BRCA1 and BRCA2 carriers with breast, ovarian and prostate cancer demonstrate a different pattern of metastatic disease compared with non-carriers: results from a rapid autopsy programme
Abstract
Aim
To catalogue and compare the pattern of metastatic disease in germline BRCA1/2 pathogenic mutation carriers and non-carriers with breast, ovarian and prostate cancer from a rapid autopsy programme.
Methods and results
The number of metastases in the major body systems and the proportion of participants with metastases were documented in 50 participants (19 germline mutation carriers). Analysis was conducted on the participants’ pattern of disease for the different cancers and mutation subgroups. The four commonly affected organ systems were the digestive (liver only) (82%), respiratory (76%), gastrointestinal (65%) and reticuloendothelial (42%). There were significant differences in the pattern of metastatic breast cancer in BRCA1/2 germline carriers compared with non-carriers. Breast cancer carriers had significantly fewer organ systems involved (median n = 3, range = 1–3) compared with non-carriers (median n = 9, range = 1–7) (P = 0.03). BRCA1/2 carriers with ovarian carcinomas had significantly more organ systems with metastatic carcinoma (median n = 10, range = 3–8) than non-carriers (median n = 5, range = 3–5) (P < 0.001). There were no significant differences in the number of involved systems in BRCA2 carriers compared with non-carriers with prostate cancer (P = 1.0). There was an absence of locoregional disease (6.5%) compared with distant disease (93.5%) among the three cancer subtypes (P < 0.001). The majority of metastatic deposits (97%) collected during the autopsy were identified by recent diagnostic imaging.
Conclusion
Even though a major limitation of this study is that our numbers are small, especially in the breast cancer carrier group, the metastatic patterns of breast and ovarian cancers may be impacted by BRCA1/2 carrier status, suggesting that tumours derived from patients with these mutations use different mechanisms of dissemination. The findings may focus clinical diagnostic imaging for monitoring metastases where whole-body imaging resources are scant.
Graphical Abstract
This study catalogued and compared the pattern of metastatic disease in germline BRCA1/2 pathogenic mutation carriers and non-carriers with breast, ovarian and prostate cancer from a rapid autopsy programme. Our findings suggest that metastatic patterns of breast and ovarian cancers maybe impacted by BRCA1/2 carrier status, suggesting that tumours derived from patients with these mutations use different mechanisms of dissemination.
Introduction
The number of clinical autopsies performed has decreased significantly in recent times due a combination of a paucity of funding, fewer autopsy facilities and a reduction in the number of trained pathologists and mortuary staff.
Pre- and postmortem magnetic resonance imaging (MRI), computerised tomography (CT) and positron emission tomography (PET) imaging is increasingly used as a surrogate for clinical autopsies, but with an awareness that imaging may not be suitable for all tumour morphologies.1, 2 This waning number of clinical autopsies has led to the establishment of research-driven rapid autopsy programmes to support innovative research in the era of precision medicine.3-5 These programmes have highlighted the complexity of clonal evolution, pathways involved in drug resistance, new treatment options based on a patient's genomic profile5-7 and generated patient-derived xenograft models to facilitate downstream in-vivo and in-vitro research activities.8-10 Others have reported cancer type-specific metastatic patterns of spread,11-14 a major limitation of these studies is that only the heavy burden of disease in common affected sites, such as bone, lung and liver, has been investigated.14-17 Thus, a thorough audit of all body systems in patients with metastatic disease, irrespective of the burden of disease at autopsy, is warranted.
CASCADE is a prospective rapid autopsy programme that includes the Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (www.kconfab.org) and the Australian Ovarian Cancer Study (www.aocs.org).18, 19 The programme has led to advances in how treatment drives genomic heterogeneity in breast cancer, the molecular events associated with acquired drug resistance in ovarian cancer and how ribosome-targeting drugs may be effective against castrate resistant prostate cancer.20-22
We have catalogued the anatomical systems with metastatic disease in CASCADE participants with a diagnosis of breast (n = 19), ovarian (n = 23) and prostate (n = eight) cancer who were germline mutation BRCA1/2 carriers and non-carriers. The aims of this study were to: (1) accurately document the systems with metastatic disease in carriers and non-carriers with breast, ovarian and prostate cancers and (2) identify if there were differences in the pattern of metastatic disease that might help to identify new avenues into the mechanisms of metastatic spread in BRCA1/2 cancers.
Methods
CASCADE programme
Participants with a strong family history of breast, ovarian or prostate cancer and/or a confirmed carrier or non-carrier of a germline BRCA1/2 mutation were enrolled into CASCADE. Information regarding institutional review board approvals, the participant information and consent form, approach to participants, the autopsy procedure and tissue collection protocol has been published.19 Demographic variables collected include age at diagnosis, date of death, germline mutation status, verified cancer and first-line treatment. Participants from the CASCADE network of the Peter MacCallum Cancer Centre, Mercy Hospital and The Royal Women's Hospital, Melbourne received uniform standardised treatment(s), including clinical trial participation with investigational agents.
