Safranal Enhances Non-Rapid Eye Movement Sleep in Pentobarbital-Treated Mice
The first two authors contributed equally to this work.
SUMMARY
Aims: Safranal (2,6,6-trimethyl-1,3-cyclohexadiene-1-carboxaldehyde, C10H14O) is an active ingredient in the saffron, which is used in traditional medicine. It has been reported to have sedative and anti-epileptic effects, but its hypnotic effects remain uncertain. The aim of this study was to evaluate effects of safranal on sleep-wake cycle. Methods: We established hypnotic-model mice treated with a low dose of pentobarbital 20 mg/kg, and administered different doses of safranal, vehicle, or diazepam. The change of sleep-wake cycle was assessed by sleep recording and c-Fos expression in the brain was analyzed by immunohistochemistry. Results: Safranal increased the duration of non-rapid eye movement (NREM) sleep, shortened NREM sleep latency, and enhanced the delta power activity of NREM sleep. Immunohistochemical evaluation revealed that safranal increased c-Fos expression in the ventrolateral preoptic nucleus (VLPO), one of the putative sleep centers, and decreased it in the arousal histaminergic tuberomammillary nuclei (TMN). Conclusion: These findings indicate that safranal enhances NREM sleep in pentobarbital-treated mice. The hypnotic effects of safranal may be related to the activation of the sleep-promoting neurons in the VLPO and the simultaneous inhibition of the wakefulness-promoting neurons in the TMN, suggesting that safranal may be a hypnotic substance.
Introduction
Insomnia is the most common symptom of psychiatric disorders, affecting more than 27% of the worldwide population. Of these, about 3–10% people are chronic and frequent users of hypnotics [1]. However, the most extensively used anti-insomnia medicines, benzodiazepines, have many side effects, such as dependence, tolerance, rebound insomnia, and amnesia [2]. Safer and more effective hypnotic drugs are needed.
The stigma of Crocus sativus L., commonly known as saffron, is an herb used in cooking and traditional medicine. It is widely cultivated in many countries, such as Iran, India, Greece, and China. Saffron extract and its active constituents have shown different activities when used to treat insomnia, Parkinson's disease, memory impairment, and depression [3–8]. Safranal is one bioactive constituent of saffron. Safranal content is in the range of 0.4–1.3% of the stigma's dry weight [9]. It has been reported that safranal can antagonize pentylenetetrazol-induced seizures by affecting the affinity of the gamma-aminobutyric acid (GABA) A receptor, and prolong loss of the righting reflex (LORR) induced by pentobarbital sodium [7, 10]. Pentobarbital is a well-known hypnotic and anesthetic, known to potentiate the effects of GABA by acting at its own receptor site on the GABA receptor-ionophore complex [11, 12].
Previous reports have shown that the sedative component of pentobarbital is mediated by GABA receptors in an endogenous sleep pathway and that sleep-wake regulatory nuclei, ventrolateral preoptic area (VLPO), tuberomammillary nucleus (TMN), locus coeruleus (LC), and striatum are involved in the sedative response to pentobarbital [13]. These findings suggest that safranal might enhance the inhibitory action of GABA on GABAA receptors and potentiate the hypnotic effects of pentobarbital. Thus, we hypothesized that safranal may affect the architecture and profiles of phenobarbital-induced sleep.
In order to verify our hypothesis that safranal may affect the architecture and profile of phenobarbital-induced sleep in mice, we recorded electroencephalogram (EEG) and electromyography (EMG) to investigate the change of non-rapid eye movement (non-REM, NREM) sleep, NREM sleep latency, the number of stage transitions between NREM sleep and wakefulness, and the delta activity of NREM sleep in mice treated with a low dose of pentobarbital. In addition, an immunohistochemical method was used to explore possible mechanisms for the hypnotic effects of safranal via observing changes of c-fos expression in the VLPO and TMN.
Materials and Methods
Animals
Male inbred C57BL/6J mice, weighing 24–30 g, 11–13 weeks old, were obtained from the Laboratory Animal Center of the Chinese Academy of Sciences (Shanghai, China). The animals were housed individually under an ambient temperature of 22 ± 0.5 °C with a relative humidity of 60 ± 2% and an automatically controlled 12-h light/12-h dark cycle (lights on at 07:00, illumination intensity ≈ 100 lux). They had free access to food and water. Experimental protocols were approved by the Medical Experimental Animal Administrative Committee of Shanghai. Every effort was made to minimize the number of animals used and any pain or discomfort that they might experience.
