Successful Isolated Liver Transplantation in a Child with Atypical Hemolytic Uremic Syndrome and a Mutation in Complement Factor H
Abstract
A male infant was diagnosed with atypical hemolytic uremic syndrome (aHUS) at the age of 5.5 months. Sequencing of the gene (CFH) encoding complement factor H revealed a heterozygous mutation (c.3644G>A, p.Arg1215Gln). Despite maintenance plasmapheresis he developed recurrent episodes of aHUS and vascular access complications while maintaining stable renal function. At the age of 5 years he received an isolated split liver graft following a previously established protocol using pretransplant plasma exchange (PE) and intratransplant plasma infusion. Graft function, renal function and disease remission are preserved 2 years after transplantation. Preemptive liver transplantation prior to the development of end stage renal disease is a valuable option in the management of aHUS associated with CFH mutations.
Abbreviations:
-
- URTI
-
- upper respiratory tract infection
-
- CVL
-
- central venous line
-
- CONS
-
- coagulase-negative Staphylococcus aureus
Introduction
Hemolytic uremic syndrome (HUS) is a clinical syndrome characterized by microangiopathic hemolytic anaemia, thrombocytopaenia and acute renal failure. In the majority of pediatric cases (∼90%) the disease follows an enteric infection with toxin-producing Escherichia coli of the O157:H7 serotype. Approximately half of the patients need renal replacement therapy during the acute phase of disease, but most make a full recovery and the mortality is less than 3% with supportive treatment (1).
The remaining 10% are referred to as atypical HUS (aHUS). Approximately 60% of aHUS are associated with mutations in genes encoding both complement regulators (CFH, CFHR 1 and 3, CFI, MCP/CD46) and activators (C3, CFB), the most frequent of which is CFH (2). Factor H is mainly synthesized in the liver and plays a pivotal role in regulating the alternative complement pathway (3). Mutations in CFH may result in either factor H deficiency or secretion of a mutant dysfunctional protein with impaired ability to control complement activation.
The outcome of aHUS is variable, but those cases due to CFH mutations often have a poor long-term prognosis with a rate of end stage renal disease (ESRD) or death in up to 70–80% (3) as well as extrarenal morbidity, for example, central nervous system involvement (4). Despite lack of clinical trials regular plasma exchange (PE) is the recommended first-line treatment following initial presentation (5). Isolated renal transplantation is associated with high rate of disease recurrence particularly in patients known to have a CFH or CFI mutation and is thus discouraged (6). Initial attempts to cure the underlying defect in children by combined liver-kidney transplantation (CLKT) were associated with a high degree of morbidity and mortality (7,8) The introduction of preoperative PE protocols has allowed this procedure to be undertaken successfully and this has been shown to prevent aHUS recurrence (9–12).
We describe a case of successful isolated liver transplantation in a child with aHUS and a heterozygous CFH mutation with prevention of recurrent aHUS and preservation of native renal function.
Case Report
Background
The patient is the second child of nonconsanguineous parents of Asian origin with no previous history of renal disease. Pregnancy and birth were uneventful. At the age of 5.5 months, after an episode of respiratory tract infection, he presented with malaise, anorexia and increased respiratory effort. On clinical examination he was apyrexial, pale, dyspnoeic and tachycardic. Laboratory investigations confirmed hemolytic anemia, thrombocytopenia and renal failure with nephrotic-range proteinuria.
Complement studies performed before first PE revealed a normal C3 level, supranormal levels of factor H (758.6 mg/L; n: 345–590 mg/L), normal expression of CD46 and no evidence of antifactor H autoantibodies. Mutation screening of CFH revealed a heterozygous mutation (c.3644G>A, p.Arg1215Gln) in CFH exon 23 (encodes short consensus repeat [SCR] 20) which has been described previously (13). Neither of his parents nor his older sister were found to carry the same change.
Immediately after admission alternate day hemodialysis and PE were commenced with an initial exchange volume of ca. 90 mL/kg Octaplas® with each session. The change to plasma infusions (60 mL/kg/week) 2 weeks later was followed by a relapse 1 month after first presentation (see Table 1). The subsequent course was complicated by a total number of four further aHUS flares with sepsis being an important trigger factor (see Table 1).
| Relapse | Maintenance PE after relapse | ||
|---|---|---|---|
| Age (months) | Trigger | Volume (mL/kg) | Frequency (times per week) |
| 6.5 | Plasma infusions started 3 weeks prerelapse | 45 | 3 |
| 8 | URTI, CVL change 5 days before | 45 | 3 |
| 11 | No obvious cause | 63 | 3 |
| 22 | CVL sepsis (CONS), PE reduced from 63 to 45 mL/kg twice weekly 1 week before | 63 | 3 |
| 61 | CVL sepsis (CONS) | 60 | 3 |
- CVL, central venous line; CONS, coagulase-negative Staphylococcus aureus; URTI, upper respiratory tract infection.
