Incretin-based therapies
Re-use of this article is permitted in accordance with the Terms and Conditions set out at http://wileyonlinelibrary.com/onlineopen#OnlineOpen_Terms
Abstract
Incretin-based therapies have established a foothold in the diabetes armamentarium through the introduction of oral dipeptidyl peptidase-4 inhibitors and the injectable class, the glucagon-like peptide-1 receptor agonists. In 2009, the American Diabetes Association and European Association for the Study of Diabetes authored a revised consensus algorithm for the initiation and adjustment of therapy in Type 2 diabetes (T2D). The revised algorithm accounts for the entry of incretin-based therapies into common clinical practice, especially where control of body weight and hypoglycemia are concerns. The gut-borne incretin hormones have powerful effects on glucose homeostasis, particularly in the postprandial period, when approximately two-thirds of the β-cell response to a given meal is due to the incretin effect. There is also evidence that the incretin effect is attenuated in patients with T2D, whereby the β-cell becomes less responsive to incretin signals. The foundation of incretin-based therapies is to target this previously unrecognized feature of diabetes pathophysiology, resulting in sustained improvements in glycemic control and improved body weight control. In addition, emerging evidence suggests that incretin-based therapies may have a positive impact on inflammation, cardiovascular and hepatic health, sleep, and the central nervous system. In the present article, we discuss the attributes of current and near-future incretin-based therapies.
Introduction
Type 2 diabetes (T2D) is reaching epidemic proportions in Westernized countries owing to the intersection of excess calorie consumption and reduced physical activity imposed on a genetically prone population. Nearly one in 10 individuals in the US have diabetes and it is projected that currently one in three newly born will eventually develop diabetes (see http://www.cdc.gov/chronicdisease/resources/publications/AAG/ddt.htm, accessed 5 October 2011). Diabetes is diagnosed now in younger people and there is much concerned speculation as to how the health economic consequences of diabetes may manifest over the upcoming decades.
Most newly diagnosed and established patients with T2D are overweight or obese and current treatment algorithms place emphasis on initial lifestyle modification entailing a low-calorie diet with regular exercise in order to improve metabolic state and reduce body weight.1 Lifestyle modification alone has a low likelihood of sustained metabolic improvement and T2D is a progressive disease, likely through the inexorable decline in β-cell function, and for this reason there is an increasing need for pharmacological intervention over time. The diabetes armamentarium has well-established therapies, each of the more historic mainstays (insulin, metformin and sulfonylureas) having accumulated between 60 and 90 years of clinical experience, and a number of additional oral agent classes introduced over the past 15 years.
In general, if glycemic goals (HbA1c <7) are not achieved or sustained with lifestyle modification, metformin is strongly positioned as first-line oral therapy, largely because of long experience in the clinic, reproducible glucose-lowering efficacy, well-accepted safety and tolerability profiles, neutral effects on body weight, and low cost. Then, in a stepwise manner, further oral agents are added with the eventual need for insulin, first in the basal long-acting form and then later the use of more intensified insulin regimens. Despite this array of oral agents and advances in insulin delivery, pharmacological intervention often falls short of treatment goals with the price of unwanted side effects, namely weight gain and hypoglycemia.
Incretin-based therapies were developed after the observation during the 1960s of an incretin signal originating from the gut that augmented the β-cell response to caloric intake2 and the later discovery that the gut hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) play a central role in this physiological response.3–7 It is evident that this physiological response is mostly relevant in the postprandial period; however, rodent data have also shown that GLP-1 may play a tonic role in regulating fasting plasma glucose. The incretin response is thought to be defective in T2D, manifesting in part with a complete lack of β-cell responsiveness to exogenously administered GIP.6,8 The β-cell maintains responsiveness to exogenously administered GLP-1, but the response may be somewhat attenuated compared with that seen in the non-diabetic state, indicating a partial incretin defect with GLP-1.9 Due to these effects on the β-cell, GLP-1 became an attractive pharmacologic target, and harnessing it in some fashion was enticing. This had to be accomplished while overcoming a key shortcoming of the native GLP-1 peptide, namely its susceptibility to rapid breakdown by the ubiquitous protease dipeptidyl peptidase-4 (DPP-4), rendering the native hormone incapable of a useful pharmacologic effect unless infused continuously. Over the past 5 years, we have seen the emergence of the DPP-4 inhibitor oral agent class and the injectable GLP-1 receptor agonist class. Both pharmaceutical preparations have overcome the instability of native GLP-1 and have been important new additions, with well-documented beneficial effects on glycemic control and short-term effects on β-cell function. However, to date, no long-term β-cell-preserving effects have been documented. Regardless, the wider physiologic role of the incretin hormones has uncovered a wide array of pharmacologic effects that may be of particular advantage to patients with T2D.
