Distinct responses of human peripheral blood cells to different misfolded protein oligomers
Funding information
Financial support was provided by the Spanish Ministry of Economy (RTI2018-098432-B-I00 to D.P. and C.R.), the Regional Ministry of Economy (PAIDI2020 CTS-677 to D.P.), Fundación ‘Ramón Areces’ (conv. 2018 to C.R.), Andalusian Regional Government-FEDER (US-1265227 to C.R.), Programa ‘Ramón y Cajal’ of the Spanish Ministry of Economy (RYC-2017-23127 to C.R.), FEBS (Short-Term Fellowship to B.M.), EMBO (ASTF 450-2013 to B.M.) and the Italian Society of Biochemistry and Molecular Biology (B.M.). This work was also supported by the Cambridge Centre for Misfolding Diseases, UK (B.M., M.V., C.M.D.).
Abstract
Increasing evidence indicates that peripheral immune cells play a prominent role in neurodegeneration connected to protein misfolding, which are associated with formation of aberrant aggregates, including soluble protein misfolded oligomers. The precise links, however, between the physicochemical features of diverse oligomers and their effects on the immune system, particularly on adaptive immunity, remain currently unexplored, due partly to the transient and heterogeneous nature of the oligomers themselves. To overcome these limitations, we took advantage of two stable and well-characterized types of model oligomers (A and B), formed by HypF-N bacterial protein, type B oligomers displaying lower solvent-exposed hydrophobicity. Exposure to oligomers of human peripheral blood mononuclear cells (PBMCs) revealed differential effects, with type B, but not type A, oligomers leading to a reduction in CD4+ cells. Type A oligomers promoted enhanced differentiation towards CD4+CD25HighFoxP3+ Tregs and displayed a higher suppressive effect on lymphocyte proliferation than Tregs treated with oligomers B or untreated cells. Moreover, our results reveal Th1 and Th17 lymphocyte differentiation mediated by type A oligomers and a differential balance of TGF-β, IL-6, IL-23, IFN-γ and IL-10 mediators. These results indicate that type B oligomers recapitulate some of the biological responses associated with Parkinson's disease in peripheral immunocompetent cells, while type A oligomers resemble responses associated with Alzheimer's disease. We anticipate that further studies characterizing the differential effects of protein misfolded oligomers on the peripheral immune system may lead to the development of blood-based diagnostics, which could report on the type and properties of oligomers present in patients.
Abbreviations
-
- AD
-
- Alzheimer's disease
-
- ConA
-
- concanavalin A
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- PBMCs
-
- peripheral blood mononuclear cells
-
- PD
-
- Parkinson's disease
INTRODUCTION
Imbalance of immune/inflammatory responses within the central nervous system (CNS) is a key factor in the aetiology of neurodegenerative diseases [1-5. Resident CNS immune cells including microglia, astrocytes and oligodendrocytes, as well as perivascular/meningeal macrophages and infiltrating borne-blood immunocompetent cells, are often associated with neuronal damage [3, 5. However, it is still difficult to characterize accurately the corresponding causative processes leading to neuronal death. This challenge is mainly due to a regional compartmentalization of glia and other immunocompetent CNS cells that might result in either deleterious, protective or bystander, functional phenotypes [1, 2. In addition, increasing evidence is establishing the notion that peripheral immune cells play a prominent role in neurodegenerative processes [4. For example, it has been shown that neuroinflammation induced by Parkinson's disease-linked α-synuclein, a protein associated with Parkinson's disease, requires peripheral monocyte infiltration [6. Furthermore, peripheral inflammatory stimulations have been recently shown to reprogramme microglial cells and modify Alzheimer's disease and other neurodegenerative conditions [7, 8. Therefore, peripheral immune responses might not only drive neuropathological diseases but also imprint resident CNS immune cells with long-lasting functional phenotypes [9, 10.
Another hallmark of neurodegenerative diseases, including Alzheimer's disease (AD), amyotrophic lateral sclerosis (ALS) and Parkinson's disease (PD), is the presence of aberrant deposits of misfolded proteins [11, 12. Although these deposits are the largest species in protein aggregation, intermediate soluble oligomeric species are thought to be the most detrimental ones for cellular functions, involving several mechanisms of action [13-18. In particular, a wide range of oligomers of disease-associated proteins, including amyloid-β (Aβ) and α-synuclein, are found extracellularly [11, 12. Such oligomers have been shown to mediate microglial-associated innate immunity in CNS by eliciting danger-associated molecular pattern (DAMP) signalling pathways [8, 15, 19. Indeed, elevated levels of proinflammatory cytokines and chemokines have been detected in the plasma and cerebrospinal fluid (CSF) of patients with AD [26, 27, PD [27, 28, ALS [17, 29 and Huntington's disease (HD) [30.