Chemicals
Safranal liquid was obtained from Sigma-Aldrich (Saint Louis, MO, USA) and diluted in paraffin oil. Pentobarbital and diazepam were purchased from the China National Medicines Corporation, Ltd. (Shanghai, China) and the People's Pharmaceutical Manufacturer (Tianjin, China), respectively. Rabbit polyclonal anti-c-Fos antibody was purchased from Santa Cruz (Santa Cruz, CA, USA). Biotinylated goat anti-rabbit IgG and avidin–biotin–peroxidase were purchased from Vector Laboratories (Burlingame, CA, USA), and 3,3′-diamino-benzidine-tetrahydrochloride (DAB) was purchased from Sigma (Saint Louis, MO, USA).
Polygraphic Recordings and Vigilance State Analysis
Under 5% chloral hydrate anesthesia (360 mg/kg, i.p.), mice were implanted with electrodes for polysomnographic EEG and EMG recordings. Two stainless steel screws (1 mm in diameter) were inserted through the skull into the cortex (antero-posterior, +1.0 mm; left–right, −1.5 mm from bregma or lambda) according to the atlas of Franklin and Paxinos [14]. These served as EEG electrodes. Two insulated stainless steel Teflon-coated wires were placed bilaterally into both trapezius muscles. These served as EMG electrodes. All electrodes were attached to a microconnector and fixed onto the skull with dental cement. The EEG and EMG recordings were carried out by means of a slip ring designed not to inhibit the behavioral movement of the mice. After a 10-day recovery period, the mice were transferred into the recording chamber for 2 h before polygraphic recording.
To easily observe the hypnotic effect of drugs, we carried out the experiments during dark period in which mice spend most of time in wakefulness so that the baseline of sleep is lower than during light period. Each animal was recorded for 24 h beginning at 19:00, the end of the light period. Cortical EEG and EMG signals were amplified, filtered (EEG, 0.5–30 Hz; EMG, 20–200 Hz), digitized at a sampling rate of 128 Hz, and recorded using SleepSign (Kissei Comtec, Nagano, Japan) as has been described [15–18]. When complete, polygraphic recordings were automatically scored offline by 4-second epochs as wakefulness, REM, and NREM sleep using SleepSign according to standard criteria [15, 16, 18, 19]. Finally, defined sleep–wake stages were examined visually and corrected when necessary.
Pharmacological treatments
Safranal was prepared as described above immediately before use and administered intragastrically (i.g.) at 20:00 at a dose of 90, 180, or 360 mg/kg (n = 6). Diazepam was diluted in saline and administered i.g. at a dose of 3 mg/kg (n = 5). Pentobarbital dissolved in saline was administered intraperitoneally (i.p.) at a sub-hypnotic dosage of 20 mg/kg at 21:00 [20, 21]. We used separate groups of mice for each dose.
c-Fos immunohistochemistry
Four groups of mice were used. Each group was given either vehicle or safranal at a dose of 90, 180, or 360 mg/kg intragastrically at 20:00. One hour later, at 21:00, all mice were given pentobarbital (20 mg/kg, i.p.). Ninety minutes after pentobarbital administration, the animals were anesthetized with 10% chloral hydrate and perfused with saline solution followed by ice-cold 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (pH 7.4) via the heart. Their brains were then removed, post-fixed in 4% PFA for 6 h, and immersed in 30% sucrose overnight. Thereafter, frozen sections were cut at 30 μm incoronal planes by use of a freezing microtome (Jung Histocut, model820-II, Leica, Germany). The sections were stored in a cryoprotectant solution at −20 °C for histological analysis. Immunohistochemistry was performed in accordance with the free-floating method described earlier [22, 23]. Sections were fixed in 4% PFA for 10 minutes and incubated with 0.3% H2O2 for 15 minutes to quench endogenous peroxidase activity. The sections were next placed in blocking solution containing 10% normal rabbit serum with 0.3% Triton X-100 in 0.01 M phosphate-buffered saline (PBS, pH 7.2) at 37°C for 30 minutes and then incubated at 4°C for 24 h with a rabbit polyclonal antibody against c-Fos at a 1:2000 dilution in PBS containing 1% normal rabbit serum and 0.3% Triton X-100. On the second day, the sections were incubated with a 1:200 dilution of biotinylated goat anti-rabbit secondary antibodies for 1 h followed by a 1:200 dilution of avidin–biotin–peroxidase for 1 h at 37°C. The peroxidase reaction was visualized with 0.05% DAB in 0.1 M phosphate buffer and 0.01% H2O2. Sections were mounted, dehydrated, and cover slipped. As controls, adjacent sections were incubated without the primary antibody to confirm that no non-specific staining had occurred. The sections were examined under bright-field illumination using a Leica DMLB2 microscope (Leica Microsystems, Wetzlar, Germany). Images were captured with a Cool SNAP-Proof digital camera (SPOT RTKE Diagnostic instruments, Sterling Heights, MI, USA).