Incretin-based therapies: Leveraging the effects of GLP-1
Much of the initial focus of GLP-1 has been its effect on the β-cell. It is known that GLP-1 enhances insulin secretion in a glucose-dependent manner, meaning that insulin secretion is most demonstrable when plasma glucose is abnormally elevated and not seen when plasma glucose is below the normoglycemic range, thereby resulting in low risk of hypoglycemia.10 The effect on the β-cell is immediate and robust, namely restoration of first-phase insulin secretion,10 a well-characterized defect seen early in T2D and not corrected by other pharmacological interventions. This response, an artifice of an experimental challenge to the β-cell, gains clinical relevance when a similar early robust response of the β-cell to a meal challenge results in a powerful effect, namely the prevention of an abnormal rise in postprandial plasma glucose. In addition, GLP-1 slows gastric emptying and reduces the secretion of glucagon in a glucose-dependent manner, both of which further contribute to the reduction of elevated postprandial plasma glucose.10 The effects of GLP-1 are not confined to the postprandial state, because clinical data demonstrate a lowering of abnormally elevated fasting plasma glucose to the normal range when the hormone is infused intravenously; this appears to be β-cell mediated, because insulin secretion increases concomitantly.10 The effects of GLP-1 on the β-cell are not confined to a glucose-dependent stimulation of insulin secretion; favorable effects on upstream insulin biosynthesis are also observed11 and, over the longer-term, an effect to potentially restore β-cell function and mass has been observed in preclinical models.12–14 This preclinical finding has raised speculation concerning the potential for a truly disease-modifying impact of GLP-1 pharmacology on T2D and also possibly on Type 1 diabetes.
The wider array of glucoregulatory effects of GLP-1 can be described as β-cell sparing because they reduce the workload of the β-cell by: (i) suppressing the catabolic hormone glucagon, which is abnormally elevated in the diabetic setting, particularly postprandially; (ii) slowing of gastric emptying postprandially, thereby allowing a more timely delivery of nutrients into the portal and then systemic circulation; (iii) suppressing appetite, reducing food intake and therefore the caloric load required to be assimilated in fuel stores; (iv) reducing body weight, likely a consequence of effects on satiety; (v) insulin-sensitizing effects, clearly seen in animal models15 but somewhat disputed in humans and, if observed, often considered to be a secondary response to weight loss; (vi) an insulin-independent effect on glucose clearance from the circulation that may be mediated through portal signaling (Table 1).15
| GLP-1 | GLP-1 receptor agonists | DPP-4 inhibitors | |
|---|---|---|---|
| Enhanced glucose-dependent insulin secretion | Yes | Yes, including restoration of first-phase insulin response to i.v. glucose | Yes |
| Suppression of inappropriately elevated glucagon secretion | Yes | Yes | Yes |
| Regulation of gastric emptying | Yes | Yes | No |
| Suppression of appetite | Yes | Yes | No |
| Weight reduction | Yes | Yes | No: neutral effects on weight |
| Improved β-cell function | Yes* | Yes | Yes |
- *Glucagon-like peptide-1 (GLP-1) exerts trophic effects on primary islet cultures, in vivoβ-cell models, and β-cell lines, promoting β-cell proliferation and neogenesis while reducing β-cell apoptosis.110–113 In addition, GLP-1 also promotes differentiation of non-insulin cells into cells able to secrete insulin.114
- DPP-4, Dipeptidyl peptidase-4.
Beneficial effects are also seen in the cardiovascular system, including protective actions in the cardiomyocyte at a molecular level and improved cardiac function. In addition, potentially beneficial anti-inflammatory, cardiorenal, and peripheral vasodilatory effects have been reported.16–18 There is also a body of work that highlights the potential downstream cardiac effects of the DPP-4-cleaved GLP-1 metabolic product GLP-1(9-36).18 It is unclear as yet whether GLP-1(9-36) serves an important physiologic role, how its actions are elicited at the receptor level, and how this cleaved product may influence what we observe with GLP-1-based pharmacology. In summary, GLP-1 pharmacology may have potential cardiovascular benefits for patients with T2D and has the potential to exert specific cardiac benefit in certain acute care settings. That said, there is currently only a limited body of supporting evidence from the clinic.