Besides immune alterations in CSF [31-36, increased levels of pathogenic oligomers haven been also found in plasma of patients affected by neurodegeneration and related to disease progression [37-42. The mechanisms involved in the oligomer efflux from the CNS to the periphery and its clearance in the periphery are unclear [43, 44, but remarkably, these soluble oligomeric species have been shown to be biologically active, inducing immunological responses in peripheral immune cells [45-49. Despite the fact that peripheral immune cells might provide an opportunity for immune manipulation and a better understanding of the neurodegenerative processes, there are virtually no studies in humans using soluble oligomers that precede the formation of mature fibrils, especially those related to functional responses of peripheral T cells and/or T-cell subsets.
Oligomers from different proteins with no sequence similarity usually share several structural characteristics, high affinity to conformation-specific antibodies, small size compared with amyloid fibrils and hydrophobic surfaces [50-52. While it has been suggested that these hydrophobic surfaces typically present in misfolded protein aggregates might be a major driver of innate immune responses [13, 53, more general links between the physicochemical features of diverse oligomers and the effects on immune system, in particular adaptive immune responses, are still largely unknown. There are some constraints that might explain this limited information on disease-associated oligomer structure and immune functional correlations including their low deficient stability during experimental assays, the absence of comparable studies carried out simultaneously and challenging structural characterization. Some of these limitations can be overcome by using the misfolded oligomers of the N-terminal domain of the HypF protein from E. coli (HypF-N) as a working model [17, 56, 57. Valuable characteristics of HypF-N oligomers are as follows: a) HypF-N is readily able to form oligomers, protofibrils and amyloid-like fibrils into structured species similar to those formed by proteins related to human diseases state [17, 57; (b) HypF-N oligomers behave similar to protein oligomers associated with amyloid diseases in terms of cell viability impairment when added in vitro to different cell lines and primary cell cultures, as well as in vivo in the CNS [56, 61; and (c) an extensive array of diverse experimental conditions is available for functional studies, as HypF-N can rapidly be converted into stable rather than transient oligomers, maintaining their structure and properties even when they are transferred to conditions very different from those that initially promoted their formation [17, 64. Because of these favourable features, HypF-N oligomers have been previously exploited successfully to establish structural and functional correlations between innate immune responses and protein aggregation in microglial cells [13. Two types of stable HypF-N oligomers that are formed in vitro under different conditions (A and B) were shown to induce different degrees of cell toxicity and to differ in the interaction with the cell membrane, even though they have apparently the same thioflavin T binding capacity – typical of amyloid fibrillar structures – and display spherical morphologies with different size diameters in the range of 2–6 nm [17. However, structural analysis of type A and type B HypF-N oligomers (referred to as type A or B oligomers) has demonstrated a differential organization of the core region, with type A oligomers showing a higher degree of surface hydrophobicity compared with type B oligomers.17
In the present work, we explore for the first time the effects of the well-characterized HypF-N oligomers on human peripheral immune cells. Remarkably, type A and type B oligomers displayed differential immunoregulatory mechanisms associated with the balance of key cytokines such as IFN-γ, IL-6, IL-10 IL-23 and TGF-β, T regulatory cell function and Th1/Th17 differentiation. Our data shed light into the principal leading characteristics associated with immune imbalance in neurodegenerative misfolding diseases and provide immune-based tools that can be potentially useful for the development of diagnostic approaches to predict disease progression and to monitor therapies of neurodegenerative diseases.
MATERIALS AND METHODS
Samples
Peripheral blood mononuclear cells (PBMCs) were obtained after informed consent from eight healthy volunteers aged 26–45 years. Participants were additionally screened by physical examination and laboratory studies. All donors were considered non-demented, non-depressed healthy individuals on the basis of their former medical records and somatic and neuropsychiatric examination. None of the volunteers was taking medications.
