Antibody detection assays for COVID-19 diagnosis: an early overview
Abstract
The ongoing pandemic of coronavirus disease 2019 (COVID-19) has not only commenced a global health emergency but also agitated various aspects of humanity. During this period of crisis, researchers over the world have ramped their efforts to constrain the disease in all possible ways, whether it is vaccination, therapy or diagnosis. Because the spread of the disease has not yet elapsed, sharing the ongoing research findings could be the key to disease control and management. An early and efficient diagnosis could leverage the outcome until a successful vaccine is developed. Both in-house and commercial kits are the preferred molecular tests being used worldwide in the COVID-19 diagnosis. However, the limitation of high prices and lengthy procedures impede their use for mass testing. Keeping the constant rise of infection in mind, the search for an alternative test that is cost-effective, simple and suitable for large-scale testing and surveillance is the need of the hour. One such alternative could be immunological tests. In the last few months, a deluge of immunological rapid tests have been developed and validated across the globe. The objective of this review is to share the diagnostic performance of various immunological assays reported so far in severe acute respiratory syndrome coronavirus 2 case detection. We consolidate the studies (published and preprints) related to serological tests such as chemiluminescence, enzyme-linked and lateral flow-based point-of-care tests in COVID-19 diagnosis and update the current scenario. This review aims to be an add-on in COVID-19 research and will contribute to congregation of the evidence for decision making.
What we know about this disease so far
The world today has been amalgamated like never before to fight a common enemy to humans: the Coronavirus pandemic. Coronaviruses (CoVs) belong to the family Coronaviridae. CoVs are responsible for severe acute respiratory syndrome (SARS) that had first emerged as an epidemic in November 2003. Almost two decades later, a novel coronavirus, SARS coronavirus 2 (SARS-CoV-2), originating in Wuhan city of China in December 2019, has led to an unprecedented global pandemic of coronavirus disease 2019 (COVID-19), a serious health challenge that our modern world was yet to see.1 Despite the same family of viral origin as of SARS, transmissibility and disease severity of COVID-19 are much higher in terms of community spread. As of September 14, 2020, over 29 million confirmed COVID-19 cases have been reported with more than 900 000 deaths worldwide2 and the numbers are mushrooming by the day. After achieving a plateau in the COVID-19 infection graph in China, a shift in the epicenter of the disease is being seen with the astonishing numbers of new cases and deaths in the United States, Brazil, India, Russia and in many European countries.
Several reports of the clinical manifestation and symptoms of the disease have been reported. However, there is limited information regarding the pathogenic mechanism of the disease. Common symptoms of COVID-19 include fever, dry cough, fatigue and pneumonia-like features. The coronavirus is reported to interact with the host epithelial cell receptor, angiotensin-converting enzyme 2 (ACE2), found on outer surfaces of most of the human body organs, and triggers endocytosis into the host cell.3 Disease severity occurs when COVID-19 virus (SARS-CoV-2) enters into the lungs through the oral or nasal passage and proliferates within the lung cells that eventually leads to the cell death. Dead cells may generate an inflammatory response through cytokines such as interleukin-6, interleukin-1, tumor necrosis factor-α, resulting in the consolidation of fluid in the alveolar and interstitial spaces and acute respiratory distress syndrome. The symptomatic clinical condition without ventilation may lead to death because of the insufficiency of oxygen in the vital organs and multiple organ dysfunction (kidney injury and cardiac arrest). Researchers have reported that the virus affects organs other than the lungs and respiratory tract, mostly the liver and kidney, even with mild infection levels.4 Chances of this affecting people of older age are more as a result of their already present comorbidities.
The focus of fighting SARS-CoV-2 has been revolving around the detection of cases, monitoring, prevention of infection and diagnosis and supportive care. A specific global recommended therapy is still not available.5 However, several strategies to combat the pandemic are being implemented throughout the world, and correlations with SARS and Middle East respiratory syndrome (MERS) are being studied thoroughly to understand the potential of previously successful treatment regimes. Previous studies in many viral infections suggested that protective antibodies do not necessarily confer neutralizing activity. Thus, the potential of human antibodies in treatment or in vaccination will depend on the fact that whether antibodies have role in disease progression or in protection from the viral infection.6 Convalescent plasma treatment has shown effective results in both SARS and MERS, wherein a strong T-cell response and higher amount of neutralizing antibodies were found in cured patients who were tested over a prolonged period.5 Based on this information, plasma of convalescent patients is being utilized to treat other critical patients. Studies reported significant reduction in mortality in critical COVID-19 patients and depicted encouraging results with lowered inflammatory response and improvement in function of various organs.7 These data equivocated with improvement in clinical symptoms as previously reported studies of SARS.
Brief introduction of the current diagnosis
In the present scenario, while a vaccine is not available for COVID-19 and there is no short and sure treatment, rapid and extensive diagnosis could be the way to reach the grassroot level of the disease and break the widespread chain of this deadly SARS-CoV-2 infection.8 Diagnosis will have a relevant role in the containment of the disease until a suitable therapy or vaccine strategy is attained. As emphasized by the chief of the World Health Organization on March 16, 2020, just 3 days after declaring COVID-19 a pandemic, the way forward is to “test, test and test.”