C3 levels remained always within the reference range. Postrelapse plasma therapy was guided by disease activity and consisted of a 5–7-day course of daily exchange followed by an alternate day maintenance protocol. There was partial recovery of renal function between theses episodes. Persistent arterial hypertension necessitated combination therapy (ACE inhibitor, betablocker and calcium antagonist). Despite prophylactic warfarin and antimicrobial lock solutions (e.g. taurolidin) multiple episodes of sepsis and thrombosis increasingly compromised his vascular access. A superior vena cava (SVC) stenosis required angioplasty. This vascular problem appeared as the limiting factor for the management of the patient with PE in the medium and long term. Infusion-related reactions (generalized rash and cardiovascular symptoms) occurred but were controllable with steroid and antihistamine priming prior to further fresh frozen plasma application.
Transplant Assessment and Risk-Benefit Considerations
Consequently, a multidisciplinary risk and transplant assessment was undertaken.
Vascular imaging studies including computed tomography and direct angiography confirmed the occlusion of important parts of the supracardiac venous system putting the patient at imminent risk of PE failure during future episodes of aHUS. This prompted an evaluation of definitive therapeutic options, either isolated liver transplantation or combined transplantation of liver and kidney. Renal dysfunction was considered moderate with an estimated glomerular filtration rate (GFR) of 56 mL/min/1.73 m2 (Schwartz equation). A kidney biopsy displayed 20% glomerulosclerosis and mild-to-moderate interstitial fibrosis.
The risk of a deterioration of native renal function after isolated liver transplantation was considered to be significantly less than the risk of failed vascular access associated with waiting until combined liver-kidney transplant was indicated. The patient was therefore listed for isolated liver transplantation.
Transplant episode
Seven months later the patient was admitted for transplantation. Immediately before transplantation he underwent PE with 800 mL of fresh frozen plasma (patient weight 16.4 kg). An orthotopic deceased donor liver transplant was undertaken in which he received a split segment 2/3 graft weighing 537 g. During the procedure he was transfused another 1650 mL of fresh frozen plasma, 200 mL of platelets, 800 mL of packed red blood cells plus 500 mL of Gelfusin (Braun Medical Ltd., Sheffield, UK). The cold ischemic time was documented as 8 h, the warm ischemic time as 24 min. The transplant itself was performed without any surgical or circulatory problems.
Posttransplant course
Anticoagulation was undertaken according to our routine posttransplant protocol with intravenous Heparin immediately postoperatively and oral Acetylsalicylic acid (3 mg/kg/day once daily) and Dipyridamol (50 mg three times per day) for 3 months posttransplantation. The posttransplant immunosuppression consisted of Tacrolimus (levels of 7–9 ng/L during the first 2 weeks, levels between 3–5 ng/L during week 3–8 and 2–4 ng/L >8 weeks after transplantation), Mycophenolate Mofetil (MMF, 20 mg/kg twice daily), Prednisolone (2 mg/kg during first week, then weaned to 0.25 mg/kg/day once daily through 8 weeks) and Daclizumab (1 mg/kg on day 1, 4 and 18 after transplantation).
Early graft function was good with normal intra- and postoperative ultrasound doppler studies. Plasma lactate concentration and prothrombin time were normal within 48 h. There was no evidence of recurrent aHUS in the postoperative period and further plasma therapy was not required. CFH concentration remained within reference range 2 months after transplantation (724 mg/L).
The posttransplant course was complicated by hypertensive encephalopathy requiring transient intravenous Labetalol and symptoms of SVC obstruction, which responded to SVC angioplasty. Blood pressure was controlled long term with four antihypertensive agents.
Two and half months after transplantation he presented with blood-stained diarrhea. Investigations showed no evidence of recurrent aHUS. Bacterial and viral screening showed only a low-grade EBV viremia (5000 cps/mL). Acetylsalicylic acid was discontinued 3 months after transplantation while the MMF was continued. The diarrhea resolved spontaneously.
Ten months after transplantation he was readmitted with a short episode of Norovirus gastroenteritis and transient prerenal dysfunction. His symptoms resolved within a few days. Again there was no evidence of recurrent aHUS.
At present, 2 years after transplantation, his liver function is normal. He remains on triple immunosuppressive therapy. His native renal function is stable (Figure 1) with a 51Cr-EDTA clearance of 51 and 53 mL/min/1.73 m2 at 1 and 2 years, respectively posttransplantation. His blood pressure is well controlled using combination treatment.