Preparation of type A and B oligomers
Wild-type and C7S/C65A HypF-N were purified as described previously [13, 17. Incubation of the purified native protein with polymyxin B-agarose to eliminate bacterial endotoxins was performed as previously described [25, 65. Protein preparations were tested for lipopolysaccharide (LPS) presence according to the toxin sensor limulus amebocyte lysate (LAL) assay kit (GenScript, Piscataway, NJ), and contamination found to be negligible in all samples (<0·02 EU/mg protein). Protein purity was assessed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) at >95%. Type A and type B oligomeric aggregates were prepared by incubating the protein for 4 h at 25℃ at a concentration of 48 µM in two different conditions: (i) 50 mM acetate buffer, 12% (v/v) trifluoroethanol (TFE) and 2 mM dithiothreitol (DTT), pH 5·5 (condition A) and (ii) 20 mM trifluoroacetic acid (TFA) and 330 mM NaCl, pH 1·7 (condition B) [13, 17. The oligomers were centrifuged at 16,100 xg for 10 min, dried under a gentle flow of nitrogen and resuspended in RPMI-1640 (Roswell Park Memorial Institute Medium, Sigma, St. Louis, MO, USA). Stock solutions of the oligomers were further diluted in the same medium to reach the desired protein concentrations.
PBMC isolation
Blood samples were diluted in phosphate-buffered saline (PBS, 1:1), gently layered on Ficoll solution (Ficoll–Paque PLUS, GE Healthcare Bio-Sciences AB, Uppsala, Sweden) at room temperature and centrifuged at 700xg for 30 min. The PBMC-containing layer was collected and washed twice in PBS plus 0·1% (w/v) bovine serum albumin (BSA). The viable cells, as determined by trypan blue exclusion, were resuspended at the concentration of 2 × 106 cells/mL in medium composed of RPMI-1640 supplemented with L-glutamine (2 mM), penicillin (100 U/mL), streptomycin (100 μg/mL) and 10% of heat-inactivated fetal bovine serum (Sigma, St. Louis, MO, USA).
PBMC treatments
Stimulation of PBMC cultures in 12-well plates was performed by adding 500 µL of cell suspension and 500 µL of type A or B oligomers diluted in complete RPMI at different concentrations or medium alone in the case of untreated controls for 24 h.
Cellular viability
Cell viability was assessed by measuring the ability of cells to metabolize tetrazolium dye MTT into formazan. The cells were seeded onto 96-well plates at a density of ∼2x105 cells/well in culture medium prior to the initiation of experimental treatments. Following the treatments as indicated, 10 μL of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) (final concentration 0·5 mg/ml) was added to each well and, after further incubation for 4 h in a humidified atmosphere (37℃, 5% CO2), 100 μL of solubilizing solution (50% dimethylformamide and 20% SDS, pH 4·8) was added. After an overnight incubation in a humidified atmosphere after complete solubilization of the formazan crystals, spectrophotometric absorbances were determined using a microplate reader at 595 nm.
CD8+ lymphocyte flow cytometry analysis
Treated PBMC cells were collected and washed twice with staining buffer PBS (plus 2% (v/v) fetal bovine serum and 2mM ethylenediaminetetraacetic acid (EDTA)). Then, cells were resuspended in staining buffer and detected with anti-CD8 antibodies labelled with fluorescein isothiocyanate (FITC) for 20 min at room temperature. After surface staining, cells were fixed in cold PBS containing 4% p-formaldehyde for 15 min at 4℃ and then washed in PBS. Cells were resuspended in staining buffer, and flow cytometry analysis was performed. Flow cytometry analysis was performed with fluorescence-activated cell sorting (FACS) using a FACSCalibur cytometer and the CellQuest Pro (BD Biosciences, San Diego, CA, USA) software.
Regulatory T-cell (Treg) flow cytometry analysis
Treated PBMCs were collected and washed twice with PBS and labelled with anti-CD4-FITC, anti-CD25-APC and anti-Foxp3-PE antibodies (BD Bioscience, San Diego, CA, USA) following the manufacturer's instructions of the human Foxp3 Buffer Set (BD Bioscience). The CD4+ cell population was calculated as the percentage of cells positive for CD4 within the total lymphocyte population. The Treg cell population was calculated as the percentage of cells positive for CD4, CD25, and Foxp3 staining (CD4+ CD25+ Foxp3+) among the total lymphocyte population. Flow cytometry analysis was performed with FACSCalibur cytometer and CellQuest Pro (BD Biosciences) software.
