Genetic and immunological features of immune deficiency and dysregulation-associated lymphoproliferations and lymphomas as a basis for classification
Corresponding Author
Daan Dierickx
Department of Hematology, University Hospitals Leuven, Leuven, Belgium
Department of Oncology, Laboratory for Experimental Hematology, KU Leuven, Leuven, Belgium
All authors equally contributed to the article.
Address for correspondence: D Dierickx, Department of Hematology, University Hospitals Leuven, Herestraat 49, Leuven 3000, Belgium. e-mail: [email protected]
Search for more papers by this authorColm Keane
Frazer Institute, University of Queensland, Brisbane, QLD, Australia
All authors equally contributed to the article.
Search for more papers by this authorYasodha Natkunam
Department of Pathology, Stanford University School of Medicine, Stanford, CA, USA
All authors equally contributed to the article.
Search for more papers by this authorCorresponding Author
Daan Dierickx
Department of Hematology, University Hospitals Leuven, Leuven, Belgium
Department of Oncology, Laboratory for Experimental Hematology, KU Leuven, Leuven, Belgium
All authors equally contributed to the article.
Address for correspondence: D Dierickx, Department of Hematology, University Hospitals Leuven, Herestraat 49, Leuven 3000, Belgium. e-mail: [email protected]
Search for more papers by this authorColm Keane
Frazer Institute, University of Queensland, Brisbane, QLD, Australia
All authors equally contributed to the article.
Search for more papers by this authorYasodha Natkunam
Department of Pathology, Stanford University School of Medicine, Stanford, CA, USA
All authors equally contributed to the article.
Search for more papers by this authorAbstract
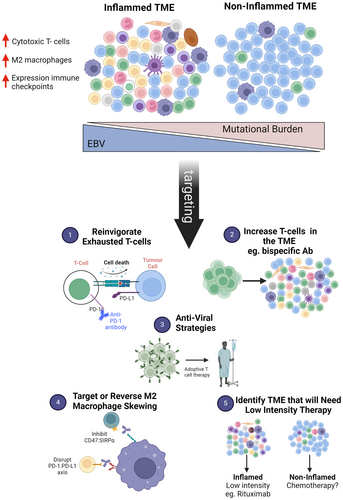
Immune deficiency and dysregulation-associated lymphoproliferative disorders and lymphomas (IDD-LPDs) encompass a heterogeneous clinical and pathological spectrum of disorders that range from indolent lymphoproliferations to aggressive lymphomas. They arise in a variety of clinical settings and are associated with oncogenic viruses such as the Epstein–Barr virus (EBV) and Kaposi sarcoma-associated herpesvirus/human herpes virus (KSHV/HHV8) in some, but not all, cases. The recognition of IDD-LPDs as distinct from LPDs in immune competent patients is essential to tailor clinical management options for affected patients. The 5th edition of the World Health Organisation classification has introduced an integrated classification of IDD-LPDs with the goal of standardising diagnoses among different settings to enhance clinical decision support. In parallel, new knowledge in the field, particularly surrounding the role of oncogenic viruses and the tumour microenvironment, has led to clearer understanding of the complex pathogenesis of IDD-LPDs and how these features can be precisely harnessed for therapeutic purposes. In this perspective, we highlight the need for multidisciplinary decision-making to augment patient care as well as key areas where evolving concepts offer challenges and opportunities for clinical management, research and future iterations of the classification.
Graphical Abstract
Conflicts of interest
D.D. has received honoraria from Takeda, Incyte, Sanofi, Novartis, Amgen, Atara Biotherapeutics, Kite/Gilead and Pierre Fabre, all paid to his institution. C.K. has received Honoria from Merck, Roche, Takeda and Beigene. Y.N. has received honoraria from Roche and Leica Biosystems, and research funding from Kite Pharma.