Statistical analysis
All results are expressed as means ± SEM (n = 5–6). For parametric data, single comparisons were evaluated using the t-test, and multiple comparisons between groups were analyzed using one-way repeated measures ANOVA followed by Fisher's probable least-squares difference (PLSD) test. Time-courses of the hourly amounts measured at each stage were analyzed against the vehicle control using the paired t-test. The total amount of sleep/wakefulness, sleep latency, number of bouts, number of stage transitions, mean duration, and number of c-Fos immunoreactive neurons were analyzed by ANOVA followed by Fisher's PLSD test. All statistical analyses were carried out using SPSS 10.0 for Windows. In all cases, P < 0.05 was taken as the level of significance.
Results
Safranal shortened NREM sleep latency and increased NREM sleep in pentobarbital-treated mice
Safranal was administered intragastrically at a dose of 90, 180, or 360 mg/kg at 20:00. Pentobarbital sodium was injected intraperitoneally at a dose of 20 mg/kg at 21:00. Diazepam at 3 mg/kg was given as a positive control. Figures 1A and B show typical examples of polygraphic recordings and corresponding hypnograms from an individual mouse given vehicle or safranal (360 mg/kg) in pentobarbital-treated (PENT) mice. During the period from 21:00 to 1:00, this mouse from the vehicle control group spent more time in wakefulness than in sleep states (Figure 1A). When safranal was given, however, the animal started sleep after a shorter amount of time, and spent more time in sleep than control mice did (Figure 1B). Similar changes were observed at lower doses (180 mg/kg) (data not shown). As shown in Figure 1C, administration of safranal shortened NREM sleep latency remarkably. NREM sleep latency is defined as the time from the administration of the vehicle or safranal to the appearance of the first NREM sleep episode lasting for at least 20 seconds. The latency-to-NREM sleep period in mice treated with safranal (180 and 360 mg/kg) and diazepam (3 mg/kg) was 1.61, 1.86, and 1.04 minutes, respectively. This was significantly shorter than the 3.47-minute latency seen after vehicle injection (F(4,28)=3.808, P < 0.05). However, there was no change in REM sleep latency after administration of safranal or diazepam to PENT mice. The short NREM sleep latency of the safranal-treated mice clearly indicates that safranal accelerated the initiation of NREM sleep in PENT mice.
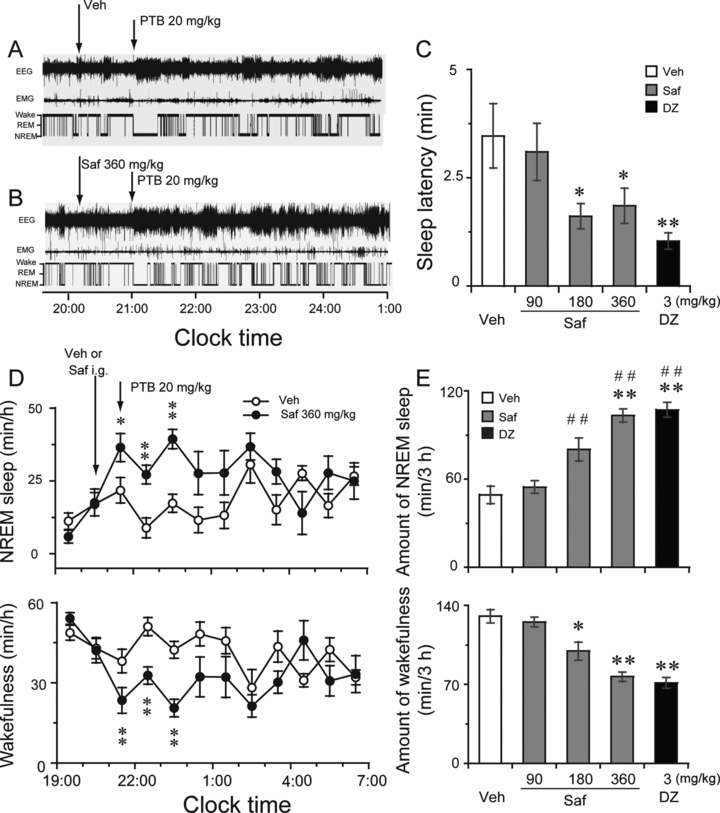
Sleep-stage distribution produced by i.g. administration of safranal in PENT mice. Typical examples of polygraphic recordings and corresponding hypnograms in a PTB-treated mouse given (A) vehicle or (B) safranal at a dose of 360 mg/kg. (C) Effects of safranal and diazepam on NREM sleep latency. Open and filled bars show the profiles of the vehicle and safranal or diazepam injection, respectively. *P < 0.05; **P < 0.01 relative to vehicle as assessed by ANOVA followed by Fisher's PLSD test. (D) Time-course changes produced by the i.g. administration of safranal at 360 mg/kg. Each circle represents the hourly mean ± SEM of wakefulness and NREM sleep. Open and filled circles indicate the vehicle and safranal group profiles, respectively. Safranal