Th1/Th17 subset flow cytometry analysis
PBMCs were treated as described, and five hours before collecting the cells, ionomycin and phorbol 12-myristate 13-acetate (PMA) (Sigma) were simultaneously added to a final concentration of 500 ng/mL for ionomycin and 50 ng/mL for PMA, respectively, in addition to a protein transport inhibitor containing monensin (GolgiStopTM, BD Biosciences). After incubation for a total of 24 h, cells were collected and then labelled with anti-CD4-FITC, anti-IL-17-APC, anti-IFN-γ-PE and anti-IL-17-Alexa Fluor 647 (BD Bioscience, San Diego, CA, USA). Briefly, cells were washed twice with staining buffer, PBS plus 2% (v/v) fetal bovine serum and 2 mM EDTA. Before staining, cells were blocked with 20% (v/v) human commercial serum to avoid non-specific binding of antibodies to Fc receptors and subsequently labelled for surface markers in staining buffer (anti-CD4-FITC). After 20 min of incubation at room temperature, cells were washed twice and were fixed in cold PBS containing 4% p-formaldehyde for 15 min at 4℃ and then washed in PBS prior to being permeabilized with BD Perm/Wash buffer by following the manufacturer's instructions. Then, PBMCs were labelled for intracellular markers (anti-IL-17-APC and anti-IFN-γ-PE). After labelling, cells were resuspended in staining buffer and flow cytometry analysis was performed. The helper T1 (Th1) cell population was calculated as the percentage of cells positive for IFN-γ staining (CD4+IFN-γ+) among the CD4+ lymphocyte population, and the helper T17 (Th17) cell population was calculated as the percentage of cells positive for IL-17 staining (CD4+IL-17+) among the CD4+ lymphocyte population. In all cases, isotype-matched antibodies were used as controls. Flow cytometry analysis was performed with a FACSCalibur cytometer using the CellQuest Pro (BD Bioscience, San Diego, CA, USA) software.
Cytokine release measurements
After treatment and incubation of cells for a total of 24 h, as explained in the specific section ‘PBMCs treatments’, culture supernatants were harvested and centrifuged at 700xg for 5 min. The supernatants from treated cultures were collected and stored at −80℃ before cytokine determination. IL-6, TNF-α, IL-1ß, IL-10, IL-23 and IL-17 levels were assayed by using the human BD OptEIA ELISA set specific for each cytokine (BD Biosciences, Franklin Lakes, NJ, USA) according to the manufacturer's protocols.
Regulatory T (Treg)-cell proliferation suppression studies
After primary stimulation of PBMCs for 24 h with 0·5 μM type A or type B HypF-N oligomers at different concentrations, CD4+ T cells (purity of 94–98%) were isolated by positive selection from total PBMC by using the CD4 isolation kit (Miltenyi Biotec). Isolated cells were labelled with anti-CD4-FITC, anti-CD127-PE and anti-CD25-APC. CD4+CD127lowCD25+ T cells were sorted by using a FACSAria cytometer and CellQuest Pro (BD Biosciences) software. Different ratios (1:0·5; 1;0·1) of sorted Tregs were added to 5x104 PBMCs previously seeded in a 96-well U-bottom plates (Nunc) and stimulated with 2·5 µg/mL concanavalin A, just before adding the sorted Treg cells. After 96 h of incubation, proliferation was analysed by measuring [3H]-thymidine incorporation within the last 8 h of the experiment. Plates were harvested with a Filter Mate, and [3H]-thymidine incorporation was determined by liquid scintillation spectroscopy using a TopCount.
Statistical analysis
Values from experiments carried out at least three times are expressed as the mean ±standard error of the mean (SEM), whereas results from two independent experiments are expressed as mean ±standard deviation (SD). For all the analysed parameters, statistical analyses were performed with the non-parametric Mann–Whitney U (two-sided)-test using the SPSS Statistics 22·0 (IBM Company, Chicago, USA). Only p values lower than 0·05 were considered statistically significant.