Chest radiography was initially used in China as a preliminary test to detect COVID-19-positive patients. However, because of an increasing number of misdiagnosis, it was suggested to be only used as a secondary line of diagnosis for patients already admitted and confirmed positive with molecular or immunological tests.9 From a global perspective, the most popular and gold standard technique that is being used for COVID-19 diagnosis is an initial symptomatic analysis followed by molecular-based nucleic acid detection from sputum, nasal and mouth swab and serum. The COVID-19 bears a single-stranded RNA genome of approximately 30 000 nucleotides. The nucleic acid testing of COVID-19 is based on the reverse transcription-PCR (RT-PCR) technique.10 Several conserved viral genomic regions, such as RNA-dependent RNA polymerase gene (RdRP gene), nucleocapsid protein gene (N gene) and envelop protein gene (E gene), have been targeted in the molecular tests. Because this test can amplify even low levels of the virus and detect the disease at an early stage, it is the most used and reliable detection method. However, the performance of RT-PCR largely depends on the target viral RNA selected and the primers used. The World Health Organization therefore publishes from time to time standard protocols for the parity of the assay. Despite the large number of RT-PCR-based tests that are being conducted throughout the globe, its accessibility to centers in remote areas restricts its mass appeal. Moreover, these tests are expensive, equipment based, time taking and require biological expertise. Missed diagnosis of a large number of clinically suspected individuals may promote the spread of the virus that may ultimately lead to faster disease progression. Moreover, in many cases molecular testing often requires more than one test for disease confirmation.11 RT-PCR is valuable in the initial phase of infection when the virus is present in the body. However, this method has limitation in identifying past, recovered and asymptomatic infections. Most of the countries have been under partial or complete lockdown for almost 5 months now, so it is essential to have a vigorous door-to-door testing to counter the pandemic. Molecular biology-based tests in such cases will become highly cumbersome and should be eased with simpler and quicker tests.
Rapid diagnosis to test as many as we can at this point of time may be considered the only hope to fight the disease. The Foundation for Innovative New Diagnostics, Geneva, Switzerland, has published the first result of independent evaluation and clinical performance of five molecular test kits on April 16, 2020. Sensitivity achieved by all the kits with 50 COVID-19-positive samples was 100%, and specificity achieved by these kits with 100 negative samples was 97–100%.12 However, there may be asymptomatic or infected patients, who may have cleared the viral load without detection. In such cases, an RT-PCR will fail to detect the infection. Therefore, it is important to develop and introduce a rapid and point-of-care test to detect COVID-19 cases and carriers. The National Health Commission of China in the seventh edition of its novel coronavirus pneumonia diagnosis and treatment plan recently recommended the use of immunoglobulin (Ig)M/IgG antibodies testing for suspicious cases.13 With the dynamics of the disease changing at the speed of its spread, it seems highly essential to get all the information at a glance related to the performance of serological tests reported worldwide to pace our health care needs. This review compiles the performance of various serological assays across the globe for the detection of COVID-19 pandemic.
Key determinants of immunoassay
Although molecular tests are the gold standard and very specific in early COVID-19 detection, their reach to a large-scale diagnosis is restricted because of their cost, feasibility and rapidness. Immunological assays could be a good complement in this regard. However, concerning diagnosis, immunoassays are different from molecular assays because immunoassays necessitate some knowledge of the protein and the antibody response generated against that protein. Therefore, immunoassays can be engaged to detect either specific viral proteins (antigens) or the antibodies developed by the host B cells in response to that antigen.