Open Research
Data availability statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
- 1 WHO Classification of Tumours Editorial Board. Haematolymphoid tumours. Vol. 11. 5th ed. Lyon, France: International Agency for Research on Cancer, 2024. https://publications.iarc.who.int/.
- 2Swerdlow SH, Webber SA, Chadburn A, Ferry JA. Post-transplant lymphoproliferative disorders. In SH Swerdlow, E Campo, NL Harris et al. eds. WHO classification of Tumours of Haematopoietic and lymphoid tissues. Lyon, France: IARC Press, 2008; 343–349.
- 3Swerdlow SH, Campo E, Pileri SA et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016; 127; 2375–2390.
- 4Penn I, Hammond W, Brettschneider L, Starzl TE. Malignant lymphomas in transplantation patients. Transplant. Proc. 1969; 1; 106–112.
- 5Hamilton MP, Sugio T, Noordenbos T et al. Risk of second tumours and T-cell lymphoma after CAR T-cell therapy. N. Engl. J. Med. 2024; 390; 2047–2060.
- 6Shen J, Hu R, Lin A et al. Characterization of second primary malignancies post CAR T-cell therapy: real-world insights from the two global pharmacovigilance databases FAERS and VigiBase. EClinicalMedicine 2024; 73; 102684.
- 7Zhang S, Zhou X, Zhang S et al. EBV-associated lymphoproliferative disease post-CAR-T-cell therapy. Front. Med. 2024; 18; 394–398.
- 8Epstein MA, Achong BG. The EB virus. Ann. Rev. Microbiol. 1973; 27; 413–436.
- 9Murata T, Sugimoto A, Inagaki T et al. Molecular basis of Epstein-Barr virus latency establishment and lytic reactivation. Viruses 2021; 13; 2344.
- 10Dierickx D, Pociupany M, Natkunam Y. Epstein-Barr virus-associated posttransplant lymphoproliferative disorders: new insights in pathogenesis, classification and treatment. Curr. Opin. Oncol. 2022; 34; 413–421.
- 11Bednarska K, Chowdhury R, Tobin JWD et al. Epstein-Barr virus-associated lymphomas decoded. Br. J. Haematol. 2024; 204; 415–433.
- 12Niller HH, Wolf H, Minarovits J. Viral hit and run-oncogenesis: genetic and epigenetic scenarios. Cancer Lett. 2011; 305; 200–217.
- 13Mundo L, Ambrosio MR, Picciolini M et al. Unveiling another missing piece in EBV-driven lymphomagenesis: EBV-encoded MicroRNAs expression in EBER-negative Burkitt lymphoma cases. Front. Microbiol. 2017; 8; 229.
- 14Abate F, Ambrosio MR, Mundo L et al. Distinct viral and mutational spectrum of endemic Burkitt lymphoma. PLoS Pathog. 2015; 11; e1005158.
- 15Kaymaz Y, Oduor CI, Yu H et al. Comprehensive transcriptome and mutational profiling of endemic Burkitt lymphoma reveals EBV type-specific differences. Mol. Cancer Res. 2017; 15; 563–576.
- 16Grande BM, Gerhard DS, Jiang A et al. Genome-wide discovery of somatic coding and noncoding mutations in pediatric endemic and sporadic Burkitt lymphoma. Blood 2019; 133; 1313–1324.
- 17Leoncini L. Epstein-Barr virus positivity as a defining pathogenetic feature of Burkitt lymphoma subtypes. Br. J. Haematol. 2022; 196; 468–470.
- 18Richter J, John K, Staiger AM et al. Epstein-Barr virus status of sporadic Burkitt lymphoma is associated with patient age and mutational features. Br. J. Haematol. 2022; 196; 681–689.
- 19Siciliano MC, Bertolazzi G, Morello G et al. Tumor microenvironment of Burkitt lymphoma: different immune signatures with different clinical behavior. Blood Adv. 2024; 8; 4330–4343.
- 20Zhang Y, Guo W, Zhan Z, Bai O. Carcinogenic mechanisms of virus-associated lymphoma. Front. Immunol. 2024; 15; 1361009.
