Human papillomavirus and p53 status define three types of vulvar squamous cell carcinomas with distinct clinical, pathological, and prognostic features
Abstract
Introduction
Based on their etiological relationship with human papillomavirus (HPV), the 2020 WHO classification has divided vulvar squamous cell carcinomas (VSCC) into two distinct types, HPV-associated and HPV-independent, and HPV-independent tumours have recently been divided according to p53 status. Nevertheless, the clinical and prognostic significance of this classification has not been clearly established. We analysed the differential clinical, pathological, and behavioural characteristics of these three types of VSCC in a large series of patients.
Methods and results
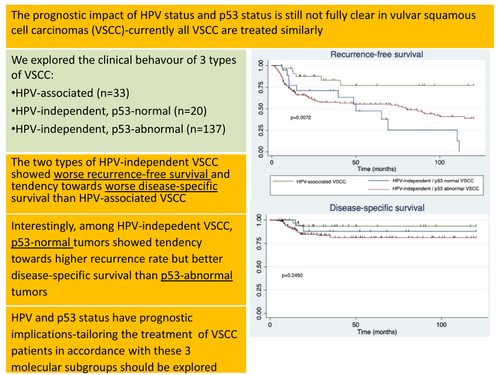
VSCC samples from patients who underwent primary surgery at the Hospital Clinic of Barcelona, Spain, during a 47-year period (January 1975 to January 2022) were analysed (n = 190). HPV detection, p16, and p53 immunohistochemical staining were evaluated. We also analysed recurrence-free survival (RFS) and disease-specific survival (DSS). Thirty-three tumours (17.4%) were HPV-associated and 157 (82.6%) HPV-independent. Of these, 20 showed normal and 137 abnormal p53 expression. The two types of HPV-independent tumours showed worse RFS in the multivariate analysis (hazard ratio [HR] = 3.63; P = 0.023 for the HPV-independent p53 normal VSCC and HR = 2.78; P = 0.028 for the HPV-independent p53 abnormal VSCC). Although the differences were not significant, HPV-independent VSCC had worse DSS than HPV-associated VSCC. Although patients with HPV-independent p53 normal tumours had worse RFS than patients with HPV-independent p53 abnormal tumours, the DSS was better for the former group. Only advanced FIGO stage was associated with worse DSS in multivariate analysis (HR = 2.83; P = 0.010).
Conclusion
The association of HPV and p53 status have prognostic implications, reinforcing a three-tier molecular classification of VSCC (HPV-associated VSCC, HPV-independent VSCC with normal p53, HPV-independent VSCC with abnormal p53).
Graphical Abstract
Currently, all VSCC are treated similarly, irrespective of HPV and p53 status. Our findings suggest that in the future, the treatment of VSCC might be tailored in accordance with these molecular subgroups (HPV-associated VSCC, HPV-independent VSCC with wildtype p53, HPV-independent VSCC with mutated p53), given their different prognosis and behaviour.
Introduction
Vulvar squamous cell carcinoma (VSCC) represents over 95% of all vulvar malignancies. The 2020 World Health Organization (WHO) classification divides this neoplasia into two distinct pathological types: human papillomavirus (HPV)-associated and HPV-independent, based on their etiological relationship with infection by this oncogenic virus.1, 2 This new classification based on etiological criteria represents a major change compared with previous classifications primarily established according to morphological characteristics.2
Several differences support this classification. HPV-associated VSCC typically affects younger women and develops from an intraepithelial precursor named high-grade squamous intraepithelial lesion (HSIL).3-5 Morphologically, both HPV-associated VSCC and HSIL characteristically show an immature basaloid appearance or warty architecture with koilocytic features.3, 4 Immunohistochemically (IHC), intraepithelial and invasive HPV-associated lesions typically show strong, diffuse, “block type” positivity for p16, and a normal pattern of p53 IHC expression.6, 7 Contrarily, HPV-independent VSCC usually arise in older women, develop from an intraepithelial precursor named differentiated vulvar intraepithelial neoplasia (dVIN), and are commonly associated with chronic inflammatory lesions such as lichen sclerosus and lichen simplex chronicus.1, 3, 4 Morphologically, both HPV-independent VSCC and dVIN characteristically have a well-differentiated appearance, with prominent keratinisation. HPV-independent lesions are typically negative for p16, and frequently show an abnormal pattern of p53 IHC expression that correlates with mutated TP53.6, 7 Several studies have shown that there is a significant morphological overlap involving not only invasive VSCC, but also intraepithelial proliferations, with occasional HPV-associated lesions showing well-differentiated, keratinizing features and occasional HPV-independent lesions showing an immature, basaloid, or warty architecture.8-11 Due to this morphological overlap between the two types of VSCC, ancillary tests, such as molecular HPV detection and/or IHC, are required for an adequate classification of these tumours.2
Recent reports have suggested that HPV-independent VSCC can be separated into two different categories based on the status of p53: HPV-independent VSCC with wildtype p53 and HPV-independent VSCC with mutated p53, and that these two variants have differential clinical and pathological features.12-14 The p53 wildtype HPV-independent VSCC are commonly of verrucous type and are preceded by specific precursors named vulvar acanthosis with altered differentiation (VAAD)15 and differentiated exophytic vulvar intraepithelial lesion (DEVIL),16 both of which have been recently classified under the term of verruciform acanthotic vulvar intraepithelial neoplasia (vaVIN).17 In contrast, p53 mutated HPV-independent VSCC are commonly of the keratinizing type and are preceded by dVIN. It has been shown that IHC staining for p53 strongly correlates with the status of the TP53 gene, with normal patterns of expression indicating a wildtype TP53 and abnormal patterns correlating with a mutated TP53.12-14
