, ultimately, can differ in living human tissues. Punctures that were performed manually, as well as using a controlled Instron tester, were both set up such that transseptal device tip was orthogonal to the septum, representing the typical clinical orientation20; this is an important factor in measuring force, puncture success and coring, and may vary in alternative patient anatomies. Further investigation can help to evaluate the clinical relevance of the observed thermal damage (e.g., charring, larger defects) and tissue cores from these experiments.
6 CONCLUSIONS
This study demonstrates the potential safety, efficacy, and efficiency benefits, in addition to the simplicity of use, of a dedicated RF TSP system. Optimized electrode design and generator settings improve puncture efficacy, eliminate embolic coring risk, and minimize thermal injury. Application of electrosurgical energy to the AcQCross needle, as an alternative to mechanical force, requires the use of high energies with at least 30 W and was associated with tissue coring and charring, and, thus, has lower potential safety and efficacy than the dedicated VersaCross system. This study did not evaluate the cost implications of the various TSP options in clinical use.
ACKNOWLEDGMENTS
The authors would like to acknowledge Boston Scientific for equipment, lab and study support, and the University Health Network Centre for Phenogenomics—Pathology Core for histopathology services.