- 21Chen BJ, Chapuy B, Ouyang J et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin. Cancer Res. 2013; 19; 3462–3473.
- 22Yoon H, Park S, Ju H et al. Integrated copy number and gene expression profiling analysis of Epstein-Barr virus-positive diffuse large B-cell lymphoma. Genes Chromosomes Cancer 2015; 54; 383–396.
- 23Kim JH, Cho H, Sung H et al. Reappraisal of the prognostic value of Epstein-Barr virus status in monomorphic post-transplantation lymphoproliferative disorders-diffuse large B-cell lymphoma. Sci. Rep. 2021; 11; 2880.
- 24Ferreiro JF, Morscio J, Dierickx D et al. EBV-positive and EBV-negative posttransplant diffuse large B cell lymphomas have distinct genomic and transcriptomic features. Am. J. Transplant. 2016; 16; 414–425.
- 25Menter T, Juskevicius D, Alikian M et al. Mutational landscape of B-cell post-transplant lymphoproliferative disorders. Br. J. Haematol. 2017; 178; 48–56.
- 26Frontzek F, Staiger AM, Wullenkord R et al. Molecular profiling of EBV associated diffuse large B-cell lymphoma. Leukemia 2023; 37; 670–679.
- 27Montes-Moreno S, Odqvist L, Diaz-Perez JA et al. EBV-positive diffuse large B-cell lymphoma of the elderly is an aggressive post-germinal center B-cell neoplasm characterized by prominent nuclear factor-kB activation. Mod. Pathol. 2012; 25; 968–982.
- 28Dojcinov SD, Venkataraman G, Pittaluga S et al. Age-related EBV-associated lymphoproliferative disorders in the Western population: a spectrum of reactive lymphoid hyperplasia and lymphoma. Blood 2011; 117; 4726–4735.
- 29Nicolae A, Pittaluga S, Abdullah S et al. EBV-positive large B-cell lymphomas in young patients: a nodal lymphoma with evidence for a tolerogenic immune environment. Blood 2015; 126; 863–872.
- 30Li Y, Xu-Monette ZY, Abramson J et al. EBV-positive DLBCL frequently harbors somatic mutations associated with clonal hematopoiesis of indeterminate potential. Blood Adv. 2023; 7; 1308–1311.
- 31Nikolich-Žugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat. Immunol. 2018; 19; 10–19.
- 32Fali T, Vallet H, Sauce D. Impact of stress on aged immune system compartments: overview from fundamental to clinical data. Exp. Gerontol. 2018; 105; 19–26.
- 33Mancuso S, Carlisi M, Santoro M, Napolitano M, Raso S, Siragusa S. Immunosenescence and lymphomagenesis. Immun. Ageing 2018; 15; 22.
- 34van den Akker EB, Makrodimitris S, Hulsman M et al. Dynamic clonal hematopoiesis and functional T-cell immunity in a supercentenarian. Leukemia 2021; 35; 2125–2129.
- 35Jaiswal S, Fontanillas P, Flannick J et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 2014; 371; 2488–2498.
- 36Maguire A, Chen X, Wisner L et al. Enhanced DNA repair and genomic stability identify a novel HIV-related diffuse large B-cell lymphoma signature. Int. J. Cancer 2019; 145; 3078–3088.
- 37Chapman JR, Bouska AC, Zhang W et al. EBV-positive HIV-associated diffuse large B cell lymphomas are characterized by JAK/STAT (STAT3) pathway mutations and unique clinicopathologic features. Br. J. Haematol. 2021; 194; 870–878.
- 38Cesarman E, Chadburn A, Rubinstein PG. KSHV/HHV8-mediated hematologic diseases. Blood 2022; 139; 1013–1025.
- 39Wilson KS, McKenna RW, Kroft SH et al. Primary effusion lymphomas exhibit complex and recurrent cytogenetic abnormalities. Br. J. Haematol. 2002; 116; 113–121.