However, there is limited knowledge of the clinical, pathological, and prognostic features of the two main types of VSCC defined by the WHO classification, i.e. HPV-associated and HPV-independent, and whereas some studies report that HPV-associated VSCC are less aggressive than HPV-independent VSCC, other studies find no significant differences.18-20 There is controversy over whether HPV-independent carcinomas should be subclassified into two distinct categories: p53 normal (wildtype) and p53 abnormal (mutated), as the number of studies describing HPV-independent tumours as separate categories are very scant. Currently, regardless of HPV and p53 status, all cases of VSCC are equally treated, with surgery (with or without adjuvant radiotherapy) being the main pillar of treatment. Primary chemoradiation is reserved for unresectable lesions or as neoadjuvant treatment in selected patients with advanced-stage disease.21 Thus, the main objective of this study was to analyse a large series of patients with VSCC and evaluate the clinical, pathological, and behavioural differential characteristics of these three types of VSCC.
Materials and Methods
Study design
We performed a retrospective study in which all VSCC from patients who underwent surgery as primary treatment at the Department of Gynaecological Oncology of the Hospital Clinic of Barcelona, Spain, during a 47-year period (January 1975 to January 2022) were retrieved from the files of the Department of Pathology. In all cases, a thorough revision of the clinical charts and the surgical pathology report was conducted, focusing on age, tumour size, location, uni- or multifocality, lymph node metastases, and International Federation of Gynaecology and Obstetrics (FIGO) staging. All available pathological material was carefully reviewed. The following inclusion criteria were required for this study: (i) histologically confirmed invasive VSCC; (ii) surgery with or without adjuvant radiotherapy as primary treatment; (iii) available paraffin block/s representative of the invasive tumour and the adjacent skin with sufficient material for IHC and HPV-DNA testing; and (iv) follow-up time of at least 6 months (or to death). Exclusion criteria were: patients who underwent primary nonsurgical treatment.
Overall, 190 patients with VSCC fulfilling the inclusion criteria were identified. Institutional ethical approval for this study was obtained (registry ref HCB/2020/1198). Informed written consent was obtained from all the patients included in the study.
Selection of the paraffin blocks for IHC and DNA testing
In all cases, HPV-DNA detection by polymerase chain reaction (PCR) and IHC staining for p16 and p53 were performed in formalin-fixed, paraffin-embedded samples (FFPE). A paraffin block showing both invasive tumour and adjacent skin, including any intraepithelial precursors if present, was selected. When no paraffin blocks representative of the two lesions were identified, two different blocks were analysed.
HPV analysis
The DNA was extracted from whole sections of FFPE blocks. HPV-DNA detection and genotyping were performed using SPF10 PCR and the LiPA25 system (v. 1, Labo Biomedical Products, Rijswijk, The Netherlands). A volume of 10 μl of isolated DNA was PCR-amplified by the INNO-LiPA HPV Genotyping Extra II Amplification (Fujirebio, Gent, Belgium) kit, which was also used for HPV genotyping. This method allows the genotyping of both high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 26, 53, 66, 70, 73, 82) and low-risk HPV types (6, 11, 40, 42, 43, 44, 54, 61, 62, 67, 81, 83, and 89).
p16 Immunohistochemistry
p16 IHC staining was performed using a CINtec Histology anti-p16INK4a (clone E6H4) monoclonal mouse antibody (Roche Cat# 705-4793, RRID:AB_2833232; Basel, Switzerland). Diffuse, block-type staining was considered as a positive result, whereas either completely negative or patchy staining was considered as negative for p16.6, 7
p53 Immunohistochemistry
IHC for p53 was performed using the CONFIRM anti-p53 (clone DO-7) primary monoclonal antibody (Roche Cat# 05278775001, RRID:AB_2892528). The results were evaluated using the recently described IHC pattern-based interpretation framework.22, 23 Staining was evaluated independently in the invasive tumour and in the adjacent skin. p53 staining patterns were classified into two major categories: normal and abnormal. Normal (wildtype) patterns, included scattered and mid-epithelial staining patterns, whereas abnormal (mutant) expression, included basal or diffuse overexpression, as well as null and cytoplasmic staining patterns.
Criteria of VSCC classification
All the study cases were classified into three main groups based on their association with HPV and the pattern of p53 IHC expression. The groups included: (i) HPV-associated VSCC, (ii) HPV-independent p53 normal VSCC, and (iii) HPV-independent p53 abnormal VSCC.