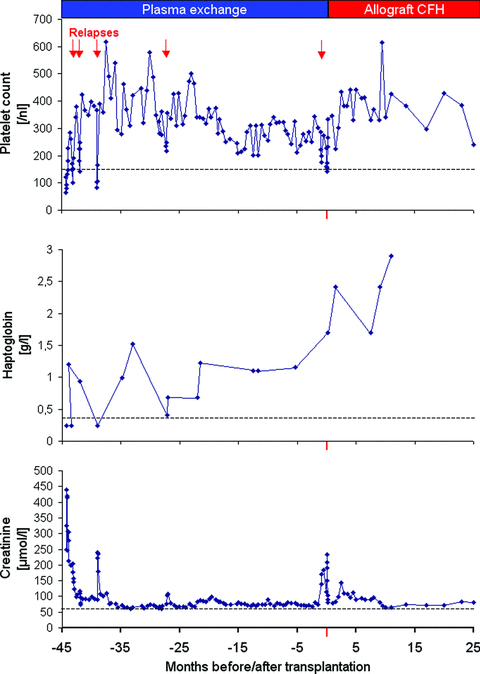
Course of disease and renal dysfunction before and after isolated liver transplantation.
Discussion
Atypical HUS is a severe disease, which in a significant number of patients results in ESRD despite aggressive treatment. Approximately 30% of patients with aHUS have mutations in CFH (2). More than 100 distinct mutations have been reported to date, most of them heterozygous. Our patient was known to have a heterozygous mutation in SCR 20 (c.3644G>A, p.Arg1215Gln). This is part of the carboxy-terminal recognition domain of factor H and is a region where mutations associated with aHUS cluster. Mutations in this region have been shown to result in impaired protection of host vascular endothelial cells against complement-mediated injury (13) while plasma complement regulatory activity is maintained.
Even in the absence of controlled trials PE is currently the recommended initial treatment for aHUS by replacing dysfunctional mutant CFH with normal CFH. It is associated with a significant decrease in mortality (3) and has been successful in inducing short-term remission, maintaining renal function and preventing relapse (5). Success of PE is very much dependent on CFH replacement through adequate exchange volumes and treatment frequency. In our patient (Table 1), the first relapse was triggered by a change to plasma infusions resulting in a lower CFH dose, the fourth one by reduction of PE volume and frequency. Lifelong maintenance and reintensification during triggering events (e.g. infective episodes) are important. Plasma-dependent patients may develop unresponsiveness after long-term therapy (3). Our patient's experience shows that plasma therapy is not always successful. He experienced significant adverse events including recurrent disease, infusion-related reactions and catheter-related complications. His pretransplant treatment was onerous and had a significant negative impact on his and his family's quality of life.
Isolated renal transplantation is associated with a high rate of disease recurrence and graft failure in patients with abnormalities in circulating complement components, especially in those with CFH and CFI mutations (2) and has thus been discouraged in this group of patients (6).
Because factor H is predominantly synthesized in the liver, CLKT seems to be a logical procedure in patients with CFH mutations and a severe aHUS phenotype. The concept is that liver transplantation will restore and normalize plasma wild-type CFH sufficiently to competitively inhibit the small amount of dysfunctional-mutant CFH synthesized by the endothelium. Recurrences with subsequent renal graft loss could thus be prevented. Nonetheless, initial reports of this procedure were associated with a high morbidity and mortality (7,8) assumed to be caused by uncontrolled complement activation during the procedure. Saland and others have since reported successful CLKT by using preoperative PE and intraoperative plasma transfusion (9–12). This modified approach both removes mutant factor H present before surgery preventing possible antagonism of transfused wild-type CFH and supplies normal factor H in the vulnerable postoperative period prior to recovery of synthetic graft function. Seven out of eight procedures following this protocol (whole and split-liver grafts) have been carried out successfully since with short-term survival rates similar to CLKT for other indications (3). Cheong et al. previously reported an isolated auxiliary partial liver transplantation in a patient with factor H deficiency. The transplant protocol did not include PE. The patient finally died 11 months after transplantation due to bacterial sepsis after an episode of EBV-related lymphoproliferative disease treated with Rituximab 3 months after transplantation. (14).
Our case demonstrates the first successful isolated liver transplantation prior to the development of ESRD with a 2-year recurrence-free follow-up. Transplantation restored wild-type CFH bioavailability, removed the need for PE and has protected his native kidneys to date. This concept of preemptive orthotopic liver transplantation (OLT) follows our experience with primary hyperoxaluria type 1 (15): selected patients with mild-to-moderate chronic kidney disease (CKD), who timely receive an isolated liver graft, avoid the metabolic and social consequences of ESRD and renal replacement therapy while postponing, and possibly preventing, the eventual need for renal transplantation. Early transplantation of OLT candidates with renal dysfunction may indeed have a beneficial effect on intermediate-term renal function (16).