RESULTS
Sensitivity of human peripheral immune cells to type A and type B oligomers
Two types of stable HypF-N oligomers, A and B, were produced under different conditions (see Materials and Methods). Both of them have previously been shown to have spherical morphologies with different diameters and found to have amyloid-specific functional properties [17. In addition, type A and type B oligomers were reported to have different effects on their ability to impair cell viability, type A oligomers being toxic to cultured neuronal cells to an extent comparable to that of the oligomers formed by Alzheimer's disease-associated Aβ42· [61 It has been shown that the distinct effects of type A and type B oligomers are due to the different degree of ordered intermolecular packing between corresponding hydrophobic regions of adjacent HypF-N molecules [17, 61. To study the biocompatibility and the effects of the two different types of oligomers on peripheral immune cells, we treated human PBMCs from healthy donors with different concentrations of pre-formed type A and type B oligomers, and measured cell viability by the MTT assay after 24-h incubation (Figure 1a). Our results showed a limited effect of both types of oligomers, with mild reductions in PBMC viability at almost all concentrations tested. Only at very high concentrations (25 μM), oligomers caused around a 20% decrease in cell viability with no differences between type A and type B oligomers (Figure 1a). The effects of the amyloidogenic oligomers are associated with their misfolded structures, as PBMC viability was not altered (>90% of MTT reduction compared with control) at either of the concentrations tested when native HypF-N was used as control (data not shown). We note that type A oligomers were previously shown to cause a 30% reduction in cell viability at 12 μM in microglial cells, while type B oligomers were innocuous at all concentrations tested [13. Although for either microglial [13 or peripheral immune cells, the oligomers do not show considerable toxicity at concentrations below 4·3 and 15 μM, respectively, it appears that peripheral immune cells – represented mostly by lymphocytes – have a different sensitivity towards HypF-N oligomers. Whether this is a phenomenon that differentiates between innate and acquired immune cells remains to be elucidated, as well as its structural bases. All subsequent PBMC experiments were done at fully biocompatible HypF-N oligomer concentrations.
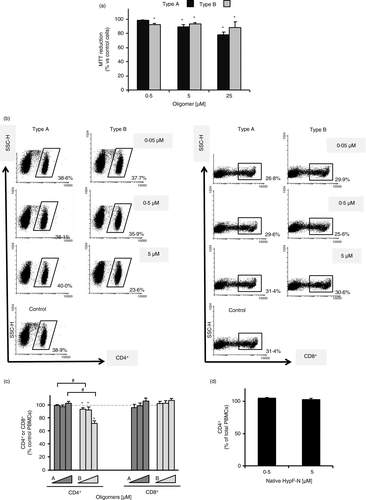
Type B oligomers decrease the populations of CD4+ T cells in PBMCs
Once we verified that PBMC viability was not significantly compromised after exposure to HypF-N oligomers, we studied the impact of these oligomers on human CD4+ and CD8+ T-cell subpopulations (Figure 1b,c). PBMCs were treated with type A or type B oligomers at different concentrations (0·05, 0·5 and 5 μM) for 24 h. After treatment, the number of immunolabelled CD4+ and CD8+ cells within total treated PBMCs was analysed by flow cytometry and compared with that of control (untreated) cells. We found that while type A oligomers did not induce any significant change in the CD4+ subpopulation of cells at any concentration tested compared with control cells, treatment with type B oligomers resulted in a significant reduction in the CD4+ subpopulation at all concentrations tested (Figure 1b,c). Moreover, at the highest concentration (5 μM), the cell viability was 72% ±3·9 compared with untreated cells. On the other hand, the CD8+ subpopulation was not modified by either of the two types of oligomers used (Figure 1b,c). To further verify that the effects seen for type B oligomers were indeed caused by their distinct conformational characteristics, a FACS CD4+ subpopulation control analysis was carried out with the native form of HypF-N (Figure 1d). No changes in CD4+ T cells were found, confirming that the change previously observed was due to the differential effect of type B oligomers.
Type A and type B oligomers differentially modify lymphocyte differentiation to regulatory T (Treg) cells and their function in PBMCs
Because Treg cells are known to be key modulators of a counterbalanced immune response and to play an important role in self-antigen tolerance and in suppressing excessive immune responses in the neurodegenerating CNS [2, 66, we analysed their possible implication in regulating the response associated with the two types of oligomeric species. First, we compared the content of CD4+CD25HighFoxP3+ Treg cells in PBMCs from donors after treatment of cultured cells with the two types of oligomers compared with basal Treg levels without treatment. Flow cytometry analysis revealed an increase of about 20% of Treg cells for PBMCs treated with either type A or type B oligomers at the lowest concentration tested (Figure 2a). However, at higher doses, an increase was observed only as a result of the treatment with type A oligomers (3·27% ±0·3), whereas an inverse dose-dependent effect on the percentage of Treg cells was observed in cells treated with type B oligomers (Figure 2b).