To categorize a tumour as HPV-associated or -independent, both p16 IHC staining and HPV testing were considered. Tumours with positive staining for p16 and/or molecular testing showing high-risk HPV-DNA, were classified as HPV-associated. The inclusion of a tumour as HPV-independent required negative p16 IHC staining and the absence of high-risk HPV DNA. These HPV-independent tumours were subclassified as p53 normal and p53 abnormal based on the IHC patterns previously described.
Histological revision of the invasive tumour
All haematoxylin and eosin (H&E)-stained slides from each tumour were reviewed by two gynaecological pathologists with expertise in vulvar pathology (N.R., A.S.). The histological variant of the invasive VSCC (keratinizing, nonkeratinizing, basaloid, warty, and verrucous) was recorded.2 The evaluation was conducted blinded to HPV testing and p16 and p53 IHC results.
Evaluation of the adjacent skin
The skin adjacent to the invasive tumour was reviewed in all cases. The presence and type of intraepithelial lesion (if present), as well as its presence in the resection margin were carefully evaluated and recorded. We specifically looked for intraepithelial precursors, including HSIL,2 dVIN,24, 25 and vaVIN (VAAD/DEVIL).15-17 The presence of inflammatory dermatoses (lichen simplex chronicus, lichen sclerosus, and lichen planus) was also recorded.26 HSIL was diagnosed when full-thickness epithelial atypia, high nuclear-to-cytoplasmic ratio, and marked nuclear pleomorphism was identified.3 dVIN was diagnosed based on significant basal atypia with preserved maturation in the upper layers.27, 28 Acanthotic and/or verruciform architecture, altered squamous differentiation with absent cytological atypia, in the context of negative or patchy p16 and normal p53 staining were used as criteria for the diagnosis of vaVIN (VAAD/DEVIL).1, 17
Treatment and follow-up
All patients underwent surgical treatment with vulvectomy or wide local excision of VSCC lesions. In 1998, our centre started to perform sentinel lymph node biopsy followed by inguinofemoral lymph node dissection in order to validate the technique.29 In 2002, we began to perform sentinel lymph node biopsy exclusively in patients with unifocal VSCC measuring <4 cm. When a positive sentinel lymph node was identified, ipsilateral inguinofemoral lymph node dissection was performed. Adjuvant radiotherapy and chemotherapy were administrated in accordance with the latest clinical guidelines for the treatment of VSCC prevailing at the time of diagnosis.
The patients were followed in accordance with the protocols of our centre with physical examination every 4–6 months for the first 2 years and annually afterwards. During follow-up, imaging techniques (magnetic resonance imaging, inguinal ultrasound, or computed tomography scan) were periodically performed in patients with advanced stage VSCC or when recurrence was suspected.
Information on surgical and adjuvant treatment, relapse of disease, and causes of death was retrieved from the clinical charts. All cases were retrospectively restaged according to FIGO 2021 criteria.
Statistical analysis
StataIC/v15.0.591 was used for all the data analyses. The clinical and histopathological data were compared among the different groups of VSCC using χ2 tests (categorical data) and analysis of variance, anova (numerical data).
Endpoints of the study were recurrence-free survival (RFS) and disease-specific survival (DSS). RFS was defined as the time from treatment (primary surgery) until the patient survived without any sign of disease recurrence (local or distant) or the date of the last follow-up. DSS was calculated as the time from the date of primary surgery to the date of death by VSCC or the date of the last follow-up. Survival analyses were conducted using the Kaplan–Meier method and survival curves were compared using the log-rank test. Survival data are expressed as median RFS and DSS. Given that these data were not computable in all study groups because the survival probability did not reach 50%, we also computed the 10-year recurrence and mortality rates (and compared them with a χ2 test). Univariate Cox regression was performed to evaluate the role of HPV and p53 status in RFS and DSS. Multivariate Cox regression was used to assess possible confounding factors involved in RFS and DSS; besides the three study groups, all variables with significance in the univariate analysis were included in the multivariate analysis. The results were statistically significant when P < 0.05.
Results
Classification of the VSCC into the three pathological types
Of the 190 patients with VSCC included in the study, 33 (17.4%) were classified as HPV-associated and 157 (82.6%) were HPV-independent. The 157 HPV-independent VSCC were subclassified as HPV-independent VSCC with normal p53 IHC expression (20 tumours, 10.5% of the total cohort of patients), and HPV-independent VSCC with abnormal p53 IHC expression (137 tumours, 72.1% of the total cohort of patients).
HPV testing and p16 and p53 IHC expression
All HPV-associated VSCC showed diffuse overexpression of p16. HPV testing was positive in 30/33 (90.9%) and negative in 3 (9.1%) HPV-associated tumours. Single HPV types were identified in 25 tumours (66.7%), whereas multiple types were detected in five cases (15.6%, four cases with two HPV types and one with four HPV types). HPV16 was the most frequently identified type (23 [69.7%] tumours, including 18 single and all five multiple infections). HPV33 was identified in four tumours (12.1%, all single infections) and HPV56 in three carcinomas (9.1%, two single infections, one multiple infection). HPV6, HPV42, HPV53, and HPV66 were identified only in multiple infections, in addition to HPV16. p53 showed a normal pattern of expression in 32/33 (96.9%, 22 [66.7%] cases showing a scattered pattern and 10 [30.3%] a mid-epithelial pattern) HPV-associated VSCC and an abnormal pattern in one (3.0%, diffuse overexpression). The latter case was positive for high-risk HPV (HPV16), showed basaloid histology, was FIGO IA stage and had favourable behaviour (no recurrence or death because of disease), in similarity with the remaining HPV-associated VSCC.