The decision to undertake an isolated liver as opposed to a CLKT in a patient with preexisting mild-to-moderate renal dysfunction needs to be based on a detailed risk-benefit analysis. In view of the scarcity of deceased-donor organs it is crucial to discriminate potential liver transplant patients with a high risk for early evolution to Stage 4 or 5 CKD needing combined transplant from those likely to maintain stable renal function after transplantation who do not need renal transplantation. This is hampered by the lack of a reliable model to predict renal function after successful liver transplantation.
There is consensus (17) that the duration and severity of renal disease pretransplant as well as calcineurin inhibitor-use posttransplant are important determinants of renal outcome after liver transplantation. In view of an expected posttransplant decline of GFR by 30 to 40% on standard calcineurin inhibitor immunosuppression, a pretransplant GFR of <40 mL/min and/or more than 30% fibrosis and glomerulosclerosis on kidney biopsy would indicate CLKT (17).
Evaluation of vascular access is a vital part of transplant assessment in children with a previous history of long standing central venous access. Similar to patients with intestinal failure assessed for intestinal transplantation failure of vascular access may become the major indication as in this case who had SVC obstruction.
In our patient, the comparable long-term outcome between isolated liver transplantation and CLKT had to be balanced against the risk of vascular access failure while waiting until CLKT was indicated. As he was considered to have a high likelihood of dialysis-free posttransplant survival beyond 1 year after liver transplantation, he was subsequently listed for isolated OLT. This approach follows our institutional protocol, which restricts CLKT to those patients expected to require kidney transplantation within 12 months of OLT.
We addressed the issue of tacrolimus-induced nephrotoxicity with the implementation of a calcineurin-inhibitor minimization protocol based on the use of IL2R-blockers and mycophenolate mofetil which allowed rapid reduction in tacrolimus dosing to 12-h trough levels of 2–4 ng/L. Aggressive blood pressure control and monitoring of further cardiovascular risk factors (triglycerides, blood sugar) were considered important.
Our patient suffered significant postoperative morbidity related to his existing vascular access difficulties and preexisting hypertension. With hindsight earlier transplantation might have ameliorated some of these. We thus feel that isolated liver transplantation warrants early consideration in the course of disease if it is to successfully protect and maintain native kidney function.
Our experience supports the efficacy of the protocol of preoperative PE and also confirms that split liver grafts can be used successfully in aHUS (12). We used a similar postoperative anticoagulation protocol to that used by Saland et al. However, this protocol is in common use in our program for liver transplantation for other indications, and we are uncertain if it has any specific benefits in transplantation for aHUS.
Further therapeutic options for aHUS may become available in the near future including human-plasma derived complement factor H as well as recombinant complement regulators modulating key activating components of the complement cascade. Eculizumab (Soliris®; Alexion Pharmaceuticals, Inc. Cheshire, CT) is a humanized monoclonal antibody with high affinity to C5 complement protein, the main mediator of the complement effector pathway. There is increasing evidence regarding its safety and efficacy in treating patients with recurrent aHUS, both in the setting of preserved native kidney function (18) as well as for recurrence postrenal transplant (19,20). Orphan designation (EU/3/09/653) was granted by European Medicines Agency in July 2009.
With the emergence of a new era of medical treatment, the possible role of transplantation in aHUS obviously warrants critical reflection. In contrast to Eculizumab, liver transplantation is effectively a curative approach as it is for a number of hepatic-based metabolic diseases. The associated immediate perioperative risks are acceptable and the long-term outcome for pediatric liver transplantation in general is excellent. To date, however, there is no long-term liver transplant experience in aHUS. The first results regarding the use of Eculizumab in aHUS are encouraging, although treatment will be lifelong and requires adequate venous access. Data regarding the long-term safety and efficacy in children with aHUS are unlikely to be available in the short term. These will address critical questions such as the emergence of anti-Eculizumab antibodies, Eculizumab dependence and recurrence of aHUS under therapy. The latter will be of particular interest as any single recurrence could potentially jeopardize the function of the native or transplanted kidney.
In summary, we describe a child with aHUS associated with a CFH mutation who underwent successful isolated liver transplantation with preservation of native renal function and an excellent short-term outcome.
Conclusion
We suggest that isolated liver transplantation should be considered as a treatment option for patients with aHUS and CFH mutations correlated with a more severe disease phenotype when PE is ineffective or accompanied by complications.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Acknowledgments
The authors would like to thank V. Frémeaux-Bacchi, Hopital Européen George Pompidou, Paris, for undertaking the factor H mutation study.
Funding source: THJ Goodship is supported by the UK Medical Research Council (Grant G0701325).