The approximately 30-kb SARS-CoV-2 genome codes for approximately 27 proteins that include four structural proteins, eight accessory proteins and 15 nonstructural proteins. The accessory proteins are involved in the replication of viral RNA and transcription. The functions of nonstructural proteins range from crucial viral activity such as replicase, protease and deubiquitinase. The viral structural proteins comprise spike glycoproteins (S), membrane glycoproteins (M), envelop proteins (E) and nucleocapsid phosphoproteins (N).14 The trimeric S protein (approximately 180 kDa monomer) is postulated to be the first viral fraction to bind with host cell receptors through the receptor-binding domain (RBD) of the S1 subunit and helps in the fusion of virus and host membranes through the S2 subunit. The M protein plays a role in viral morphogenesis and budding through its glycosylated N-terminal domain, three transmembrane domains and a long C-terminal domain. E protein is the smallest structural protein that constitutes the viral assembly, release and pathogenesis. The N protein is the most abundant viral protein (approximately 40 kDa), and thus could be an ideal candidate for diagnosis.15 A strong immune response has been demonstrated against this protein detected in both blood and urine samples. Despite its abundance, S proteins have also been used in COVID-19 diagnosis because of their specificity.16 Studies have been reported to map the SARS-CoV-2 proteins and their antibody interaction through proteome microarray. Krishnamurthy et al.17 demonstrated the antibody response against all the four SARS-CoV-2 proteins, S1, RBD, S2 and N, with infected serum samples and found 98.1% sensitivity. Wang et al.18 have identified specific antibodies to M, N and S proteins of the virus. More than 80% of COVID-19 patients showed antibodies against four immunodominant epitopes of N, S and Orf3a (accessory protein) residue. Interestingly, no antibodies were detected to E protein. In another proteome microarray, 29 convalescent sera samples of COVID-19 were used to demonstrate the IgM/IgG response against 18 SARS-CoV-2 protein constructs. The result showed 100% antibody response mainly against N and S1 proteins, where S1 proved to be the best in differentiating COVID-19 patients from controls.19
The immunoassays that are being used prominently for recent COVID-19 diagnosis are mainly chemiluminescence immunoassays (CLIAs), ELISAs and rapid diagnostic tests such as lateral flow immunoassays (LFIAs). These detect mainly the viral structural proteins or seroconverted IgM and IgG antibodies in blood or serum. On March 13, 2020, the Foundation for Innovative New Diagnostics had called for an expression of interest for evaluation of antigen and antibody-based immunoassays from the COVID-19 test manufacturers. Seven ELISAs, five antigen detection methods and 27 antibody detection rapid diagnostic tests were selected in the first phase.20 Immunological assays listed on the website comply either with research use only or in vitro diagnostics guidelines. In vitro diagnostics assays are more stringent than research use only in certification and validation with real clinical samples.
Antibody dynamicity through CLIA
Since the emergence of COVID-19 pandemic, many researchers have investigated the antibody response during the disease through CLIA. Jin et al.21 demonstrated the dynamic variance of IgM and IgG antibodies in COVID-19 patients retrospectively. Positivity of IgM antibodies increased initially then decreased over time, whereas IgG positivity increased to reach 100% and every time it was higher than IgM. Another retrospective study by Hu et al. observed maximum concentration of antibodies on days 19–21 with the highest detection rate found to be 73.6% on days 16–18 for IgM and 98.6% on days 19–21 for IgG. In addition, in critical COVID-19 patients the results suggest a significantly higher concentration of IgG than in mild and moderate cases.22 A serological study in Italy observed 100% sensitivity for IgG versus 88% for IgM on day 12.23 A study from China reported 97.7% and 95.6% sensitivity and 95.2% and 96.6% specificity of IgM and IgG antibodies, respectively.24 In the United States, 100% sensitivity and specificity were achieved with the commercial IgG CLIA test on day 17 from the onset of disease.25 In another study, seropositivity in confirmed COVID-19 patients was found within 7–12 days of disease onset and continued with disease progression. The overall specificity of IgM and IgG with non-COVID-19 suspected cases, other diseases, medical staff and healthy controls was found to be 97%.11 Interestingly, Zeng et al.26 have shown comparatively higher IgG antibody in females during the early phase of SARS-CoV-2 infection which tended to be elevated in severe cases in comparison to males. A case–control retrospective study suggested maximum IgM level in the 4th week of disease onset and higher in severely diseased patients in comparison to COVID-19 patients with mild and moderate disease.27 Similarly, Xiao et al.28 revealed 100% positivity of IgM and IgG in the 3rd week of disease onset. However, from the 5th week onward, IgG remained positive but IgM continued to decline. By contrast, a study using peptide derived from S protein detected IgG earlier than IgM in CLIA and as early as 2 days from the onset of COVID-19 symptoms. The combination of IgM and IgG showed 81.52% positivity in comparison to 57.2% and 71.4% for IgM and IgG, respectively, with 100% specificity to other diseases and healthy controls.8 Four CLIA tests were evaluated and found to be different in performance with maximum sensitivity and specificity of 92% and 99.23% with total antibody.29 One of the studies comparing the two known antigens in CLIA suggested outperformance of RBD over N protein with responses of IgA and IgM earlier than IgG.30 In addition, the severity of the disease was positively correlated with the level of IgA antibody in many cases. Overall, sensitivities and specificities of IgA, IgM and IgG tests with laboratory-confirmed patients were 98.6%, 96.8% and 96.8% and 98.1%, 92.3% and 99.8%, respectively. However, after combining the three antibodies, the sensitivity and specificity reached to 99.5% and 100%, respectively. A multicentric study recently reported the result of an immunoassay with COVID-19 patients from 10 hospitals in Wuhan using recombinant fusion protein constructed with fragments of N and S proteins. Virus-specific IgG showed 96.62% and 86.54% sensitivity with RT-PCR-confirmed and suspected cases, respectively, whereas IgM showed 85.88% and 73.08%, respectively. Moreover, IgM and IgG showed 97.33% and 97.43% specificity, respectively, for hospitalized patients with other diseases and 99.49% and 99.15%, respectively, with normal healthy individuals.31 Liu et al.32 have demonstrated the superiority of antibody test compared with the nucleic acid test in moderate, severe and critical COVID-19 cases of Wuhan retrospectively. For moderate cases positivity for IgM and IgG was 79.55% and 83.18%, respectively, which was higher than RT-PCR (65.91%). Severe cases showed 82.69% and 100% positive ratio for IgM and IgG, respectively, in comparison to 71.15% with RT-PCR, whereas in critical cases these ratios were 72.97% and 97.30% for IgM and IgG, respectively, in comparison to RT-PCR (67.57%). The sensitivity and specificity obtained through CLIA are summarized in Table 1.