All HPV-independent VSCC showed negative staining for p16. HPV detection was negative in 155/157 (98.7%) HPV-independent tumours and positive in 2/157 (1.3%). These two cases were single type infections caused by low-risk HPV types (HPV6 and HPV61; one case each). p53 showed a normal pattern of expression in 20/157 HPV-independent VSCC (12.7%, all cases showing a scattered pattern) and an abnormal pattern in 137 (87.3%). In the subset of HPV-independent tumours with abnormal p53 IHC, diffuse overexpression was the most frequent pattern (92/137; 67.6%), followed by a null pattern (20/137; 14.6%), basal overexpression (15/137; 10.9%), and cytoplasmic expression (10/137; 7.3%). The two HPV-independent VSCC with a positive low-risk HPV-DNA test showed an abnormal p53 IHC; all HPV-independent tumours with normal p53 IHC staining had a negative result in the HPV testing.
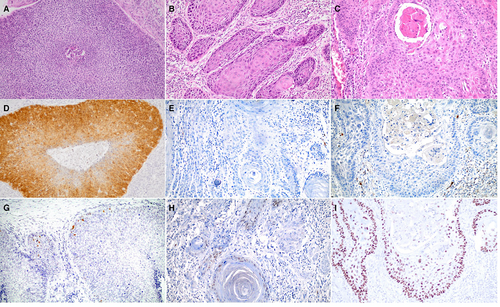
Figure 1 shows the H&E stain, as well as the p16 and p53 IHC staining of a representative example of each category group defined in the study.
Treatment
All patients were treated with primary surgery, either vulvectomy (85 women, 44.7%) or wide local excision (105 women, 55.3%). One hundred sixty patients (84.2%) underwent surgical lymph node evaluation either by sentinel lymph node biopsy (n = 62), inguinofemoral lymph node dissection (n = 54), or both sentinel and inguinofemoral dissection (n = 44). There were no differences in the type of surgery (vulvectomy versus wide local excision, P = 0.09) or in the type of lymph node evaluation (sentinel lymph node biopsy versus inguinofemoral lymph node dissection versus both procedures, P = 0.26) between study groups. Among the 30 patients who did not undergo surgical lymph node evaluation, seven patients presented FIGO stage IA VSCC (negativity of lymph nodes was assumed), and 23 patients did not undergo surgical nodal evaluation because of poor performance status. There were no differences in the proportion of patients who did not undergo surgical nodal evaluation among the study groups. Sixty-three (34.2%) patients received adjuvant radiotherapy and nine (4.7%) adjuvant chemotherapy. There was no significant difference in the proportion of patients receiving adjuvant treatment (30.3% versus 15.0% versus 37.2%; P = 0.131) in HPV-associated and HPV-independent VSCC with normal and abnormal p53. Sixteen patients (8.4%) who should have received radiotherapy according to current guidelines21, 30 did not, but the differences in this proportion were not significant among the study groups.
Clinical features
Table 1 shows the clinical features and prognostic factors of the three types of VSCC. Patients with HPV-associated VSCC were significantly younger than those with HPV-independent (p53 normal and abnormal) tumours. HPV-associated VSCC were smaller and less deeply invasive than the two types of HPV-independent tumours. No differences in terms of percentage of HPV-associated and -independent tumours were observed over the time lapse of the study.
| HPV-associated VSCC (n = 33) | HPV-independent VSCC | P | ||
|---|---|---|---|---|
| With normal p53 (n = 20) | With abnormal p53 (n = 137) | |||
| Age (years) | 63.6 ± 15.9 | 76.7 ± 14.8 | 75.8 ± 11.1 | <0.001 |
| Tumour size (mm) | 20.5 ± 15.3 | 33.7 ± 19.4 | 28.3 ± 18.3 | 0.024 |
| Surgical margin affected by invasive tumour | 6 (18.2) | 0 (0.0) | 10 (7.3) | 0.046 |
| Surgical margin affected by premalignant lesion | 12 (36.4) | 7 (35.0) | 37 (27.0) | 0.485 |
| Location | ||||
| Central | 26 (78.9) | 13 (65.0) | 81 (59.1) | 0.108 |
| Lateral | 7 (21.2) | 7 (35.0) | 56 (40.8) | |
| Multifocal carcinoma | 6 (18.2) | 6 (30.0) | 28 (20.4) | 0.607 |
| Lymph node metastasis n (%) | ||||
| Yes | 8 (24.2) | 5 (25.0) | 47 (34.3) | 0.219 |
| No | 18 (54.6) | 11 (55.0) | 78 (56.9) | |
| Not assessed (surgically) | 7 (21.2) | 4 (20) | 12 (8.8) | |
| FIGO staging n (%) | ||||
| Early (I/II) | 26 (78.79) | 16 (80.0) | 92 (67.6) | 0.260 |
| Advanced (III/IV) | 7 (21.2) | 4 (20.0) | 45 (32.8) | |
- FIGO, International Federation of Gynaecology and Obstetrics.