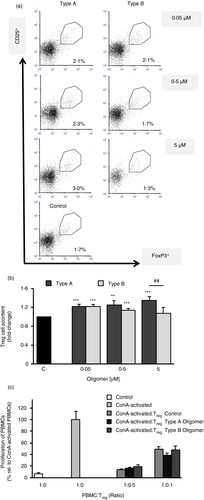
After observing an enhanced differentiation towards Treg cells after the treatment with type A oligomer treatment, the functional suppressive capacity of Tregs was analysed (Figure 2c). We performed a proliferation assay of T cells in the presence or absence of Treg cells and observed that for a ratio of 1:0·1 PBMC:Tregs, type A oligomer-treated Tregs showed a higher suppressive trend on lymphocyte proliferation than Tregs treated with oligomers type B or untreated Tregs, although this change did not reach statistical significance.
Type A and type B oligomers alter the interleukin secretion profile of PBMCs
Cytokine release from PBMCs treated with type A or type B oligomers for 24 h was quantified from cell supernatants by ELISA (Figure 3). We first analysed three key proinflammatory cytokines associated with protein misfolding diseases [1, 2: IFN-γ, which is involved in the activation of macrophages and in differentiation to Th1 cells; IL-6, which is found at higher levels in almost all inflammatory responses and is primarily produced at sites of acute and chronic inflammation and reported to be essential for Th17 differentiation; and IL-23, which plays an important role in the development and maintenance of effector Th17 cells and is a central link between innate and adaptive immune responses [66. Both type A and type B oligomers induced higher levels of IFN-γ compared with untreated cells, with the exception of type A oligomers at the lowest concentration tested, 0·05 μM (Figure 3a). The secretion of IL-6 and IL-23 was stimulated by both type A and type B oligomers in a dose-dependent manner, and at all the concentrations tested, the levels of these cytokines were significantly higher than the untreated cells. As previously observed in microglial cells [13, the proinflammatory response triggered by type B oligomers is more dramatic than the one observed after the treatment with type A oligomers (Figure 3a).

Finally, we analysed IL-10 and transforming growth factor-β (TGF-β), two anti-inflammatory cytokines found to be dysregulated in many neurodegenerative diseases [3, 66. IL-10, with immunoregulatory properties, is able to directly regulate innate and adaptive Th1 and Th2 responses by limiting T-cell activation and differentiation in the lymph nodes and suppressing proinflammatory responses in tissues. Again, secretion of IL-10 by PBMCs treated with type A and type B oligomers was higher for type B oligomers at 0·05 μM and 0·5 μM than for type A oligomers (Figure 3b). However, this difference was less evident at 0·5 μM, while at the highest oligomer concentration (5 μM), the cytokine secretion was comparable for both treatments, showing that even though for both types of aggregates the secretion was dose-dependent, this effect was stronger for type A oligomer treatment (Figure 3b). When TGF-β secretion was analysed, only type A oligomers led to an increase in TGF-β levels for the two lower concentrations compared with control (Figure 3b). Only when PBMCs were challenged with 5 μM of type A or type B oligomers, both treatments increased the secretion of this cytokine, but unexpectedly, this secretion profile was reversed, and type B oligomer -induced levels of TGF-β were higher than type A oligomers (Figure 3b).