- 40Manzano M, Patil A, Waldrop A, Dave SS, Behdad A, Gottwein E. Gene essentiality landscape and druggable oncogenic dependencies in herpes viral primary effusion lymphoma. Nat. Commun. 2018; 9; 3263.
- 41Gomez F, Fisk B, McMichael JF et al. Ultra-deep sequencing reveals the mutational landscape of classical Hodgkin lymphoma. Cancer. Res. Commun. 2023; 3; 2312–2330.
- 42Maura F, Ziccheddu B, Xiang JZ et al. Molecular evolution of classic Hodgkin lymphoma revealed through whole-genome sequencing of Hodgkin and reed Sternberg cells. Blood Cancer Discov. 2023; 4; 208–227.
- 43Alig SK, Shahrokh Esfahani M, Garofalo A et al. Distinct Hodgkin lymphoma subtypes defined by noninvasive genomic profiling. Nature 2024; 625; 778–787.
- 44Venanzi A, Marra A, Schiavoni G et al. Dissecting clonal hematopoiesis in tissues of classical Hodgkin lymphoma patients. Blood Cancer Discov. 2021; 2; 216–225.
- 45de Jong D, Roemer MG, Chan JK et al. B-cell and classical Hodgkin lymphomas associated with immunodeficiency: 2015 SH/EAHP workshop report-part 2. Am. J. Clin. Pathol. 2017; 147; 153–170.
- 46Soltani S, Zakeri A, Tabibzadeh A et al. A review on EBV encoded and EBV-induced host microRNAs expression profile in different lymphoma types. Mol. Biol. Rep. 2021; 48; 1801–1817.
- 47Briercheck EL, Ravishankar S, Ahmed EH et al. Geographic EBV variants confound disease-specific variant interpretation and predict variable immune therapy responses. Blood Adv. 2024; 8; 3731–3744.
- 48Trappe R, Oertel S, Leblond V et al. Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol. 2012; 13; 196–206.
- 49Dierickx D, Habermann TM. Post-transplantation lymphoproliferative disorders in adults. N. Engl. J. Med. 2018; 378; 549–562.
- 50Morscio J, Dierickx D, Ferreiro JF et al. Gene expression profiling reveals clear differences between EBV-positive and EBV-negative posttransplant lymphoproliferative disorders. Am. J. Transplant. 2013; 13; 1305–1316.
- 51Morscio J, Finalet Ferreiro J, Vander Borght S et al. Identification of distinct subgroups of EBV-positive post-transplant diffuse large B-cell lymphoma. Mod. Pathol. 2017; 30; 370–381.
- 52Green MR, Rodig S, Juszczynski P et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin. Cancer Res. 2012; 18; 1611–1618.
- 53Kinch A, Sundström C, Baecklund E, Backlin C, Molin D, Enblad G. Expression of PD-1, PD-L1, and PD-L2 in posttransplant lymphoproliferative disorder after solid organ transplantation. Leuk. Lymphoma 2019; 60; 376–384.
- 54Leivonen SK, Friman T, Autio M et al. Characterization and clinical impact of the tumour microenvironment in post-transplant aggressive B-cell lymphomas. Haematologica 2023; 108; 3044–3057.
- 55Baron M, Labreche K, Veyri M et al. Epstein-Barr virus and immune status imprint the immunogenomics of non-Hodgkin lymphomas occurring in immune-suppressed environments. Haematologica 2024. https://doi.org/10.3324/haematol.2023.284332. Online ahead of print.
- 56Stubbins RJ, Lam R, Zhu J et al. Tumour infiltrating lymphocytes predict survival in solid organ transplant recipients with monomorphic post-transplant lymphoproliferative disorders. Clin. Lymphoma Myeloma Leuk. 2022; 22; 744–752.
- 57Taylor JG, Liapis K, Gribben JG. The role of the tumour microenvironment in HIV-associated lymphomas. Biomark. Med 2015; 9; 473–482.