- Bold values indicate significant of P < 0.05.
Among patients with surgical evaluation of lymph nodes, 60 (35.9%) presented lymph node metastases (including four cases of isolated tumour cells). One hundred thirty-four patients (70.5%) showed an early FIGO 2021 stage (I or II), whereas 56 (29.5%) were diagnosed at an advanced FIGO 2021 stage. No significant differences were observed among the three groups in terms of percentage of cases with metastatic lymph nodes or advanced FIGO stage, or involvement of the surgical margins by premalignant lesion (Table 1).
Histological subtypes and prognostic factors
The histological features of VSCC and the associated lesions identified in the adjacent skin are shown in Table 2. Twenty-eight out of 33 (84.8%) of the HPV-associated tumours and only 28/157 (17.8%) HPV-independent tumours were of basaloid, warty, mixed warty/basaloid, or nonkeratinizing subtypes (P < 0.001). Among the HPV-independent tumours, 75% (119/157) were of the keratinizing subtype. Five out of the 20 (25%) HPV-independent tumours with p53 normal staining were verrucous carcinomas.
| HPV-associated | HPV-independent VSCC | P | ||
|---|---|---|---|---|
| VSCC (n = 33) | With normal p53 (n = 20) | With abnormal p53 (n = 137) | ||
| Histological type | ||||
| Keratinizing | 5 (15.2%) | 12 (60.0%) | 107 (78.1%) | <0.001 |
| Verrucous | 0 (0.0%) | 5 (25.0%) | 5 (3.7%) | |
| Basaloid | 19 (57.6%) | 2 (10.0%) | 14 (10.2%) | |
| Warty | 3 (9.1%) | 1 (5.0%) | 6 (4.3%) | |
| Mixed basaloid/warty | 4 (12.1%) | 0 (0.0%) | 1 (0.7%) | |
| Non-keratinizing | 2 (6.1%) | 0 (0.0%) | 4 (2.9%) | |
| Depth of infiltration (mm) | 5.5 ± 4.5 | 4.9 ± 3.1 | 7.7 ± 5.4 | 0.012 |
| Lympho-vascular invasion | 6 (18.2%) | 3 (15.0%) | 22 (16.1%) | 0.944 |
| Intraepithelial precursors | ||||
| dVIN | 0 (0.0%) | 7† (35.0%) | 75‡ (54.7%) | <0.001 |
| HSIL | 28* (84.8%) | 0 (00.0%) | 0 (0.0%) | |
| vaVIN (VAAD/DEVIL) | 0 (0.0%) | 6 (30.0%) | 0 (0.0%) | |
| No intraepithelial precursor | 5 (15.2%) | 7 (35.0%) | 62 (45.2%) | |
| Associated inflammatory lesions | ||||
| Lichen sclerosus | 0 (0.0%) | 10§ (52.6%) | 59¶ (43.1%) | <0.001 |
| Lichen simplex chronicus | 0 (0.0%) | 1 (5.3%) | 14** (10.2%) | |
| Lichen planus | 0 (0.0%) | 1 (5.3%) | 2 (1.5%) | |
| No lesion | 33 (100%) | 7 (36.8%) | 62 (45.3%) | |
- dVIN, differentiated vulvar intraepithelial neoplasia; vaVIN, verruciform acanthotic vulvar intraepithelial neoplasia; VAAD, vulvar acanthosis with altered differentiation; DEVIL, differentiated exophytic vulvar intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion.
- Bold values indicate significant of P < 0.05.
- * Two lesions with dVIN-like features were identified in this group.
- † Four lesions with HSIL-like features were identified in this group.
- ‡ Twenty-one lesions with HSIL-like features were identified in this group.
- § Two cases showed lichen sclerosus and lichen simplex chronicus.
- ¶ Seven cases showed lichen sclerosus and lichen simplex chronicus.
- ** One case showed lichen simplex chronicus and lichen planus.
HPV-associated VSCC more frequently showed an intraepithelial precursor in the adjacent skin than HPV-independent carcinomas (28/33 [93.9%], versus 88/157 [56.1%]; P = 0.002). HSIL was identified in 28/33 (84.8%) HPV-associated VSCC and in all cases showed strong and diffuse positive staining for p16. The most frequent precursor in HPV-independent VSCC was dVIN (82/157 [52.2%]). A high frequency (30.0%) of vaVIN (VAAD/DEVIL) lesions were identified in the group of HPV-independent p53-normal VSCC. All precursors identified in HPV-independent VSCC were negative for p16.
No inflammatory skin lesions were identified in HPV-associated VSCC, whereas a high percentage of these conditions, mainly lichen sclerosus, were identified in HPV-independent carcinomas.