Glucagon-like peptide-1 (GLP-1) has been an attractive pharmacologic target for some time and it has now been harnessed in two very different forms: the DPP-4 inhibitor class and the GLP-1 receptor agonists. The former is represented by the introduction of three oral agents in the US and a fourth available in Europe. The GLP-1 receptor agonist class first emerged as the twice a day injectable exenatide and, since then, efforts have been directed at leveraging GLP-1 actions in a less frequent dosing platform.
Glucagon-like peptide-1 receptor agonists: Clinical review
Exenatide (exendin-4) was the first available GLP-1 receptor agonist and was originally isolated from the salivary secretion of the Gila monster.19 Exenatide has approximately 50% amino acid sequence identity to GLP-1, with which it shares many glucoregulatory effects. However, unlike GLP-1, exenatide is resistant to DPP-4 degradation, which gives it a half-life of 2.4 h in the circulation, making it amenable to twice-daily injectable subcutaneous administration.20
Early mechanistic work with the exenatide molecule established many of the attributes already seen with GLP-1: glucose-dependent actions on both β- and α-cells,21 restoration of first- and second-phase insulin secretion,21 slowing of gastric emptying,22 satiety effects at mealtime,23 and powerful glucose lowering in both the fasting and postprandial states (Table 1).22 In the original major long-term clinical trials with exenatide as an adjunct to oral agents, 10 μg exenatide twice daily (b.i.d.) reduced HbA1c (approximately 1%) and body weight (approximately 3 kg) in patients with T2D.24–27 This was a unique moment in the treatment of T2D because although historic interventions elicited glycemic improvement, this was invariably coupled with weight gain or, at best, weight neutrality. Trial extension data out to 3 years with the completer population showed exenatide elicited sustained reductions in HbA1c (approximately 1%) and body weight (approximately 5 kg).28
The efficacy of exenatide has also been compared with basal and biphasic insulin regimens in patients with T2D.29–32 Comparing 10 μg exenatide b.i.d. with optimally titrated insulin glargine in trials of up to 52 weeks duration,29,30,32 and a further study comparing 10 μg exenatide b.i.d. or insulin lispro 70/30 for 52 weeks,31 revealed equivalent reductions in HbA1c between exenatide and insulin in the range of 0.8–1.4%.29–32 However, the means by which overall glycemic improvements were achieved differed between the two interventions: insulin treatment had a greater lowering effect on fasting plasma glucose, whereas a greater reduction in postprandial glucose excursion was observed with exenatide.29–32 Also of note, exenatide reduced body weight in all studies compared with the weight gain seen with insulin treatment.29–32
A long-acting, sustained-release formulation of exenatide is currently in development for use as a once-weekly injection.33 This technology consists of unaltered exenatide encased within a biodegradable medical polymer, namely poly(d,l-lactic-co-glycolic acid), that allows gradual, controlled drug delivery over an extended period of time.34 Early clinical work has shown that the once-weekly platform elicits a greater effect on fasting plasma glucose and HbA1c (approximately 1.7% lowering) than that seen with exenatide b.i.d., but similar effects on body weight.33 In subsequent longer-term clinical trial work, 2 mg exenatide once weekly has consistently resulted in HbA1c lowering in the 1.5–2.0% range, with weight effects similar to those seen with exenatide b.i.d. Studies have compared exenatide once weekly with exenatide b.i.d., maximal dosing of either pioglitazone or sitagliptin, and optimally titrated glargine insulin.35–37 In each study, exenatide once weekly has established superiority with regard to lowering HbA1c, coupled with reproducible weight reduction. Exenatide once weekly is currently under review with the US Food and Drug Administration (FDA).