| Country | COVID-19 cases/non-COVID-19 cases | Antibodies | Days from disease onset | Sensitivity % | Specificity % | References |
|---|---|---|---|---|---|---|
| China | 43/33 | IgM/IgG | Median 16 days | 48.1/88.9 | 100/90.9 | 21 |
| China | 276/367 |
IgM/IgG IgM + IgG |
— |
57.2/71.4 81.52 |
100 | 8 |
| China | 34 | IgM/IgG |
3rd week 5th week |
100/100 94.1/100 |
— | 28 |
| China | 3/736 | IgM/IgG | 7–12 days | 100 | 97.3–100 | 11 |
| China | 133 | IgM/IgG | — | 72.9–82.6/93.1–100 | 65.9–71.1 | 32 |
| China | 87/483 | IgA/IgM/IgG | — | 98.6/96.8/96.8 | 98.1/92.3/99.8 | 30 |
| China | 555/1558 | IgM/IgG | 3–35 days | 73.0–85.8/86.5–96.6 | 97.3–99.4/97.4–99.1 | 31 |
| China | 221 | IgM/IgG | 16–21 days | 73.6/98.6 | — | 22 |
| United States | 125/1020 | IgG | 17 days | 100 | 99.9 | 25 |
| China | 50/130 | IgM/IgG | 13 days | 92 | 99.23 | 29 |
| Italy | 87 | IgM/IgG | 12 days | 88/100 | — | 23 |
| China | 47/300 | IgM/IgG | — | 97.7/95.6 | 95.2/96.6 | 24 |
- CLIA, chemiluminescence immunoassay; COVID-19, coronavirus disease 2019; Ig, immunoglobulin.
Antibody ELISA in COVID-19 diagnosis
Because conventional ELISA is a cumbersome and time-consuming process, diagnostic companies provide precoated ELISA either withresearch use only or in vitro diagnostics labeling to detect virus-specific antibodies in human samples. However, the sensitivity and specificity of the ELISA kit largely rely on the type of viral protein used. Nonetheless, the seroconversion of antibodies in COVID-19 patients depends on the onset of symptoms. Therefore, the day on which the test is being conducted is an important consideration. Liu et al.33 have used an ELISA format to detect IgM/IgG antibodies in a patient’s serum against SARS-CoV-2 N protein. The test showed 81.5% sensitivity with IgM and/or IgG on day 10 of disease onset compared with 64.3% with viral RNA detection. The antibody positivity increased from 50% to 80% when tested before 5 days and after 10 days of symptom onset. Zhao et al.34 demonstrated that within 7 days the RNA test has 66.7% sensitivity and the antibody test has only 38.3%. However, after 12 days of disease onset, antibody detection overtook the RNA test and was found to be more than 90% sensitive. Adams et al.35 have shown 85% sensitivity and 100% specificity of IgM or IgG ELISA using the trimeric S protein and 100% sensitivity of IgG after 10 or more days of disease onset.
The dynamics of antibodies against SARS-CoV-2 showed that out of total antibodies, IgM can be detected first in the sample followed by IgG. A similar kind of study revealed that antibodies increased from day 0 to day 5 and positivity rate was observed from 50% to 81% for IgM and from 81% to 100% for IgG.10 Serological test with combined IgM and IgG detection demonstrated overall increased sensitivity as evident from a study where 44.4% and 82.5% sensitivities were observed for IgM and IgG, independently, whereas in combination sensitivity reached 87.3%.36 Guo et al.37 demonstrated the sensitivities for IgM antibodies in PCR-positive confirmed and PCR-negative suspected cases, which were found to be 75.6% and 93.1%, respectively. Moreover, a combination of IgM ELISA with PCR significantly increased the positivity to 98.6% compared with the PCR test alone (51.9%). A cohort study in Hong Kong, however, suggested the early seroconversion of IgG than IgM antibodies in confirmed cases. Moreover, antibodies after 14 days of disease onset showed 100% sensitivity for IgG and 94% for IgM against RBD antigen whose seropositivity was observed earlier than N protein.38 Liu et al.39 confirmed that the S protein has higher sensitivity and earlier antibody response than the N protein in COVID-19 diagnosis. For S protein-based ELISA, the sensitivity was found to be below 50% on days 0–5 and 90.7% on days 11–15 for IgM and/or IgG detection with 100% specificity among healthy controls. A study in France compared two in-house ELISAs with N and S SARS-CoV-2 proteins and found early antibody response of patients for N protein than the S protein.40 However, a similar study in China showed better sensitivity of the N protein for IgM and IgG detection as compared to the S protein.24 Okba et al.41 reported the higher specificity of S1 viral protein than S protein and showed the lower specificity of commercial serological ELISA against other coronavirus samples collected from different countries. An ELISA in the United States with S1 domain of SARS-CoV-2 showed 96.6% and 100% sensitivity for IgA and IgG detection, respectively.42 Lassaunière et al.43 recently evaluated three commercially available serological ELISA kits in Denmark and observed 65–90% sensitivity with 93–100% specificity. In Germany, Kohmer et al.44 validated two commercial ELISA kits and found 58.8–70.6% sensitivity between days 5 and 9 and 93.8–100% between days 10 and 18. However, three commercial ELISAs tested in France demonstrated similar sensitivity of 86.7% with 80–100% specificity.45 Commercially available ELISA is cheaper than PCR diagnosis with high-throughput competence and lenient sample requirement; however, it is not ideal for rapid testing in field conditions. The performance of ELISA for COVID-19 diagnosis in different countries is listed in Table 2.