Clinical outcomes
The mean follow-up duration was 53.1 ± 43.5 months. There were no differences in terms of time of follow-up among groups (P = 0.932). Figure 2 shows the Kaplan–Meier curves for RFS and DSS for HPV-associated versus HPV-independent VSCC with normal and abnormal p53 IHC expression tumours, and early FIGO versus advanced FIGO stages. With a median RFS of 50 months, patients with HPV-independent p53 normal VSCC showed the worst RFS in the log rank test, followed by patients with HPV-independent p53 mutant VSCC and by those with HPV-associated VSCC. Although the differences were not statistically significant, HPV-independent VSCC showed a tendency to a worse DSS than HPV-associated VSCC. Remarkably, patients with HPV-independent normal p53 tumours had a better DSS than patients with HPV-independent abnormal p53 tumours, in spite of having a worse RFS. Patients with an advanced FIGO stage showed a significantly worse DSS.
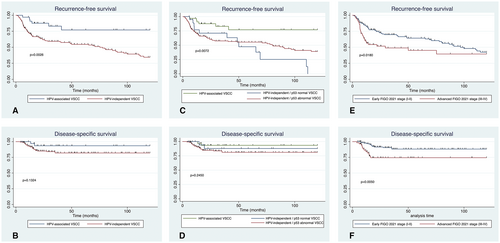
The 10-year recurrence rate of patients with HPV-associated VSCC was significantly lower than that of patients with HPV-independent abnormal p53 and p53 normal carcinomas (6/33 [18.8%] versus 67/137 [48.9%] versus 12/20 [60.0%], P = 0.002). Patients with HPV-independent p53 abnormal VSCC presented the highest mortality rate, followed by those with HPV-independent p53 normal VSCC, and HPV-associated VSCC, although the differences did not reach statistical significance (21/137 [15.3%] versus 2/20 [10%] versus 2/33 [6.1%], P = 0.334).
The univariate and multivariate analyses for RFS and DSS are shown in Table 3. In the univariate analysis, age at diagnosis older than 80, HPV-independent tumours (both p53 normal and abnormal), advanced FIGO 2021 stage, the presence of metastatic lymph nodes and surgical margins affected by premalignant lesion were associated with a worse RFS. HPV-independent p53 normal tumours showed a higher hazard ratio for recurrence (3.63, P = 0.023) than HPV-independent abnormal p53 tumours (2.78, P = 0.028). In the multivariate analysis, the two types of HPV-independent tumours, metastatic lymph nodes, and surgical margins affected by premalignant lesion were associated with impaired RFS. Regarding DSS, an advanced FIGO 2021 stage and metastatic lymph nodes were associated with worse prognosis in the univariate analysis, and an advanced FIGO stage was associated with worse DSS in the multivariate analysis.
| Recurrence-free survival | Disease-specific survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| VSCC type | ||||||||
| HPV-associated | 1 | 1 | ||||||
| HPV-independent with normal p53 | 4.17 (1.56–11.12) | 0.004 | 3.32 (1.10–10.04) | 0.034 | 1.84 (0.26–12.05) | 0.543 | 1.82 (0.26–12.92) | 0.549 |
| HPV-independent with abnormal p53 | 3.21 (1.39–7.41) | 0.006 | 2.78 (1.11–6.94) | 0.028 | 3.05 (0.71–13.01) | 0.132 | 2.85 (0.67–12.19) | 0.157 |
| FIGO stage | ||||||||
| Early (stage I-II) | 1 | 1 | ||||||
| Advanced (stage III-IV) | 1.72 (1.09–2.69) | 0.019 | * | 2.94 (1.3–6.47) | 0.007 | 2.83 (1.29–6.23) | 0.010 | |
| Lymph node metastases | ||||||||
| No | 1 | 1 | ||||||
| Yes | 1.90 (1.18–3.06) | 0.008 | 1.85 (1.14–2.96) | 0.012 | 3.36 (1.41–8.02) | 0.006 | * | |
| Surgical margin affected by tumour | ||||||||
| No | 1 | 1 | ||||||
| Yes | 1.24 (0.60–2.57) | 0.561 | 0.47 (0.06–3.44) | 0.453 | ||||
| Surgical margin affected by premalignant lesion | ||||||||
| No | 1 | 1 | ||||||
| Yes | 1.56 (1.01–2.42) | 0.046 | 1.70 (1.05–2.74) | 0.030 | 0.90 (0.38–2.16) | 0.816 | ||
| Lympho-vascular invasion | ||||||||
| No | 1 | 1 | ||||||
| Yes | 1.55 (0.88–2.73) | 0.130 | 2.15 (0.85–5.40) | 0.104 | ||||
| Size | ||||||||
| Less than 25 mm | 1 | 1 | ||||||
| More than 25 mm | 1.38 (0.89–2.12) | 0.147 | 1.97 (0.49–4.36) | 0.093 | ||||
| Depth of invasion | ||||||||
| Less than 5 mm | 1 | 1 | ||||||
| More than 5 mm | 1.40 (0.91–2.16) | 0.124 | 1.59 (0.71–3.55) | 0.255 | ||||
| Age at diagnosis | ||||||||
| Younger than 80 years | 1 | 1 | ||||||
| Equal or older than 80 years | 1.56 (1.00–2.45) | 0.049 | 1.51 (0.91–2.50) | 0.108 | 1.41 (0.63–3.14) | 0.401 | ||
- VSCC, vulvar squamous cell carcinomas; FIGO, International Federation of Gynaecology and Obstetrics; HPV, human papillomavirus; HR, hazard ratio.