was given at 20:00 followed by pentobarbital at dose of 20 mg/kg at 21:00. *P < 0.05; **P < 0.01, relative to the vehicle control as assessed by two-tailed paired t-test. (E) Total time spent in wakefulness, and NREM sleep 3 hours after administration of safranal and diazepam. Open and filled bars show the profiles of the respective vehicle, safranal, and diazepam injections. Values are means ± SEM (n = 5–6). **P < 0.01; ##P < 0.01 relative to vehicle control and safranal at 90 mg/kg, respectively, as assessed by ANOVA followed by Fisher's PLSD test. Veh, vehicle; PTB, pentobarbital; Saf, safranal; DZ, diazepam.
Figures 1D and E summarize the time-courses of the hourly and cumulative amounts of NREM sleep experienced by the PENT mice 3 h after pentobarbital (20 mg/kg) injection in subjects pretreated with the vehicle or safranal (90, 180, or 360 mg/kg) and diazepam (3 mg/kg), respectively. Relative to the vehicle control, safranal at 360 mg/kg markedly increased the amount of NREM sleep during those 3 h, commencing the second hour after the safranal injection (Figure 1D). This augmentation in sleep time was accompanied by a reduction in wakefulness (Figure 1D). Similar time-course profiles were observed with lower doses, but the effect on sleep was small and lasted only about 1–2 h after administration (data not shown). We calculated the total time spent in NREM sleep during the 3 h following safranal or diazepam administration in PENT mice (Figure 1E). Safranal given at 180 or 360 mg/kg and diazepam at 3 mg/kg significantly increased the total amounts of NREM sleep by 62.6%, 109.7%, and 117.5%, respectively, during that 3-hour period, relative to the vehicle control. However, safranal at 90 mg/kg was not found to affect the cumulative amount of NREM sleep during this time. In addition, there was essentially no significant difference in REM sleep after the administration of safranal (data not shown). ANOVA analysis revealed that safranal increased NREM sleep (F(4,28)= 21.316, P < 0.01) and the effects of safranal at 360 mg/kg were stronger than those of safranal at 90 and 180 mg/kg (P < 0.01, vs. safranal 360 mg/kg) but not significantly different from that of diazepam at 3 mg/kg (P > 0.05). These results indicate that safranal increased NREM sleep in PENT mice.
Changes in the number of bouts, mean duration of episodes, stage transition, and power density of NREM sleep by safranal in PENT mice
As shown in Figures 2 A and B, safranal changed the total number and mean duration of NREM sleep episodes, but there was no change in REM sleep. Safranal (180 or 360 mg/kg) increased the number of NREM bouts by 2.7- or 1.9-fold, respectively. The mean duration of NREM sleep in PENT mice was shorter after administration of safranal at 360 mg/kg and 180 mg/kg during the 3-hour period. However, the administration of safranal at 90 mg/kg did not affect either the number or mean duration of NREM or REM sleep bouts (Figures 2 A and B). Safranal at 180 and 360 mg/kg increased the number of state transitions from wakefulness to NREM sleep and from NREM sleep to wakefulness (Figure 2D). Neither a change in the number of transitions from NREM to REM nor in that from REM to wakefulness was found.