| Country | COVID-19 cases/non-COVID-19 cases | Antibodies | Days from disease onset | Sensitivity % | Specificity % | References |
|---|---|---|---|---|---|---|
| China | 238/120 | Ab/IgM/IgG | 5 days | 81.5 | 94.2–100 | 33 |
| China | 173 | Ab/IgG/IgM | <15 days | 100, 94.3, 79.8 | — | 34 |
| China | 178 | IgM/IgG |
0 days 5 days |
50/81 81/100 |
— | 10 |
| China | 63/35 | IgM/IgG | 1–28 days | 87.3 | 100 | 36 |
| China | 214/100 | IgM/IgG |
0–5 days 11–15 days |
45.5 90.7 |
100 | 39 |
| China | 208/285 | IgA/IgM/IgG | 5–14 days median | 92.7/85.4/77.9 | 100 | 37 |
| China | 23 | IgM/IgG | >14 days | 88–94/94–100 | — | 38 |
| Denmark | 30/82 | IgG/IgM, IgA | — | 65–90 | 93–100 | 43 |
| UK | 40/142 | IgM/IgG | <28 days | 85 | 100 | 35 |
| France | 51/200 | IgM/IgG | 5–14 days | 65–69 | 100 | 40 |
| Germany | 17/13–26 | IgA, IgG |
5–9 days, 10–18 days |
58.8–70.6 93.8–100 |
95.2–95.7 | 44 |
| France | 15/20 | IgA, IgG | >15 days | 86.7 | 80–100 | 45 |
| China | 47/300 | IgM/IgG | — | 89.1–7.9/95.7–97.9 | 97–99.7/85.7–99.7 | 24 |
| United States | 30/57 | IgA/IgG | 3–4 days> | 96.66/100 | 92.98/98.24 | 42 |
- Ab, antibody; COVID-19, coronavirus disease 2019; Ig, immunoglobulin.
Rapid point-of-care test in SARS-CoV-2 infection
In many infectious diseases, LFIA-based immunochromatographic tests are popular for field-adaptable, quick and user-friendly diagnosis. The test mainly uses colloidal gold as a tracer to detect either pathogens’ antigen or its specific antibodies in the biological samples such as serum, urine and oral fluid of the patients. In the current pandemic, apart from RT-PCR, clinicians search for simple and rapid diagnosis suitable for field settings. Many laboratories, therefore, report LFIA-based serological tests for COVID-19 diagnosis.
The diagnostic indexes of LFIA-based IgM/IgG tests were demonstrated at different time points after onset of the symptoms, wherein at 0–7 days, 8–15 days and 16 days or more after symptom onset these tests showed 18.8%, 100% and 100% sensitivity and 77.8%, 50% and 64.3% specificity, respectively.13 A similar study by Pan et al.46 with RT-PCR-confirmed cases showed increase in sensitivity from 11.1% to 96.8% in LFIA within the first week and after 2 weeks of disease onset, respectively. However, in RT-PCR-negative suspected cases, the detection capacity by LFIA was 43.6%. Confirmed SARS-CoV-2 infection in China suggested 23% and 63.8% detectability of IgM and IgG by LFIA test, respectively, in an early phase of illness which were lower than that with RNA test from sputum (92.3%). Between 8 and 14 days, the sensitivity of IgM and IgG increased to 50% and 87.5%, respectively. At 15 days or more, the sensitivity rose to 52.2% and 91.3% for IgM and IgG, respectively, compared with 60.8% for the molecular test.47 A study in Germany with IgM/IgG LFIA showed 62.5% and 93.8% sensitivities from 5 to 9 days and 10 to 18 days of PCR test, respectively.44 Another LFIA in Brazil showed 77.1% sensitivity and 98% specificity after at least 10 days when tested positive for RT-PCR.48 A case report from Taiwan investigated IgG response against SARS-CoV-2 in LFIA and observed its appearance after 18–21 days of exposure or the 11th day of illness.49 A study from Spain suggested the use of antibody tests after at least 14 days of disease onset; the authors found 73.9% sensitivity at this time point compared with 12.5% and 33.3% at 0–7 days and 7–13 days, respectively.50
Li et al.51 developed and evaluated IgM- and IgG-based lateral flow tests using the recombinant RBD domain of S protein. Results interpreted with both IgM and IgG bands showed 88.66% sensitivity and 90.63% specificity which were better than single IgM and IgG tests. One similar study showed 82.4% sensitivity including both serum IgM and IgG as compared with 57.1% and 81.3% for IgM and IgG alone with 100% specificity in each case.36 Similarly, a study in the United States with defined cases estimated 91.8% sensitivity and 99.5% specificity.36 Moreover, Hu et al.52 have detected 63.4% of IgM/IgG positives in the blood of clinically suspected cases through LFIA compared with 46.3% from nasal or pharyngeal swab specimens through RT-PCR.