- Bold values indicate significant of P < 0.05.
- * Not included in multivariate model because of collinearity.
Discussion
Our study confirms that HPV-associated and HPV-independent VSCC are different entities, not only in terms of clinical and pathological features, but also in terms of prognosis. Thus, the results of our study support the WHO 2020 classification that has divided this neoplasia into two distinct types based on their etiological relationship with HPV.1, 2 Our results are also in keeping with recent reports indicating that HPV-independent VSCC should be separated into two different types based on the status of p53: HPV-independent VSCC with wildtype (normal p53 IHC expression) and with mutated p53 (abnormal p53 IHC expression), because these two pathological types have differential clinical and pathological features and a different behaviour.12-14
As shown in previous studies, HPV-associated VSCC are clinically distinct: they arise in younger women and are smaller and less invasive at diagnosis than HPV-independent VSCC.12, 14, 31 These parameters have classically been identified as prognostic factors in VSCC.12, 32 Remarkably, in spite of the small size and superficial invasion observed in HPV-associated tumours, no differences were observed in terms of lymph node metastases or FIGO staging at diagnosis when compared to HPV-independent tumours, indicating the potential aggressiveness of the HPV-associated tumours.
HPV-associated and HPV-independent tumours were also histologically different: most HPV-associated tumours showed basaloid or warty features, whereas most HPV-independent tumours were of the keratinizing variant.3, 11 In addition, each tumour type was associated with a specific precursor lesion: HSIL for the HPV-associated, vaVIN (VAAD/DEVIL) for the HPV-independent tumours with normal p53, and dVIN for the HPV-independent tumours with abnormal p53. However, a significant percentage of tumours showed paradoxical morphological features in the invasive tumour (15% of HPV-associated tumours presented keratinizing morphology and 20% of HPV-independent VSCC showed basaloid features) as well as in the precursor lesion, emphasizing the need for ancillary techniques (HPV-DNA detection and/or p16 IHC) to confidently classify VSCC.11 Interestingly, a high proportion (25%) of patients with HPV-independent p53 normal tumours had a verrucous carcinoma, a subtype of VSCC rarely exhibiting lymph node metastasis or fatal outcomes.15, 33, 34
The most relevant results of this study are related to the prognosis of the three pathological types. Several studies have evaluated the relationship between HPV and prognosis in VSCC with controversial results. While some authors have reported that HPV-association is not a prognostic factor,19, 35 recent studies indicate that HPV-associated VSCCs have an improved prognosis.14, 20, 31 Curiously, a previous study by our group did not identify differences in prognosis between HPV-associated and -independent VSCC.18 However, this study included a lower number of patients with a shorter median follow-up time (3.8 years), and classification of the HPV status of VSCC was exclusively based on HPV testing. In the present study we included a large series of patients treated primarily with surgery at a single institution and with a long follow-up period. Women with HPV-associated tumours had a better RFS in the univariate and the multivariate analyses. Some authors suggest that a possible explanation for this phenomenon lies in the better response of the HPV-associated tumours to radiotherapy.12, 31 Indeed, it has been shown that HPV-associated VSCC present better response to radiotherapy,36, 37 a phenomenon which has also been described in HPV-associated tumours of the head and neck.37, 38
The status of the surgical margins and the radicality of the surgical excision may have an impact on the risk of recurrence, which might lead to bias.39, 40 Interestingly, McAlpine et al.31 noted no differences in outcome between HPV-associated and -independent VSCC for the cohort of patients treated before 1995, when treatment was mainly radical surgery, whereas HPV-independent tumours showed worse prognosis in recent years, when more conservative surgical strategies have been adopted.31 In our cohort, the proportion of patients who underwent vulvectomy versus wide local excision were not different between the study groups (P = 0.09), but patients with HPV-associated VSCC showed a higher rate of margin involvement by invasive carcinoma. In univariate analysis, surgical margin affected by the tumour was not associated with worse RFS or DSS, but surgical margin affected by premalignant lesion was associated with worse RFS both in the univariate and the multivariate analysis. This association has been previously reported by other authors41, 42 and highlights the importance of completely excising the premalignant lesion in addition to the invasive tumour.
The different etiopathogenic pathways involved in HPV-associated and -independent VSCC3 confer biological plausibility to the differences identified in prognosis, with different molecular alterations involved in each diagnostic category.43 A large study based on next-generation sequencing has shown that alterations in the PI3K/mTOR pathway are involved in HPV-associated VSCC, while HPV-independent tumours harbour mutations in TP53, TERT, CDKN2A, and CCND1.14, 44-47 Although some of these studies have evaluated the prognostic role of the above-mentioned mutations,14, 45, 48 the limited number of tumours included in these molecular studies precludes reaching strong conclusions. However, in our study only lymph node metastases and an advanced FIGO stage (III or IV) were associated with a worse DSS.