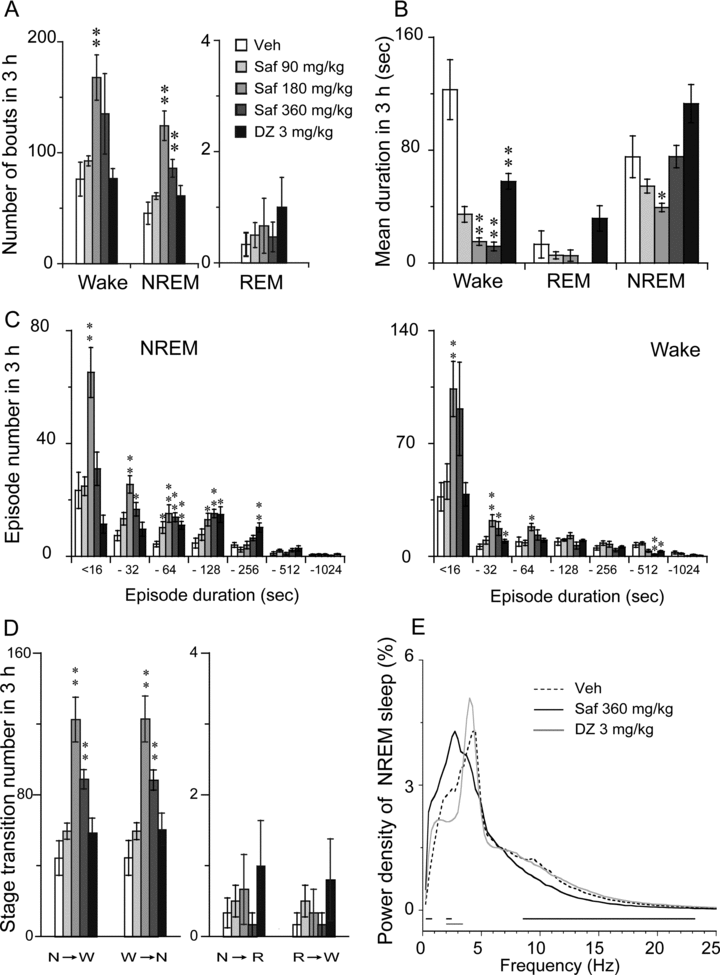
Characteristics of sleep–wake bouts: (A) number of bouts, (B) mean duration, (C) number of episodes, (D) stage transition, and (E) power density during NREM sleep in the 3 hours after the administration of diazepam (3 mg/kg) and safranal at 90, 180, or 360 mg/kg in PENT mice. W, N, and R represent the stages of wakefulness, NREM, and REM sleep, respectively. Open and filled bars show the profiles for the respective vehicle and safranal or diazepam. The horizontal bars (E) indicate statistical differences (P < 0.05, PLSD test) between the vehicle control and safranal or diazepam. Values are expressed as mean ± SEM (n = 5–6). *P < 0.05; **P < 0.01 relative to vehicle as assessed by ANOVA followed by Fisher's PLSD test.
To better understand the effects of safranal on sleep characteristics, we observed sleep bout distribution as a function first of bout and then of episode duration (Figure 2C). Relative to the vehicle group, safranal at 90 mg/kg had little effect on the durations of wakefulness or NREM sleep during the 3 hours after administration of pentobarbital sodium. Higher doses of safranal (180 or 360 mg/kg) increased the number of bouts of NREM sleep that had durations of 4–16, 16–32, 32–64, and 16–128 seconds and the number of wakefulness bouts with durations of 4–16, 16–32, and 32–64 seconds (Figure 2C). Similarly, diazepam increased the number of NREM sleep bouts with durations of 32–64, 64–128, and 128–256 seconds and decreased the number of bouts of wakefulness with durations of 16–32 and 256–512 seconds (Figure 2C).