- 58Gandhi MK, Hoang T, Law SC et al. EBV-associated primary CNS lymphoma occurring after immunosuppression is a distinct immunobiological entity. Blood 2021; 137; 1468–1477.
- 59Kaulen LD, Denisova E, Hinz F et al. Integrated genetic analyses of immunodeficiency-associated Epstein-Barr virus- (EBV) positive primary CNS lymphomas. Acta Neuropathol. 2023; 146; 499–514.
- 60Jacobson CA, Abramson JS. HIV-associated Hodgkin's lymphoma: prognosis and therapy in the era of cART. Adv. Hematol. 2012; 2012; 507257.
- 61Hartmann S, Jakobus C, Rengstl B et al. Spindle-shaped CD163+ rosetting macrophages replace CD4+ T-cells in HIV-related classical Hodgkin lymphoma. Mod. Pathol. 2013; 26; 648–657.
- 62Saito S, Kaneko Y, Yamaoka K, Tokuhira M, Takeuchi T. Distinct patterns of lymphocyte count transition in lymphoproliferative disorder in patients with rheumatoid arthritis treated with methotrexate. Rheumatology (Oxford) 2017; 56; 940–946.
- 63Carreras J, Yukie Kikuti Y, Miyaoka M et al. Genomic profile and pathologic features of diffuse large B-cell lymphoma subtype of methotrexate-associated lymphoproliferative disorder in rheumatoid arthritis patients. Am. J. Surg. Pathol. 2018; 42; 936–950.
- 64Gion Y, Doi M, Nishimura Y et al. PD-L1 expression is associated with the spontaneous regression of patients with methotrexate-associated lymphoproliferative disorders. Cancer Med. 2022; 11; 417–432.
- 65Shiraiwa S, Kikuti YY, Carreras J et al. 9p24.1 genetic alteration and PD-L1 expression are characteristic of de novo and methotrexate-associated Epstein-Barr virus-positive Hodgkin lymphoma, but not methotrexate-associated Hodgkin-like lesions. Am. J. Surg. Pathol. 2022; 46; 1017–1024.
- 66Harada T, Iwasaki H, Muta T et al. Outcomes of methotrexate-associated lymphoproliferative disorders in rheumatoid arthritis patients treated with disease-modifying anti-rheumatic drugs. Br. J. Haematol. 2021; 194; 101–110.
- 67Hunter NB, Vogt S, Ambinder RF. Treatment of HIV-associated lymphomas: the latest approaches for optimizing outcomes. Oncology (Williston Park) 2017; 31; 872–877.
- 68Reshef R, Vardhanabhuti S, Luskin MR et al. Reduction of immunosuppression as initial therapy for posttransplantation lymphoproliferative disorder. Am. J. Transplant. 2011; 11; 336–347.
- 69Mahadeo KM, Baiocchi R, Beitinjaneh A et al. Tabelecleucel for allogeneic haematopoietic stem-cell or solid organ transplant recipients with Epstein-Barr virus-positive post-transplant lymphoproliferative disease after failure of rituximab or rituximab and chemotherapy (ALLELE): a phase 3, multicentre, open-label trial. Lancet Oncol. 2024; 25; 376–387.
- 70Nikiforow S, Whangbo JS, Reshef R et al. Tabelecleucel for EBV+ PTLD after allogeneic HST or SOT in a multicenter expanded access protocol. Blood Adv. 2024; 8; 3001–3012.
- 71Volaric A, Saleem A, Younes SF et al. Epstein-Barr virus latency patterns in polymorphic lymphoproliferative disorders and lymphomas in immunodeficiency settings: diagnostic implications. Ann. Diagn. Pathol. 2024; 70; 152286.
- 72Bollard CM, Gottschalk S, Torrano V et al. Sustained complete responses in patients with lymphoma receiving autologius cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J. Clin. Oncol. 2014; 32; 798–808.