Several recent studies have suggested that HPV-independent VSCC could be divided into two distinct clinical-pathological and molecular entities, HPV-independent p53-normal tumours and HPV-independent p53-abnormal tumours.12-14 Morphologically, the former group of VSCC has been associated with vaVIN (VAAD/DEVIL) precursor lesions.14, 16, 17, 48 Moreover, some authors have molecularly described this subgroup of tumours that have a higher frequency of mutations in PIK3CA, NOTCH1, and HRAS, suggesting that they might have a specific oncogenic pathway.14, 48 Interestingly, in our study patients with HPV-independent, p53 normal tumours, had a high recurrence rate―which is in contrast with the results of the series reported by Nooij et al.,14 who described a lower recurrence rate in patients with HPV-independent, normal p53 tumours than in patients with tumours with abnormal p53. Remarkably, in spite of having a worse RFS, the patients with HPV-independent p53 normal tumours had a better DSS than those with HPV-independent p53 abnormal tumours, indicating that this subgroup might have an intermediate prognosis.
The findings of our study raise the question as to whether all patients with VSCC should undergo the same treatment and follow-up. This is the current approach in most institutions, where all patients with VSCC are treated similarly, irrespective of HPV and p53 status, and with surgery being the main pillar of treatment.30 Increasing evidence indicating that several types of VSCC have different prognosis and different response to radiation therapy, plus the feasibility of presurgical evaluation of HPV, p16, and p53 status, based on IHC and molecular HPV analysis,11, 22, 23, 49 suggest that the treatment of VSCC might be stratified based on the different risk in the near future. To the best of our knowledge, no prospective studies have evaluated the possibility of tailoring VSCC treatment in accordance with HPV or p53 status. However, several investigators12, 50 consider HPV or p53 status when assessing the response to treatment of patients with VSCC.
The single HPV-associated VSCC with p53 abnormal diffuse overexpression is intriguing, as p53 protein is usually degraded in HPV-driven cancers.51 In this tumour both a high-risk HPV PCR positive result and p16 overexpression were present and, thus, the HPV-associated status seems to be unquestionable. A similar case of p16-positive and abnormal p53 with diffuse overexpression has been identified in a recent series of vulvar cancers.13 In addition, sequencing studies have also reported occasional HPV-associated VSCC with TP53 mutations.14, 52 The favourable clinical behaviour of this tumour might indicate that p53 alteration does not confer an adverse outcome in HPV-related VSCC. The main strength of our study is that it is one of largest series of patients with VSCC treated in a single centre in which the prognostic role of HPV and p53 IHC status have been evaluated.
The study also has some limitations. Its retrospective nature and the wide inclusion period of the study might have led to information bias. Indeed, although all patients were treated in accordance with our cancer centre protocols, these were modified during the study period, and this could have affected the outcomes. Nonetheless, the frequency of these patients was balanced among groups.
In conclusion, our results suggest that the association of HPV and p53 status have clinical and prognostic implications, reinforcing the three-tier molecular classification of VSCC, HPV-associated VSCC, HPV-independent VSCC with normal (wildtype) p53, HPV-independent VSCC with abnormal (mutated) p53, described by other authors. Further prospective studies are needed to explore the possibilities of tailoring the treatment of VSCC patients in accordance with these molecular subgroups.
Acknowledgements
Project “PI20/00368; Caracterización genómica de los carcinomas de vulva independientes de virus del papiloma humano y de sus precursores”, funded by Instituto de Salud Carlos III and co-funded by the European Union (ERDF) “A way to make Europe”. ISGlobal receives support from the Spanish Ministry of Science and Innovation through the “Centro de Excelencia Severo Ochoa 2019-2023” Program (CEX2018-000806-S), and support from the Generalitat de Catalunya through the CERCA Program.
Authors contributions
Nuria Carreras-Dieguez: study design, investigation, methodology, statistical analysis, writing of the first draft, writing, and editing; Adela Saco: investigation, methodology, writing, and editing; Marta del Pino: investigation, writing, and editing, statistical analysis; Lorena Marimon: investigation, methodology, writing, and editing; Ricardo López del Campo: investigation, writing, and editing; Carolina Manzotti: investigation, writing, and editing; Pere Fusté: investigation, writing, and editing; Clàudia Pumarola: investigation, writing, and editing; Aureli Torné: study design, resources, investigation, methodology, writing, and editing, resources, project management; Adriana Garcia: study design, investigation, writing, and editing, Natalia Rakislova: study design, resources, investigation, methodology, writing, and editing, project management.
Funding information
Funded by the Spanish Instituto de Salud Carlos III (FIS, PI20/368; NR).
Conflict of interest
The authors declare no conflicts of interest.
Patient consent statement
Study consent was obtained from all the patients enrolled in the study.
Open Research
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.