The first biopsy from the clinical pathology report was the diagnosis date. The participants’ first-line treatment and cancer characteristics were sourced from clinical pathology reports, imaging, surgical notes, radiotherapy and chemotherapy schedules.
All participants were in a palliative phase when consented to CASCADE. Confirmation of a participant's germline mutation status was performed in the Peter MacCallum Molecular Pathology NATA-accredited (ISO:15189) laboratory. Variants were assigned class C4–C5 status according to a five-tier clinical classification by ENIGMA (http://www.enigmaconsortium.org). Participants’ non-carrier variant status was defined as complete genetic screening of BRCA1, BRCA2, PALB2, TP53, ATM 7271 and CHEK2 1100delC, and no C4-5 variant has been detected. The BRCA1/2 variants detected were varied and not clustered into a single region of the gene: 18 frameshift, nonsense, missense mutations, one large genomic rearrangement (Table 1).
| Participant ID | Mutation nomenclature |
|---|---|
| Breast cancer carrier participants | |
| Participant 1 | BRCA1 exon 21_24del |
| Participant 2 | BRCA2 c. 3847_3848delGT exon 11 |
| Participant 3 | BRCA2 c.250C>T exon 3 |
| Ovarian cancer carrier participants | |
| Participant 4 | BRCA1 c. 546 G>T |
| Participant 5 | BRCA1 c. 3519 G>T |
| Participant 6 | BRCA2 c. 771_775delTCAAA exon 9 |
| Participant 7 | BRCA2 c.4211 C>G exon 11 |
| Participant 8 | BRCA2 c.5722_5723delCT exon 11 |
| Participant 9 | BRCA2 c.1313dupA exon 10 |
| Participant 10 | BRCA1 c.5503C>T exon 24 |
| Participant 11 | BRCA1 c.5345G>A |
| Participant12 | BRCA1 5212G>A exon 20 |
| Participant 13 | BRCA1 c.2681_2682 del AA exon 11 |
| Participant 14 | BRCA2 c.5946delT exon 11 |
| Participant 11 | BRCA1 c.5345G>A |
| Participant 12 | BRCA1 5212G>A exon 20 |
| Prostate cancer carrier participants | |
| Participant 15 | BRCA2 c.767insAT exon 7 |
| Participant 16 | BRCA2 c.8813_8814 insT (STOP 2868) |
| Participant 17 | BRCA2 c.5303_5304del exon 11 |
| Participant 18 | BRCA2 c.978_983del4 exon 9 |
| Participant 19 | BRCA2 c.8575del exon 20 |
The pattern of metastatic disease within 10 body systems [central nervous system (CNS), musculoskeletal, reproductive (including the breast), urinary, endocrine, reticuloendothelial, gastrointestinal (including peritoneal), respiratory, cardiovascular and digestive (liver only)] was formally documented during a full autopsy at the Victorian Institute of Forensic Medicine (VIFM), Melbourne, and cross-checked against a tissue action sheet that records all metastatic sites collected. Imaging that occurred just prior to the autopsy was utilised.19 The majority (98%) of participants had clinical imaging performed, on average, 8 weeks (mean = 13.1, range = 1–55 weeks) before death. Locoregional recurrence was defined as: breast involving ipsilateral breast, chest wall, lymph nodes, ovarian involving the pelvic sidewalls, para-aortic or pelvic lymph nodes and prostate involving the prostatic bed. The time from death to the autopsy was on average 6.8 h (range = 3–17 h).
Participants
The study cohort of 50 comprised kConFab (n = 30) and AOCS (n = 20) participants with a verified diagnosis of breast cancer (n = one BRCA1, n = two BRCA2 carriers, n = 16 non-carriers), ovarian cancer (n = six BRCA1, n = five BRCA2 carriers, n = 12 non-carriers) and prostate cancer (n = five BRCA2 carriers, n = three non-carriers). The autopsies were performed between February 2012 and September 2020. Table 2 shows the clinicopathological features of the cohort.
