Capsular Warning Syndrome Caused by Spontaneous Middle Cerebral Artery Dissection
Capsular warning syndrome (CWS) denotes repetitive (≥3 times within 24 h) transient cerebral ischemia causing stereotyped unilateral motor, sensory, or sensorimotor deficits that simultaneously involve face, arm, and leg, without cortical signs 1-3. CWS is an important subgroup of subcortical transient ischemic attack as it is associated with a high early risk of lacunar infarction, reported to be up to 40% within 10 days 1. The exact pathophysiology of CWS has not been clarified. It has mainly been attributed to small-vessel single-penetrating artery disease. We here report an unusual case of CWS caused by dissection of the middle cerebral artery (MCA), which will expand our understanding of possible mechanisms involved in CWS.
A 47-year-old right-handed man with untreated hypertension developed sudden onset of right hemiplegia, hemisensory loss, and dysarthria. He had no headache, vertigo, or nausea. The symptoms completely resolved within 20 min. He experienced 2 similar episodes, which recovered spontaneously within 10 min, en route to our emergency room (ER). Soon after arriving to the ER, he experienced a fourth episode. On arrival, his blood pressure was 173/91 mmHg and heart rate was 64/min. Neurological examination revealed moderate dysarthria, right-sided hemiparesis (2/5), and reduced sensation on the right side, with no neglect, dyspraxia, or dysphasia. All the deficits recovered spontaneously within 10 min.
He was a current smoker, with a history of 10 cigarettes per day for 20 years. He denied any history of diabetes mellitus, coronary disease, atrial fibrillation, previous episodes of transient ischemic attack, or stroke. Serum total cholesterol, triglyceride, low-density lipoprotein cholesterol, lipoprotein A1, and lipoprotein B were all normal. Blood coagulation studies showed normal prothrombin time, activated partial thromboplastin time, and international normalized ratio. Plasma fibrinogen level was 1.53 g/L (normal range, 1.8–4.2 g/L). Glycosylated hemoglobin, C-reactive protein, and urine analysis were within normal range.
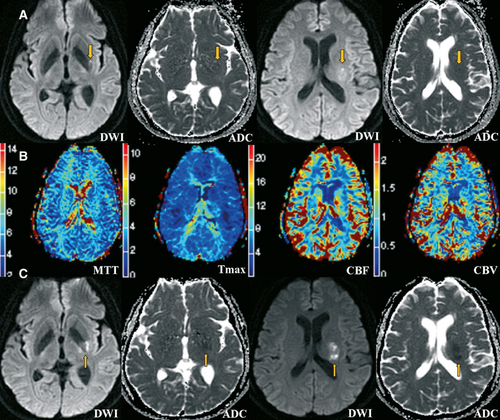
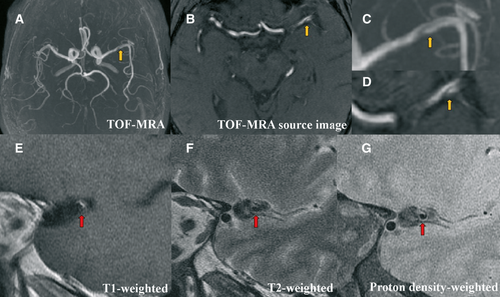
Brain magnetic resonance imaging (MRI) (3.0 T) was performed immediately. Diffusion weighted imaging (DWI) revealed a small lesion with restricted diffusion in the left internal capsule with extension into the corona radiata (Figure 1A). Perfusion-weighted imaging showed no significant abnormalities in cerebral blood flow, cerebral blood volume, mean transit time, and time to maximum in the left MCA territory (Figure 1B). Time-of-flight magnetic resonance angiography demonstrated a string-like hypointense signal in the left proximal MCA (Figure 2), which was suggestive of dissection.
He was given aspirin 200 mg and atorvastatin 40 mg immediately, and then admitted to our ward where he was given intravenous fluids. However, he had 2 further episodes while lying supine. Unfortunately, the symptoms did not completely recover after the last episode and he was left with right hemiparesis (3/5), right hemisensory loss, and dysarthria.
Electrocardiography, transthoracic echocardiography, doppler ultrasound of the internal carotid, and vertebral arteries were all normal. Brain MRI was repeated on day 5. The sagittal vessel wall imaging of the left MCA demonstrated abnormal intramural signal consistent with a diagnosis of MCA dissection (Figure 2). The DWI lesion was enlarged, with normal perfusion (Figure 1C).
The patient was discharged on aspirin and clopidogrel. Over the following month, he made a moderate recovery, with persistent weakness in the hand only. The modified Rankin score was 1 at 6 months. He has had no recurrent symptoms or deterioration.
Capsular warning syndrome is characterized by recurrent stereotyped episodes of unilateral transient ischemic attack without cortical dysfunction such as neglect, dyspraxia, or dysphasia 1. Our case fits the description of CWS. To our knowledge, this is the first report of CWS as the exclusive presentation of a patient with MCA dissection. A previous report described a case of repeated reversible ischemia caused by MCA dissection; however, both cortical and subcortical brain tissues were involved 4.
Capsular warning syndrome has been reported as the presentation of various conditions such as MCA stenosis 5, microscopic polyangiitis 6, and dural sinus thrombosis 7. However, the exact pathophysiology of the transient symptoms in CWS has not yet been determined. Small-vessel single-penetrator disease is the most widely accepted hypothesis. Other mechanisms, such as hemodynamic failure (hypotension) 8 and peri-infarct depolarization 2, have also been postulated. In our case, the lesion involved the posterior limb of the internal capsule which is supplied by the lenticulostriate arteries. We therefore postulate that CWS occurred when the free intimal end of the dissected MCA occluded the ostia of a lenticulostriate artery or served as an embolic source.
Digital subtraction angiography used to be the gold standard for detecting dissection. However, MRI is being increasingly used in the assessment of patients with dissection of carotid and vertebral arteries 9-11. MRI has the advantage of demonstrating the characteristics of the vessel wall, as well as the lumen. For detecting MCA dissection, we found that axial MR images of the vessel were less useful than those in carotid or vertebral dissection, owing to its horizontal orientation. We thus used high spatial resolution MRI to take a sagittal view of the MCA, which clearly depicted the vessel wall. In particular, sagittal MRI also visualized the intramural hematoma, seen as a crescentic rim of hyperintense signal on the T1-weighted image and hypointensity on the T2-weighted image at the fifth day after onset. MRA and sagittal views of the MCA with high-resolution MRI may be useful as the initial imaging study to the diagnosis of MCA dissection in clinical practice.
Acknowledgments
The authors thank Dr. Bruce Campbell from the department of neurology, Royal Melbourne Hospital, Australia, and Dr. Magdy Selim from the department of neurology, Beth Israel Deaconess Medical Center, Boston, for the critical reading of the manuscript. Dr. Lou is supported by grants from the National Natural Science Foundation of China (81070915 and 81171095), the Science and Technology Department of Zhejiang Province (2008C14078) and Health Bureau of Zhejiang Province (WKJ2010-2-010).
Conflict of Interest
The authors declare no conflict of interest.