Liraglutide is a modified analog of human GLP-1, with an arginine to lysine amino acid substitution (position 34) and the attachment of a glutamic acid residue with palmitic acid (C-16 fatty acid) to an existing lysine residue (position 26). These modifications render liraglutide 97% homologous to human GLP-1, resistant to DPP-4 cleavage and with an increased half-life of 13 h in the circulation, making it suitable for once-daily administration by subcutaneous injection.38 In clinical trials, the maximally recommended dose of liraglutide (1.8 mg) reduced HbA1c by 1.0–1.5% and body weight by approximately 2 kg in different trials in which it was compared with metformin, glimepiride, rosiglitazone, or insulin glargine.39–43 Of note, liraglutide was also compared with exenatide b.i.d. in a recent 26-week trial and was found to have a significant advantage over exenatide b.i.d. in terms of HbA1c lowering, but equivalent effects on body weight.44 Liraglutide was recently approved as a once a day GLP-1 agonist at doses of 0.6–1.8 mg/day.44
In addition to exenatide b.i.d., exenatide once weekly, and liraglutide, an array of additional GLP-1 receptor agonists are in earlier stages of clinical development.45–51 Taspoglutide is a highly modified analog of human GLP-1 (93% homology) with two aminoiso-butyric acid substitutions at positions 8 and 35 and a sustained-release formulation, which makes it resistant to degradation by DDP-4 and potentially suitable for once weekly administration.45 However, the development of taspoglutide has recently been suspended owing to serious hypersensitivity reactions and gastrointestinal side effects reported in clinical trials. Albiglutide is a GLP-1 genetic dimer fused to human albumin, which confers resistance to DPP-4, making this agent potentially suitable for once weekly administration.46–48 Lixisenatide (AVE-0010) is a modified exenatide molecule with six C-terminal lysine residues, which confers resistance to degradation by DPP-4 and favors once or twice daily administration.49–51
Dipeptidyl peptidase-4 inhibitors: Clinical review
Dipeptidyl peptidase-4 inhibitors differ greatly from the GLP-1 receptor agonists in that they are small molecules, rather than peptides, and thus can be administered orally rather than by subcutaneous injection. They inhibit the action of DPP-4 to cleave GLP-1 to its inactive form and therefore elevate levels of native GLP-1 in the circulation. Dipeptidyl peptidase-4 is a ubiquitous enzyme in the circulation, where, in addition to GLP-1, it cleaves other peptides, such as native GIP; it is inferred that the glucose-lowering effects of this class of drugs are through actions on GLP-1, but perturbation of other peptide systems cannot be excluded.52
There are nuances in the pharmacologic characteristics of the drugs within the class (e.g. the first commercially available DPP-4 inhibitor, sitagliptin, binds non-covalently to the enzyme, whereas vildagliptin and saxagliptin bind covalently to DPP-4), which provide each agent with unique reversible binding properties and potential for differences in their pharmacologic action.
The clinical efficacy of the DPP-4 inhibitors is modest compared with that seen with the GLP-1 receptor agonists (Table 1). In long-term clinical trials, sitagliptin reduced HbA1c in the region of 0.6–0.7%, with a modest reduction in fasting plasma glucose.53–59 Trials of sitagliptin also demonstrated weight neutrality.53–59 These findings have been reproduced in clinical work with vildagliptin and saxagliptin, and there is evidence that earlier-stage DPP-4 inhibitors in development also have similar effects.60–66
Comparison of GLP-1 receptor agonists and DPP-4 inhibitors
Several recent trials have directly compared incretin-based therapies, to some degree addressing questions concerning relative efficacy and safety of short- versus long-acting GLP-1 receptor agonists and DPP-4 inhibitors.
For example, exenatide and sitagliptin were compared in two 2-week cross-over trials.23,67 Compared with sitagliptin, exenatide improved 2-h postprandial glucose and insulin secretion, reduced the abnormal rise in postprandial glucagon secretion, reduced plasma triglycerides, slowed gastric emptying, and reduced caloric intake.
In another study, exenatide once weekly was compared with sitagliptin over a period of 26 weeks.36 Exenatide once weekly reduced HbA1c and body weight by 1.5% and 2.3 kg, respectively, compared with decreases of 0.9% and 1.5 kg, respectively, seen with sitagliptin.36 Exenatide once weekly also improved systolic blood pressure, the albumin:creatinine ratio, and B-type natriuretic peptide compared with sitaglitpin, which had no significant effect on these parameters.36
Once-daily liraglutide was compared with sitagliptin in another 26-week study.68 Liraglutide reduced HbA1c and body weight by 1.5% and 3.4 kg, respectively, compared with decreases of 0.9% and 1.0 kg, respectively, seen with sitagliptin. Liraglutide also improved fasting plasma glucose (but not postprandial glucose), HOMA-B, C-peptide concentrations, the pro-insulin:insulin ratio, and total cholesterol compared with sitagliptin.68
On balance, in head-to-head to studies, the GLP-1 receptor agonist class has demonstrated a more potent glycemic-lowering effect than the DPP-4 inhibitors, coupled with weight loss rather than weight neutrality. Mechanistically, it would appear the islet actions and effects on gastric emptying are more potent with the pharmacologic concentrations achieved with the injectable therapies, coupled with effects on satiety and food intake, which likely contribute to the weight loss observed.