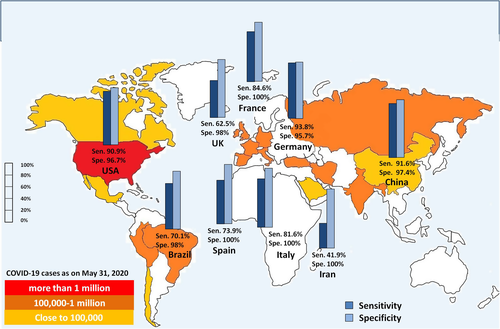
A study by the National COVID Testing Scientific Advisory Panel in the UK evaluated nine commercially available different LFIA tests for COVID-19 detection. The overall sensitivities of LFIA tests after 10 days of disease onset ranged from 55% to 70% as compared with RT-PCR with 95–100% specificities. The report suggested an inadequacy of the LFIA test to diagnose COVID-19 with the current performance.35 Low sensitivity of LFIA test was also observed in two separate studies in Iran and Germany with 47.9% and 36.4% sensitivities, respectively.53, 54 Another study in Denmark validated four commercially available lateral flow tests with a similar number of confirmed COVID-19 cases and found sensitivities ranging from 83% to 93% with 100% specificity for all LFIA.43 Ten LFIA tests were validated in the United States with RT-PCR-positive samples and found 81.8–100% sensitivity and 84.3–100% specificity after 20 days of disease onset.55 Zainol et al.56 reported a review on nine commercial rapid LFIA tests and observed 72.7–100% sensitivity and 98.7–100% specificity of the antibody tests. A study in France with six point-of-care tests showed sensitivity ranges between 76.9% and 93.3% and specificity between 65% and 100%.45 The performance of a commercial IgM/IgG LFIA test was also reported with hospitalized and emergency department COVID-19 patients in Italy. At a median of 7 days, only 63.3% of patients showed positivity with LFIA. Patients from the emergency department showed only 18.45% and 8.3% positivity with RT-PCR-positive and RT-PCR-negative cases, respectively; thus, use of LFIA was not recommended for suspected COVID-19 cases.57 A study in Japan with a commercial one-step test did not recommend the antibody test alone for initial diagnosis.58 Research conducted throughout the world so far has mixed response for utility of LFIA test in COVID-19 diagnosis. Despite the drawback of the high level of false positives and negatives, conducting a greater number of tests will be all we can do until a gold standard test is found.59 The overall percentage of sensitivity and specificity of LFIA in different countries is compiled in Table 3. Figure 1 depicts the diagnostic performance of the immune assays reported from different countries.
| Country | COVID-19 cases/non-COVID-19 cases | Antibodies | Days from disease onset | Sensitivity % | Specificity % | References |
|---|---|---|---|---|---|---|
| China | 397/128 | IgG/IgM | — | 88.66 | 90.6 | 51 |
| China | 90/89 | IgG/IgM | 0–7 days | 18.8 | 77.8 | 13 |
| 8–15 days | 100 | 50 | ||||
| >16 days | 100 | 64.3 | ||||
| China | 38 | IgM/IgG | 0–7 days | 23/53 | — | 47 |
| 8–14 days | 50/87.5 | |||||
| ≥15 days | 52.2/91.3 | |||||
| China | 91/35 |
IgM/IgG IgG + IgM |
— |
57.1/81.3 82.4 |
100 | 36 |
| China | 41 |
IgM/IgG IgG + IgM |
2 weeks |
29.3/46.3 63.4 |
— | 52 |
| China | 86 | IgM/IgG | 1–7 days | 11.1 | — | 46 |
| 8–14 days | 92.2 | |||||
| >15 days | 96.8 | |||||
| Italy | 80/30 | IgM/IgG | Median 7 days | 63.3 | 100 | 57 |
| Denmark | 30/82 | IgM/IgG | 7–23 days> | 80–100 | 80–100 | 43 |
| Spain | 55/45 | IgM/IgG | <7 days | 12.5 | 100 | 50 |
| 7–13 days | 33.3 | |||||
| >14 days | 73.9 | |||||
| United States | 37/30 | IgM/IgG | — | 91.8 | 99.5 | 70 |
| UK | 40/142 | IgM/IgG | 0–28 days | 55–70 | 95–100 | 35 |
| Japan | 112/48 | IgM/IgG | <1 week | 27.8 | 98 | 58 |
| 1–2 weeks | 48 | |||||
| >2 weeks | 95.8 | |||||
| United States | 11/108 | IgM/IgG | >20 days | 81.8–100 | 84.3–100 | 55 |
| Iran | 114/198 | IgM/IgG | 5–53 days | 47.9/47 | 99/100 | 53 |
| Germany | 10/13 | IgM/IgG | 5–9 days | 62.5 | 100 | 44 |
| 10–18 days | 93.8 | |||||
| France | 15/20 | IgM/IgG | >15 days | 76.9–93.3 | 65–100 | 45 |
| Germany | 27/22 | IgM/IgG | Median 18.5 days | 36.4 | 88.9 | 54 |
| Brazil | 83/100 | IgM/IgG | 10 days | 77.1 | 98 | 48 |
- COVID-19, coronavirus disease 2019; Ig, immunoglobulin; LFIA, lateral flow immunoassay; POC, point of care.