We then determined the EEG power spectra during NREM sleep in PENT mice. The power of each 0.25-Hz bin was first averaged across the sleep stages individually and then normalized as a group by calculating the percentage of each bin from the total power (0–24.75 Hz) of the individual animal. As shown in Figure 2E, safranal at a dose of 360 mg/kg increased the EEG power density of NREM sleep within the range of 0.5–0.75 Hz and 2.0–2.5 Hz but decreased it in the range of 8.5–23.25 Hz. Safranal at 90 mg/kg was not found to affect NREM sleep. However, 3 mg/kg diazepam decreased the EEG power density of NREM sleep in the frequency range of 2–3.5 Hz. Meanwhile, safranal (180 and 360 mg/kg) increased the percentage of mean slow wave activity (SWA) of NREM sleep in the frequency range of 0.25–4 Hz. The lower doses of safranal (90 mg/kg) and diazepam were not found to affect SWA in PENT mice (data not shown). These findings indicate that safranal can increase the EEG power density and SWA in NREM sleep.
Effects of safranal on c-Fos expression in the VLPOs and TMNs of PENT mice
To investigate the effects of safranal on the VLPO and TMN in PENT mice, we counted the number of c-Fos positive neurons in the VLPO and TMN. Figure 3 (A–H) shows representative photomicrographs of c-Fos expression in the VLPO and TMN of vehicle- (A and C) and 360 mg/kg safranal- (B and D) treated PENT mice. Analysis of the number of c-Fos-immunoreactive nuclei showed that safranal (180 or 360 mg/kg i.g.) increased expression of c-Fos in the VLPO (F(3, 19)= 25.753; P < 0.01) and decreased it in the TMN (F(3, 19)= 4.691; P < 0.05), both relative to vehicle control (Figure 3I). These findings indicate that safranal activated the VLPO sleep center and inhibited the arousal TMN to increase NREM sleep in PENT mice.
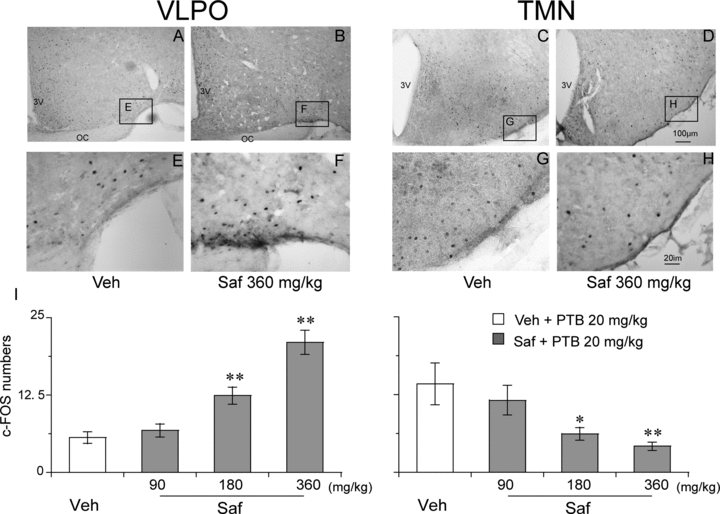
Effects of safranal on c-Fos expression in the VLPO and TMN in PENT mice. (A–H) Representative photomicrographs of c-Fos expression in the VLPO and TMN of vehicle- (A and C) and 360 mg/kg safranal-(B and D) treated PENT mice (E–H: high-magnification views of the rectangular areas marked in “E–H” from A–D). Scale bars: A–D, 100 μm; E–H, 20 μm. (I) Numbers of c-Fos expression in the VLPO and TMN of mice 90 minutes after treatment with vehicle or safranal. Values are expressed as means ± SEM (n = 5), *P < 0.05; **P < 0.01 relative to vehicle as assessed by ANOVA followed by Fisher's PLSD test.
Discussion
Crocus sativus L. stigmas, commonly known as saffron, is a rare food spice. It is widely cultivated in many countries, especially central and eastern Asia. The safranal that can be isolated from saffron is one of the spices more pharmacologically active and important ingredients. In the present study, we demonstrated that safranal significantly potentiated the hypnotic effects of pentobarbital in mice by increasing NREM sleep and shortening the latency of NREM sleep. This is consistent with the previous studies that safranal decreased locomotor activity (LMA) and prolonged duration of LORR in mice [7].
LORR was used as an index to study the acute sedative-hypnotic effects of other compounds in combination with pentobarbital (50 mg/kg, i.p.) in mice. The study suggested that safranal may have sedative-hypnotic effects but whether it affects the sleep-wake profile under baseline conditions still remains unknown. Quantified EEG analysis proved to be a very sensitive and exact method of examining sleep behavior [24]. In the present study, sleep recording showed that safranal did not affect sleep-wake profiles in either the amount or quality of sleep under baseline conditions (data not shown), but it significantly potentiated the hypnotic effects of pentobarbital. Another two ingredients in saffron, crocin and crocetin, directly increased the duration of NREM sleep in mice when given i.p. and did not change EEG power density, indicating that crocin and crocetin can promote sleep in a pattern similar to physiological sleep [25].