Safety and tolerability of incretin-based therapies
The introduction of new therapeutic approaches and new pharmaceutical entities requires an appropriate assessment of safety as well as efficacy. The safety profile of GLP-1 receptor agonists seems fairly consistent across different platforms, with some exceptions. First and foremost, the most common issues reported with incretin-based therapies are tolerability issues. Nausea is the most commonly reported event, and tends to be a short-lived phenomenon in the early days or weeks of therapy, but there is a minority of patients who have more severe symptoms and will not tolerate therapy. Other gastrointestinal side effects (e.g. diarrhea) are seen less commonly. Among the more established programs, exenatide once weekly has an improved tolerability profile than exenatide b.i.d. and liraglutide seems similar to exenatide b.i.d., although the overall rate of nausea is reported to be greater with exenatide b.i.d.35,44 Both liraglutide and exenatide b.i.d. require titration steps to mitigate nausea (two steps for liraglutide, one for exenatide b.i.d., compared with none for exenatide once weekly). The DPP-4 inhibitors, unlike the GLP-1 receptor agonists, are well tolerated with respect to gastrointestinal side effects in most patients.
The risk of hypoglycemia is an important consideration for diabetes therapeutics. Because of the very nature of the glucose dependency of the actions of GLP-1 on β- and α-cells, hypoglycemia should be an uncommon event. Mechanistically, this was demonstrated in an elegant stepped hypoglycemic clamp setting during a concomitant infusion of exenatide, where the stimulatory actions on β-cells and suppressing actions on α-cells were over-ridden promptly once plasma glucose dropped below 90 mg/dL,21 thus allowing the primary counter-regulatory responses to hypoglycemia (i.e. suppression of insulin secretion from β-cells and a robust glucagon release from α-cells) to occur unabated. These findings were reaffirmed in the clinic in an early Phase 3 trial in which exenatide b.i.d. was added on a background metformin therapy, with no increase in hypoglycemia despite a reduction in HbA1c of approximately 1%.25 The lack of an increased risk of hypoglycemia in such a clinical setting has been reproduced with both the longer-acting form of exenatide and liraglutide and is a clear differentiating feature of this injectable therapy, especially when compared with its injectable counterpart, insulin.35–37,39–44 However, it should be noted that the addition of GLP-1 receptor agonists to background sulfonylurea therapy increases the risk of hypoglycemia, necessitating titration of concomitant sulfonylurea dosing, although severe hypoglycemic events are extremely uncommon.24 The DPP-4 inhibitors have no evidence of increasing the risk of hypoglycemia, much like the peroxisome proliferator-activated receptor γ agonists and metformin, and unlike the β-cell secretagogue agents, the sulfonylureas and the meglinitides.