A hunt for asymptomatic cases
The world has faced bigger pandemics in the past. The virus has infected humans before in the form of SARS and MERS. Then why the scare and why call the pandemic unprecedented? The severity of a disease depends on its transmission rate and death rate. The transmission rate of COVID-19 is suspected to be very high especially through asymptomatic cases, with an average reproduction number (R0) value of about 3.28.60 Chances of disease spread increase as a result of these undetected asymptomatic cases in the form of leaps and bounds through contacts or expelling air droplets.3 Immunoassays are currently being tested throughout the world especially with hospitalized patients and in a small subset of population.61 However, all coronavirus infections do not show symptoms or may be mild ones. Seropositivity in mild cases was found to delayed or absent as compared with high antibody response in severe cases against SARS-CoV-2 infection.62, 63 Therefore, several studies have estimated much more increase in the COVID-19 prevalence within the community than the official figure. Li et al.64 demonstrated human-to-human SARS-CoV-2 transmission from asymptomatic individuals when studying two family clusters in China. A case report by Zhu et al.65 also highlighted a SARS-CoV-2 infection from an asymptomatic contact. A study in Germany also suggested the transmission of COVID-19 from an asymptomatic individual during his incubation period.66 Since the inception of the COVID-19 infection, most of the studies are limited to symptomatic cases and not many details have been reported to access the asymptomatic cases. According to a report from the National Health Commission in China, 78% of the total identified SARS-CoV-2 cases within 24 h on April 1, 2020, were asymptomatic.67 Moreover, a study from the Diamond Princess Cruise ship estimated that about 17.9% of asymptomatic cases occurred with SARS-CoV-2 infection.68 It is, therefore, suspected that the undetected asymptomatic cases are underestimated and may result in an increase in the total number of reported SARS-CoV-2 infections.69 A community-based study in Santa Clara County of the United States using rapid antibody test recognized population prevalence of 2.49–4.16% for COVID-19. There are an estimated 50–80 times higher number of cases than confirmed cases.70 Another study in the United States with 4856 individuals detected 87 (1.79%) SARS-CoV-2 IgG positives in the population.25 A similar study in Iran found 22% prevalence of COVID-19 with higher number of confirmed cases than expected.71 A population-based survey in Brazil with 4188 patients showed an increase in positivity from 0.04% to 0.133% within 2 weeks of mid-April.72 Recently a systemic review published with preliminary data of 83 asymptomatic cases in nine articles observed significant contribution of asymptomatic and presymptomatic patients in the spread of SARS-CoV-2 infection.73 Samples collected from the oral swab, bronchoalveolar lavage fluid and stool in one of the studies reported comparatively higher viral load in nasopharyngeal samples. However, this variation in viral load is negligible in symptomatic and asymptomatic cases. COVID-19 cases were confirmed through RT-PCR in some individuals without having any disease symptoms.74 Interestingly, an asymptomatic case report from China showed positive nucleic acid test with urine sample before throat swab, suggesting the need for alternative means of diagnosis too for asymptomatic cases.75
Because health care workers are the front runners in this pandemic, many studies have focused on their screening for asymptomatic infection. A study with health care workers having mild respiratory symptoms from nine hospitals in the Netherlands identified 4.1% of SARS-CoV-2-positive cases.76 Another test with residents of health care providers in Washington reported a 30.3% prevalence of infection in the facility.77 Seroprevalence of SARS-CoV-2 infection in health care workers from two community clinics in Japan observed 9.1% positivity rate in comparison to the overall rate of 5.9%.78 Samples collected randomly from health professionals in Spain depicted 11.2% of cumulative prevalence in antibody quantification and 97–100% positive predictive value.79 A study reported from Italy showed 5.25% seropositivity within the asymptomatic health care providers.80 By contrast, another study from Italy screened health workers and observed discordant results between two immunological assays in reporting sensitivity.81 Interestingly, a study with health care workers in Switzerland showed negative virus-specific antibodies in serum, whereas it was detected in nasal fluids and tears.62 It is evident that asymptomatic cases have a significant role in COVID-19 spread. More studies are needed to understand the immune response and spread pattern in asymptomatic infection. To deal with the extra challenge of asymptomatic carriers, a widespread testing is needed within the hotspot community. The test should ideally be performed on-site and rapid, in which antigen- or antibody-based immunoassays could be a critical success factor for mass-level testing of COVID-19.