Pentobarbital has been used as sedative-hypnotic, anticonvulsant, and anesthetic, and these effects vary with the dosage. Higher doses of pentobarbital induce anesthesia or deep inhibition in the central nervous system, but lower doses induce sedation [24, 26, 27]. We chose a sub-hypnotic dosage of pentobarbital (20 mg/kg, i.p.) to determine whether safranal has synergic effects of sleep with pentobarbital by recording EEG in mice. We found that safranal enhanced NREM sleep and shortened its latency. Similar reports using this model showed that extract of Ganoderma lucidumthe, a traditional Chinese herb, had hypnotic effects in pentobarbital-treated rats, as indicated by EEG, but not in normal rats [28]. Diltiazem, an l-type Ca2+ channel blocker, exerted potentiating effects on pentobarbital-induced hypnosis in mice, but it did not change sleep architecture when used alone [26]. Combinations of pentobarbital and sleep recording show that some compounds, such as herbs and foods, can be considered hypnotic substances. This model is useful for screening hypnotic candidates.
Safranal has been found to exert anticonvulsant activity in the pentylenetetrazol model and the effect may be mediated, at least partly, through a benzodiazepine binding site of GABAA receptor. This is because pretreatment with flumazenil (5 nmol, i.c.v.), an antagonist at benzodiazepine binding site of GABAA receptor, prior to safranal administration can abolish the protective effects of safranal against seizures [10]. In contrast to previous reports, our study demonstrated that pretreatment with flumazenil (1 mg/kg, i.p.) prior to safranal at 360 mg/kg, i.g. did not affect the total amount of NREM sleep during three hours after the administration in PENT mice, compared to vehicle control (safranal, 74.7 ± 12.0 min vs. control, 70.3 ± 10.0 min, P > 0.05, n = 6). There are two possibilities: First, in PENT mice, safranal-induced hypnosis may be dependent on the pentobarbital-induced sleep, but flumazenil has no effect on the sleep architecture of pentobarbital. Second, unlike seizures, the sleep-wake cycle is a complex phenomenon regulated by many regions of the brain and involving intricate synaptic circuits whose functions depend upon multiple neurotransmitter systems, including GABA, adenosine, histamine, NE, DA, and 5-HT [29].
A great deal of evidence shows that the VLPO contains an essential population of sleep-promoting neurons, which are more active during sleep, as indicated by the expression of c-Fos [30]. The VLPO has been shown to play a critical role in the promotion of NREM sleep [31]. The VLPO neurons send inhibitory GABA and galanin fibers into the arousal histaminergic TMN [29,33]. Safranal was found to increase c-Fos expression in the VLPO and to inhibit it in the TMN in PENT mice, suggesting that activation of VLPO might play important roles in the hypnotic effects of safranal. Similarly, VLPO has been reported to be involved in the diltiazem-potentiating effects on pentobarbital-induced hypnosis [26].
In addition to increased duration of sleep, safranal enhanced the delta power density of NREM sleep in PENT mice, suggesting that safranal might improve the quality of sleep. Safranal has hypnotic effects in the presence of pentobarbital and may provide a starting point for the development of alternative supplements to improve sleep quality. Therefore, safranal combined with hypnotic drugs at low dosage, such as diazepam, will improve sleep with little side effect compared to hypnotic drugs when used alone.
Conclusion
Safranal increased NREM sleep and shortened NREM sleep latency in PENT mice. The hypnotic effects of safranal may be related to the activation of the sleep-promoting neurons in the VLPO and the simultaneous inhibition of the wakefulness-promoting neurons in the TMN. These findings are of significant pharmacological relevance as a combination of safranal and low dose sleeping aid may be useful for certain medical applications in the treatment of insomnia.
Acknowledgments
This study was supported in part by grants-in-aid for scientific research from the Shanghai Municipal Government Foundation (09JC1402500, 10XD1400400, B119), the China National Science and Technology Foundation (2009ZX09303-006, 2009CB5220004, 31121061), and the Program of Basic and Applied Researches for Innovations in Bio-oriented Industry of Japan.
Conflict of Interest
The authors declare no conflict of interest.