More serious but less common safety issues have been highlighted that pertain to the pancreas, kidney, thyroid C-cells, and immunogenicity. Pancreatitis first emerged as a potential safety consideration with the first GLP-1 receptor agonist exenatide b.i.d. and then subsequently the first DPP-4 inhibitor sitagliptin. Pancreatitis was not noted during the original regulatory clinical trial work conducted prior to approval for either compound, but instead during post-marketing safety surveillance, when both therapies had been prescribed in a much larger population. In order to understand the significance of these phenomena, a thorough appreciation of the limitations of post-marketing safety surveillance is key, namely an imprecise numerator (i.e. reporter bias, data quality of safety events and confounders) and denominator (i.e. difficulty in capturing overall population drug exposure). It was speculated that pancreatitis may be a condition seen more often in the T2D population (irrespective of treatment) and this has been confirmed by an independent epidemiological assessment, showing that patients with diabetes have a near threefold increased risk of pancreatitis compared with those without diabetes.69 Associated epidemiological data have also reported no increased risk of pancreatitis when one examines patients initiated on either sitagliptin, exenatide b.i.d., insulin, or metformin.70,71 Studies in animal models have also tried to shed light on this safety issue with mixed findings across a variety of models. Although it is too early to examine the post-marketing experience of liraglutide, a small number of cases of pancreatitis were reported during clinical development; consequently, liraglutide has a similar pancreatitis warning to that seen with exenatide.72
In addition, clinically relevant doses of liraglutide and exenatide once weekly and toxicological doses of exenatide b.i.d. have been reported to cause thyroid C-cell adenomas and/or carcinomas in rodents during 2-year carcinogenicity studies (with unknown relevance in humans). Data from limited studies suggest that the human and rodent thyroid may respond differently to GLP-1 because human thyroid cells seem to lack or have low levels of GLP-1 receptors,73,74 but additional data are needed. As a result of thyroid safety concerns, liraglutide received regulatory approval as an adjunct to diet and exercise, but not as a first-line agent.72,75 The implications of these safety concerns as they relate to other GLP-1 receptor agonist-based therapies are currently unknown. In clinical studies, there have been no reported cases of thyroid cancer during treatment with liraglutide,39–44 exenatide b.i.d.,24–27 or exenatide once weekly.35–37 However, further preclinical studies and a 15-year cancer registry for liraglutide have been initiated.75
In general, DPP-4 inhibitors have a relatively clear safety record, with lower reported rates of adverse events than GLP-1 receptor agonists. The incidence of hypoglycemia with DPP-4 inhibitors has been low, but is higher in patients also using sulfonylurea.76
Potential safety concerns with DPP-4 inhibitors include post-marketing reports of hypersensitivity reactions, including anaphylaxis, angioedema, rash, urticaria, and exfoliative skin conditions such as Stevens–Johnson syndrome.76 It is speculated that these conditions may result from the blockade of GIP degradation, as well as that of other peptides, such as the glucagon secretin family hormones, pancreatic polypeptide proteins, neuropeptides, and chemokines. Moreover, membrane-bound DPP-4, originally identified as CD26, is involved in immune system function and plays a role in cell signaling, the regulation of extracellular matrix binding, and ion transport.77
Incretin-based therapies: Beyond glycemic control
Incretin-based therapies represent an important step forward in the treatment of T2D, addressing, at least in part, a previously neglected aspect of diabetes pathophysiology, namely the incretin defect. Incretin-based therapies demonstrate a capacity to sustain long-term improvements in glycemic control and, with GLP-1 receptor agonists, significant reductions in body weight. Moreover, incretin-based therapies have the potential for disease modification by eliciting fundamental improvements in β-cell function, primarily noted with the GLP-1 receptor agonist class.30,78
Emerging data, primarily with GLP-1 receptor agonists, again also reports the improvement of several key cardiovascular risk factors, including lipid profiles and blood pressure. In clinical trials, exenatide b.i.d. treatment was associated with significant improvements in lipid profiles, including high-density lipoprotein–cholesterol (HDL-C), triglycerides, and total cholesterol in patients who were treated for more than 3 years in open-label extension studies.28 Data from the liraglutide clinical development program also reported significant improvements in total cholesterol, low-density lipoprotein–cholesterol (LDL-C), and triglycerides.39–44 Moreover, HDL-C, LDL-C, triglycerides, and total cholesterol were reported to improve with up to 2 years of treatment with exenatide once weekly.79–81
In addition, effects on diastolic and systolic blood pressure have been observed during the exenatide b.i.d., liraglutide, and once-weekly development programs.79–82 A recent pooled analysis of six exenatide b.i.d. clinical trials of ≥26 weeks duration demonstrated significant reductions in systolic blood pressure with exenatide compared with placebo or insulin.82 Similarly, a pooled analysis of patients participating in two exenatide once weekly trials for 52 weeks also demonstrated significant improvements in systolic blood pressure compared with sitagliptin and pioglitazone.80 A meta-analysis from six 26-week trials during liraglutide clinical development reported improved endpoint systolic blood pressure.83 It is significant that improvements in cardiovascular risk have been reported to occur prior to, or be weakly correlated with, reductions in body weight in analyses of data from exenatide b.i.d. and once weekly, as well as the liraglutide clinical development programs, suggesting direct effects of GLP-1 receptor agonists on blood pressure.79–83