Discussion
Like any previous pandemic, the answer to curing the disease will be an appropriate vaccine strategy. Thus, it is highly essential to know the changes in the immune responses in patients to help design the appropriate vaccine protocol or for any kind of therapeutic intervention. It is reported that overreaction of the immune system in SARS-CoV-2 infection is responsible for the severity of the cases. Then again, antibodies isolated from recovered patients have shown the ability to neutralize the virus. Because of these disparities, it is of utmost importance to find out the determinants accountable for the ramped-up immune system during infection versus leverage of the antibody response. The world cannot be kept at a standstill and lockdown for an indefinite period waiting for a vaccine. The lockdowns are being gradually withdrawn and populations are exposed little by little to the virus so that a natural immunity is developed in our populations without causing a massacre. Until then, the most appropriate solution to combat this death knell will be extensive diagnosis, identification of the asymptomatic carriers and symptomatic patients through random testing, marking and isolating them. While RT-PCRs are good options for detecting the virus, their cumbersome protocols, time and expense do not make them suitable candidates for mass testing, emphasizing the need for alternative tests. Rapid tests are quintessential for detecting a mass population because of their ease of use, less time consumption and low cost, and thus may be used complementary to RT-PCRs largely for the false-negative case detection in symptomatic cases.
Unlike RT-PCR, rapid tests have their limitations in terms of performance and sensitivity. It has been speculated that the production of antibodies may be affected in an early phase of the disease by a reduction in B cells. Because most of the serological tests have been reported at different time points, it is difficult to access the correct timeline for seroconversion against SARS-CoV-2 infection. The studies so far indicate that the seropositivity of the antibody test within the first week of disease onset is not satisfactory in many cases. However, sensitivity escalates to 80–100% in the second week combining both IgM and IgG in the interpretation. However, it is not yet clear how long the SARS-CoV-2-specific antibodies persist within the asymptomatic or oligo-symptomatic individuals. Presence of antibodies as an indicator of protection against COVID-19 reinfection is not yet established, especially when certain virus-specific antibodies have shown to promote the infection rather than protection. Studies also suggested that not all asymptomatic and even oligo-symptomatic patients necessarily undergo seroconversion. Thus, diagnosis of mild or asymptomatic infections might be difficult because of robust immune response either not being detectable by the serological tests or transient within few days.
Immunological tests are showing varying degree of performances; however, limitations of false positives and false negatives of the tests can be overcome by employing two independent tests that detect different antibodies or use different antigens or protocols. Researchers have suggested the benefit of currently available rapid and affordable immunological test even if it is not ideally 100% sensitive and specific, but it can provide high accuracy after test and retest. However, a test with known sensitivity and specificity may have different positive predictive value according to the prevalence of the disease in that region. Because positive predictive value determines the probability of a test to find true positives in a population level, diagnostic ability of a serological test must be considered with respect to the population area. Serological tests in the point-of-care format can be used mainly for surveillance within the community. However, deregulation or misuse of these rapid tests can create an extra panic in society.
The current phase can very well be called the testing times, where various diagnostic tests, vaccines, drugs are in trials and tremendous effort is being exerted by many across the globe to put an end to this pandemic. The human race has faced various virus attacks and has forever fought to strive through the battle. This fight too will be a long one, and the key to success will be continuous efforts. Herein, our review aims to enumerate all the important diagnostic findings together to help strategize ways to deal with this global pandemic. However, the solution to managing COVID-19 cannot be limited to any one approach; instead, it has to be a holistic one. A good vaccine and safe therapeutics will have a long-term effect to combat SARS-CoV-2, and trials with the same have begun in several parts of the world. Researchers over the globe believe that COVID-19 case surveillance through serological rapid tests could be a potential strategy to mitigate the disease. However, longitudinal studies dealing with the role of B cells and antibody production in symptomatic, recovered and asymptomatic cases will brief the story.
Acknowledgments
The authors thank CSIR-Indian Institute of Chemical Biology, Kolkata. This work has, in part, received funding from UK Research and Innovation via the Global Challenges Research Fund under grant agreement ‘A Global Network for Neglected Tropical Diseases’ grant number MR/P027989/1, Sir J. C. Bose Fellowship, India, Fellowships from Council of Scientific and Industrial Research and Indian Council of Medical Research, India.
Author Contribution
Sarfaraz Ahmad Ejazi: Conceptualization; Data curation; Formal analysis; Writing-review & editing. Sneha Ghosh: Data curation; Formal analysis; Visualization; Writing-review & editing. Nahid Ali: Conceptualization; Funding acquisition; Project administration; Supervision; Writing-review & editing.
Conflict of Interest
The authors declare that they have no competing interests.