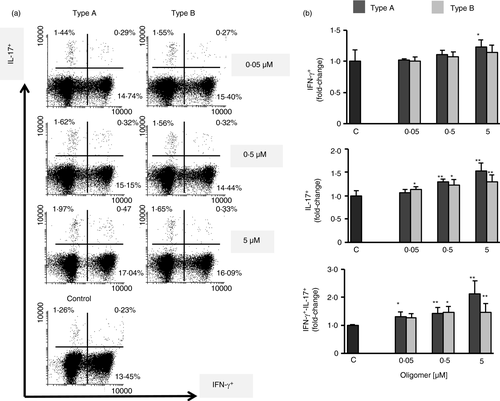
Type A and type B oligomers significantly modulate Th1 and Th17 lymphocyte differentiation
It is known that environmental factors such as systemic inflammation affect CNS homeostasis and prime microglial response, modifying the progression of associated diseases [2, 10. The entry of activated effector T cells, especially Th1 (IFNγ+) or Th17 (IL-17+), into the CNS in which inflammatory changes are ongoing might exacerbate the inflammatory cascade [2, 67. Therefore, we analysed the CD4+ T-cell polarization towards Th1 or Th17 effector cells in lymphocytes treated with type A or type B oligomers (Figure 4). We observed that treatment with both types of oligomers induced differentiation of CD4+ T cells to Th1 and Th17 subsets, as measured by flow cytometry analysis of treated cells (Figure 4a). Of note, even though the differences between the two types of oligomers were not statistically significant, this effect was higher for lymphocytes treated with type A oligomers at all concentrations tested. Furthermore, we noticed incremented numbers of CD4+ T cells that were positive for both IFN-γ and IL-17 and the differences between both oligomers were more evident than in the other subsets, this increase being much higher for type A oligomers than for type B oligomers mainly at the higher doses.
DISCUSSION
Protein misfolded oligomers are currently thought to be the most harmful species leading to different cell dysfunction in several neurodegenerative disease [51, 52, including in particular, by eliciting neuroinflammation [8, 19. In this context, the peripheral immune system is emerging as an important player in the onset and setting of neuroinflammatory responses [4, 6, 9, 10. Indeed, the modulation of the peripheral immune profile has been shown to alter the innate immunity in the CNS in neurodegenerative disease [8. In this sense, the study of the correlation between the structural and functional properties of the oligomers and the peripheral immune responses may not only lead to a better understanding of the disease mechanisms, but also lead to the identification of potential therapeutic and diagnostic approaches. Unfortunately, the low stability and difficult characterization of oligomers have made it very challenging to carry out systematic studies. In the present work, we have exploited the advantages of the well-characterized HypF-N oligomers to analyse, for the first time, peripheral immune responses to highly stable soluble oligomers of two different oligomer types, referred to as type A and type B. Type A and type B oligomers have different biophysical characteristics and have shown to recapitulate biological features of disease-linked oligomers [11, 13, 17, 64, 68, 69.
Our experiments assessing cell viability showed limited effects of both type A and type B oligomers in human PBMCs. Oligomers were biocompatible even at relatively high concentrations. Type A and type B exposure of PBMCs caused a 20% decrease in MTT activity only at very high concentrations (25 μM), with essentially no differences associated with either type A or type B oligomers. We note that our previous observations in microglial cells showed a higher sensitivity and specificity to type A oligomers compared with PBMCs when cell viability was analysed [13. Although further studies are required to explain this difference in terms of structural and functional correlations, our results might imply that acquired and innate immunity cells have differential sensitivities towards amyloid soluble oligomers in terms of cell viability.
The absence of major toxicity effects allowed us to explore the consequences of type A and B oligomers on PBMC functional readouts and cell subsets. Flow cytometry analysis showed that CD8+ T-cell subpopulation was not modified by either of the two types of oligomers used. However, while type A oligomers did not induce any significant change in the percentage of CD4+ subpopulation of cells at either oligomer concentration, the treatment with type B oligomers resulted in a significant reduction in the content of the CD4+ subpopulation at all concentrations tested. Interestingly, a significant reduction in human CD4+ T cells had been reported in the context of PD pathology [70-72 whereas no significant changes have been found in the percentage of viable CD4+ T cells nor increases in proliferative responses after in vitro treatment with Aβ peptides [45, 73. In studies where patients with AD were stratified according to the severity of the