- 73McLaughlin LP, Rouce R, Gottschalk S et al. EBV/LMP-specific T-cells maintain remissions of T- and B-cell EBV lymphomas after allogeneic bone marrow transplantation. Blood 2018; 132; 2351–2361.
- 74Perrine SP, Hermine O, Small T et al. A phase 1/2 trial of arginine butyrate and ganciclovir in patients with Epstein-Barr virus-associated lymphoid malignancies. Blood 2007; 109; 2571–2578.
- 75Jones RJ, Iemridee T, Wang X et al. Lenalidomide, thalidomide, and pomalidomide reactivate the Epstein-Barr virus lytic cycle through phosphoinositide 3-kinase signaling and Ikaros expression. Clin. Cancer Res. 2016; 22; 4901–4912.
- 76Dalton T, Doubrovina E, Pankov D et al. Epigenetic reprogramming sensitizes immunologically silent EBV+ lymphomas to virus-directed immunotherapy. Blood 2020; 135; 1870–1881.
- 77Martinez OM, Krams SM. The immune response to Epstein Barr virus and implications for posttransplant lymphoproliferative disorder. Transplantation 2017; 101; 2009–2016.
- 78Pociupany M, Snoeck R, Dierickx D, Andrei G. Treatment of Epstein-Barr virus infection in immunocompromised patients. Biochem. Pharmacol. 2024; 225; 116270.
- 79Sehn LH, Salles G. Diffuse large B-cell lymphoma. N. Engl. J. Med. 2021; 384; 842–858.
- 80Vase MØ, Maksten EF, Bendix K et al. Occurrence and prognostic relevance of CD30 expression in post-transplant lymphoproliferative disorders. Leuk. Lymphoma 2015; 56; 1677–1685.
- 81Pearse WB, Petrich AM, Gordon LI et al. A phase I/II trial of brentuximab vedotin plus rituximab as frontline therapy for patients with immunosuppression-associated CD30+ and/or EBV + lymphomas. Leuk. Lymphoma 2021; 62; 3493–3500.
- 82Amengual JE, Pro B. How I treat posttransplant lymphoproliferative disorder. Blood 2023; 142; 1426–1437.
- 83Paranji S, Steinbarg A. First use of upfront polatuzumab vedotin in post-transplant lymphoproliferative disorder: a case report. Cureus 2024; 16; e56409.
- 84McKenna M, Epperla N, Ghobadi A et al. Real-world evidence of the safety and survival with CD19 CAR-T-cell therapy for relapsed/refractory solid organ transplant-related PTLD. Br. J. Haematol. 2023; 202; 248–255.
- 85Veloza L, Teixido C, Castrejon N et al. Clinicopathological evaluation of the programmed cell death 1 (PD1)/programmed cell death-ligand 1 (PD-L1) axis in post-transplant lymphoproliferative disorders: association with Epstein-Barr virus, PD-L1 copy number alterations, and outcome. Histopathology 2019; 75; 799–812.
- 86d'Izarny-Gargas T, Durrbach A, Zaidan M. Efficacy and tolerance of immune checkpoint inhibitors in transplant patients with cancer: a systematic review. Am. J. Transplant. 2020; 20; 2457–2465.
- 87Alzahrani N, Al Jurdi A, Reilla LV. Immune checkpoint inhibitors in kidney transplantation. Curr. Opin. Organ Transplant. 2023; 28; 46–54.
- 88Lurain K, El Zarif T, Ramaswami R et al. Real-world multicenter study of PD1- blockade in HIV-associated classical Hodgkin lymphoma across the United States. Clin. Lymphoma Myeloma Leuk. 2024; 24; 523–530.
- 89Chaganti S, Maycock S, McIlroy G et al. Ibrutinib as part of risk-stratified treatment for posttransplant lymphoproliferative disorder: the phase 2 TIDaL trial. Blood 2024; 144; 392–401.