Although the impact of these improvements in cardiovascular risk factors on eventual events has not yet been demonstrated directly, the cardiovascular risk profile associated with exenatide b.i.d. treatment has been assessed in a meta-analysis of 12 clinical trials of ≤52 weeks duration. Compared with a pooled comparator group, the relative cardiovascular event risk associated with exenatide treatment was 0.80 [95% confidence interval (CI) 0.53–1.22].84
Increasingly, liver fat content is being recognized as another cardiovascular risk factor that often accompanies T2D, because 80% of patients with diabetes are thought to have concurrent fatty liver disease.85 There is early evidence that GLP-1 receptor agonists have the potential to improve hepatic health,28,79,80 as measured by hepatic enzyme concentrations, with implications for the treatment of non-alcoholic steatohepatitis and non-alcoholic fatty liver disease, particularly in patients with T2D. Up to 3.5 years of treatment with exenatide b.i.d. or 1 year with exenatide once weekly improved alanine transaminase (ALT) and aspartate aminotransferase (AST), measures of hepatic function that are commonly used surrogates of liver inflammation/steatosis.28,79,80 Exenatide b.i.d. treatment has also been demonstrated to reduce liver fat content in individual patients. For example, a case study reported that a male patient who had been treated with exenatide for 44 weeks experienced a reduction in liver fat of 73% that was associated with improvements in liver function (ALT – 26 IU/L and AST – 5 IU/L).86 This was accompanied by improvements in blood pressure, LDL-C, HDL-C, triglycerides, and HOMA-IR,86 consistent with the emerging link between liver fat/liver function and cardiovascular risk factors.87
Because of the observed effects on cardiovascular risk markers in patients with T2D and the demonstration of GLP-1 receptor localization to cardiac and vascular tissues, several investigations have addressed the question of potential direct effects of GLP-1 receptor agonists on the cardiovascular system. Exenatide has been reported to have cardioprotective effects, measured by preserved cardiac function and reduced infarct size in animal models of ischemia–reperfusion injury in pigs and rodents.18,88,89 Liraglutide was reported to have no cardioprotective effect in a similar pig ischemia–reperfusion injury model,90 but it did improve survival in mice following induced myocardial infarction, reducing the number of cardiac ruptures and infarct size and improving cardiac output.91 Both exenatide and liraglutide have been reported to modulate the expression of cardioprotective genes and to reduce activation of the apoptotic pathway enzyme caspase-3 during ischemic insults.88,91
Exenatide b.i.d. has been reported to reduce C-reactive protein, a measure of inflammation,92,93 total adiponectin, and the oxidative stress marker malondialdehyde compared with insulin glargine in studies of ≤52 weeks duration.93,94 In addition, 52 weeks of exenatide b.i.d. treatment reduced resistin and retinol binding protein-4 (measures of insulin resistance), as well as C-reactive protein concentrations, compared with glibenclamide.95
In comparison with GLP-1 receptor agonists, there are significantly less data available describing the effects of DPP-4 inhibitors on cardiovascular risk. In clinical trials, sitagliptin was reported to elevate HDL-C, whereas vildagliptin was reported to lower cholesterol and triglycerides.58,66 A systematic assessment of cardiovascular risk across eight randomized trials in the saxagliptin clinical development program reported no increased relative risk (RR 0.43; 95% CI 0.23–0.80) of a compound endpoint (cardiovascular death, myocardial infarct, and stroke) compared with placebo, metformin, or glyburide.96
Glucagon-like peptide-1 acts through a well-described gut–central nervous system axis to exert central effects on food intake and satiety. Glucagon-like peptide-1 receptors are located in the brain and emerging evidence suggests that GLP-1 may have effects beyond modulating food intake, including modulating sleep patterns,97 limiting inflammation,98,99 and neuroprotection.100–102 It should be noted that this evidence is from animal models and that no human data are available; consequently, the potential benefits to humans are speculative in nature.
There is strong evidence to indicate that disrupted sleep (reduced sleep duration and/or quality) leads to reduced glucose tolerance, reduced insulin sensitivity, and dysregulation of appetite.103 Consequently, disrupted sleep has been reported to be associated with the increase in risk of developing T2D.104,105 It has been hypothesized that altered gut–brain satiety and insulinotropic signaling may contribute, in part, to these observed metabolic defects. In support of this, preliminary evidence from animal models is consistent with the existence of a link between GLP-1 receptor signaling and circadian/sleep systems. The clock mutant mouse, which is deficient in a central circadian regulator and displays disrupted circadian control, is associated not only with symptoms of the metabolic syndrome and diabetes, but also with increased sensitivity to the effects of exenatide on the regulation of food intake and weight loss.97 It is possible that effects of exenatide on glucose tolerance and appetite regulation could be associated with an improvement of sleep duration and quality in patients with disordered sleep, with or without diabetes.