disease, it was found that CD4+ and CD8+ T-cell levels were unmodified at a mild stage, whereas CD4+ T cells were increased and CD8+ T cells decreased, in patients restricted to severe stages of the disease [74, 75. An important subset of CD4+ T cells is represented by regulatory T (Treg) cells. Alterations in the frequency and/or suppressive function of peripheral CD4+CD25HighFoxP3+ Treg cells have been associated with disruption of peripheral tolerance leading to autoreactive T cells in the context of CNS neuroinflammation in protein misfolding diseases [2, 66. We found that both type A and B oligomers increased the content of CD4+CD25HighFoxP3+ Treg cells in PBMCs, although only type A oligomers led a trend towards enhancement in Treg-mediated immune suppression of PBMC proliferative response. The higher levels of TGF-β after type A oligomer exposure of PBMCs, compared with type B oligomers, are consistent with the driving role of TGF-β in the process of Treg differentiation, although further experimental evidence is required [76, 77. In this sense, in the context of AD, it has been demonstrated that an increase in peripheral Treg suppressive activity leads to impairment of IFN-γ-mediated clearance of Aβ accumulation and worsen the AD pathology [78-82. Higher levels of peripheral IFN-γ secretion and lower Treg activity, comparable to what we observed after exposure of PBMCs to type B oligomers, have been associated with α-synuclein-mediated effects in preclinical studies [83. Although both type A and type B oligomers promoted a polarization towards Th1 and Th17 cells, the effect was more pronounced for type A oligomers, resembling a characterized phenomenon in AD pathology related to activation of peripheral CD4+ IL-17+IFN-γ+ T cells [84. Even though to what extent CD4+ T-cell subsets are dysregulated in PD is not completely clear, recent studies demonstrated that reduced number of circulating CD4 T cells is due to reduced Th17 and Treg subsets [71, 85, a situation partially mirrored in this study by type B oligomer treatment of human PBMCs. Finally, little is known about peripheral T helper cell subsets in AD or PD and reciprocal counterbalance between Tregs and Th17 cell populations. Recent evidence had shown an increase in Th17 cells, while Tregs remained unaltered in AD patients with mild cognitive impairment or even slighted elevated in mild cognitive impairment patients [86, 87. Thus, an increase in Th17 cell populations might be compatible with a moderate increase or the absence in a counterbalance in peripheral Treg populations. In this sense, we might not rule out the possibility that type A/B oligomers might partly reflect some of the events related to early stages of AD or PD.
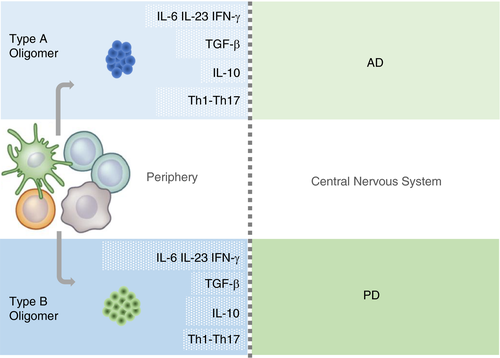
In conclusion, our study provides evidence for the first time about peripheral immune responses to structurally defined protein misfolded oligomers (Figure 5). While type B oligomers recapitulated some of the T-cell biological responses associated with PD in peripheral immunocompetent cells, type A oligomers resembled typical responses associated with AD. These observations might be relevant to develop approaches to investigate the relationships between oligomer structural characteristics and immune-elicited responses, and ultimately establish accurate blood-based diagnostic methods for neurodegenerative disorders.
ACKNOWLEDGMENTS
Financial support was provided by the Spanish Ministry of Economy (RTI2018-098432-B-I00 to D.P. and C.R.), the Regional Ministry of Economy (PAIDI2020 CTS-677 to D.P.), Fundación ‘Ramón Areces’ (conv. 2018 to C.R.), Andalusian Regional Government-FEDER (US-1265227 to C.R.), Programa ‘Ramón y Cajal’ of the Spanish Ministry of Economy (RYC-2017-23127 to C.R.), FEBS (Short-Term Fellowship to B.M.), EMBO (ASTF 450-2013 to B.M.) and the Italian Society of Biochemistry and Molecular Biology (B.M.). This work was also supported by the Cambridge Centre for Misfolding Diseases, UK (B.M., M.V., C.M.D.). MML-S and BM conducted experiments and generated critical reagents. MML-S, BM, FC, MV and CMD analysed data and wrote the paper. CR and DP designed research, analysed data and wrote the paper.
CONFLICT OF INTEREST
The authors state no conflict of interests.
AUTHOR CONTRIBUTIONS
MML-S and BM conducted experiments and generated critical reagents. MML-S, BM, FC, MV and CMD analysed data and wrote the paper. CR and DP designed research, analysed data and wrote the paper.
PERMISSION TO REPRODUCE (FOR RELEVANT CONTENT)
Granted.