Consistent with preclinical studies indicating that exenatide may have protective and/or regenerative effects on the β-cells in the pancreas, it appears GLP-1 receptor agonism may have similar effects in the brain. Glucagon-like peptide-1 receptors are located in the brain, where both GLP-1 and exenatide gain access via the circumventricular organs. In addition, GLP-1 receptor-knockout mice display reduced synaptic plasticity and disordered learning and memory, suggestive of a role for GLP-1 receptors in normal neural function.106 Glucagon-like peptide-1 receptor agonism has been shown to have neurotrophic and neuroprotective effects on neuronal cell types, including the promotion of neurite outgrowth in cultured cells,100,101 protection of cultured neurons from apoptosis induced by trophic factor deprivation,102 oxidative insult, amyloid-β (Aβ) peptide exposure, and excitotoxic stimulation.107 Furthermore, in animal models of Alzheimer’s disease, GLP-1 receptor agonism has been shown to reduce levels of Aβ peptide in the brain and reduce oxidative damage.108 In a mouse model of Parkinson’s disease [1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)], exendin-4 protected dopaminergic neurons against MPTP-induced neurodegeneration, thereby preserving dopamine levels and improving motor function.109 Together, these findings, as well as a growing body of additional data, provide the basis for speculation that GLP-1 receptor agonism may have beneficial effects in patients with neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease.
The incretin-based therapies are currently labeled for the treatment of patients with T2D. However, the reported improvements in extraglycemic effects of the class render incretin-based therapies, particularly the longer-acting GLP-1 receptor agonists, as potential candidates for the treatment of individuals at risk of not just prediabetes and the metabolic syndrome, where weight and cardiovascular outcomes are key concerns, but also a number of additional disease states, including sleep disorders and neurodegenerative disease (Fig. 1).
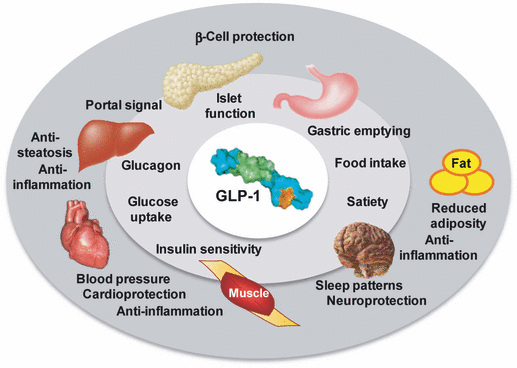
Glucagon-like peptide-1 (GLP-1) has established effects on glycemic control and body weight, and is reported to have positive impacts on cardiovascular risk, inflammation, sleep, and hepatic health. In addition, GLP-1 has been reported to have neuroprotective, neurotrophic, and cardioprotective effects. (See text for details).
Conclusions
The emergence of the first incretin-based therapies, exenatide and sitagliptin, has impacted the treatment of T2D such that they have become important considerations in the treatment armamentarium. These two pioneer therapies are now followed by additional DDP-4 inhibitor agents and GLP-1 receptor agonists as the incretin-based therapies become increasingly established. Both classes exhibit important glucose-lowering effects and unique positive attributes. Drugs in the DPP-4 inhibitor class are administered orally and exhibit good tolerability and an acceptable safety profile, with the attraction of a low hypoglycemic potential; in addition, they are weight neutral. The GLP-1 receptor agonists are injectable, have short-term gastrointestinal tolerability effects, but appear to have more glucose-lowering potential than the DPP-4 inhibitors. They elicit significant weight loss in many patients and are associated with positive effects on cardiovascular risk factors.
The GLP-1 receptor agonist class holds great promise with the introduction of once-daily therapy (liraglutide) and the possibility of once-weekly and even once- monthly platforms in development. Long-term clinical data are required to determine whether the potential positive effects of incretin-based therapy on β-cell health and the cardiovascular system are fully realized.