| No. of patients (n = total) | BRCA1 C4-5 carriers | BRCA2 C4-5 carriers | BRCA non-carriers* | Significance of difference between carriers and non-carriers in age at diagnosis and age at death (Fisher's P-value) |
|---|---|---|---|---|
| Breast cancer | n = 1 | n = 2 | n = 16 | |
| Mean/median age at diagnosis (range) | 37/37 (37) | 38/38 (33–43) | 42.5/42.5 (34–54) | |
| Mean/median age at death (range) | 43/43 (43) | 45.5/45.5 (43–48) | 51.0/50.5 (38–69) | |
| Mean/median duration from diagnosis to death (years) | 6.3/6.3 | 7.4/7.4 | 8.6/6.4 | |
| T-stage† at first diagnosis (%) | ||||
| ≤ T2 | 7 (43.7)§ | Age at diagnosis P = 0.227 | ||
| T3–T4 | 1 (100) | 2 (100) | 9 (56.2)¶ | Age at death P = 0.0486 |
| Morphology‡ (%) | ||||
| Infiltrating ductal carcinoma | 1 (100) | 13 (81.2) | ||
| Infiltrating ductal and lobular carcinoma | 1 (50) | 1 (6) | ||
| Mucinous carcinoma | 1 (50) | |||
| Ductal and lobular carcinoma in situ (high grade) | 1 (6) | |||
| Ductal carcinoma in-situ (high-grade) | 1 (6) | |||
| First-line treatment (%) | ||||
| Mastectomy | 0/1 (0) | 2/2 (100) | 11 (56) | |
| Neoadjuvant chemotherapy | 1/1 (100) | 1/2 (50) | 4 (25)** | |
| Adjuvant chemotherapy | 0/1 (0) | 1/2 (50) | 8 (50)†† | |
| Radiotherapy | 1/1 (100) | 0/2 (0) | 2 (12) | |
| Risk-reducing oophorectomy pre-breast cancer diagnosis | 0/1 (100) | 0/2 (100) | 0 (100) | |
| Risk-reducing oophorectomy post-breast cancer diagnosis. Normal morphology | 1/1 (100) | 2/2 (100) | 5 (31) | |
| Oophorectomy never performed, metastatsis in the peritoneal cavity detected at autopsy | 3/11 (19) | |||
| Ovarian cancer | n = 6 | n = 5 | n = 12 | |
| Mean/median age at diagnosis (range) | 51.8/49 (36–62) | 53.6/53 (38–68) | 64.9/63 (41–85) | |
| Mean/median age at death (range) | 55.8/52.5 (38–67) | 61.2/ 63.5 (51–76) | 68.3/65 (43–87) | |
| Mean/median duration from diagnosis to death (years) | 4.11/4.6 | 5.5/5.9 | 3.3/4.2 | |
| T-stage at first diagnosis (%) | ||||
| ≤ T2 | 3 (25) | Age at diagnosis P = 0.0201 | ||
| T3–T4 | 6 (100) | 5 (100) | 8 (67) | |
| Unknown | 1 (8) | Age at death P = 0.0395 | ||
| Morphology (%) | ||||
| High-grade serous adenocarcinoma | 6 (100) | 5 (100) | 10 (83) | |
| High-grade serous adenocarcinoma with mixed endometrial cancer | 1 (8) | |||
| Mucinous | 1 (8) | |||
| First-line treatment (%) | ||||
| TAH-BSO‡‡ omentectomy | 4 (67) | 2 (40) | 7 (58) | |
| Omentectomy and oophorectomy | 1 (17) | 5 (42) | ||
| BS0, omentectomy | 1 (17) | 4 (33) | ||
| Biopsy only | 3 (60) | 6 (50) | ||
| Neoadjuvant chemotherapy | 5 (83) | 2 (40) | ||
| Adjuvant chemotherapy | 1 (17) | 3 (60) | ||
| Prostate cancer | n = 5 | n = 3 | ||
| Mean/median age at diagnosis (range) | 64.4/63 (48–78) | 52.6/52.5 (49–56) | ||
| Mean/median age at death (range) | 71/67 (53–81) | 58/59 (54–64) | ||
| Mean/median duration from diagnosis to death (years) | 6.5/6.4 | 5.9/6.5 | ||
| T-stage at first diagnosis (%) | ||||
| ≤ T2 | 2 (40) | Age at diagnosis P = 0.113 | ||
| T3–T4 | 3 (60) | 3 (100) | Age at death P= 0.0987 | |
| Morphology and Gleason score | ||||
| Adenocarcinoma | 5(100) | 3 (100) | ||
| 1 (20) 5 + 4 | 1 (33) 4 + 5 | |||
| 1 (20) 4 + 4 | 1(33) 4 + 3 | |||
| 1(20) 4 = 5 | 1 (33) 3 + 4 | |||
| 1 (20) 4 + 3 | ||||
| 1 (20) UK | ||||
| First-line treatment | ||||
| Radical prostatectomy | 0 | 2 (67) | ||
| Radiotherapy | 1 (20) | 1 (33) | ||
| Anti-hormone | 3 (60%) | 2 (67) | ||
| Adjuvant chemotherapy | 1 (20) | |||
- BSO, bilateral salpingo-ophorectomy; TAH, total abdominal hysterectomy.
- * Participants had complete sequencing and MLPA of the BRCA1, BRCA2, PALB2, TP53, ATM 7271, CHEK2 1100delC gene and no C4–C5/pathogenic mutation was detected.
- † Highest T-stage used from clinical or pathology report.
- ‡ Breast cancer subtype immunohistochemistry; ×1 BRCA1 carrier basal phenotype, ×2 BRCA2 carriers, ×1 Luminal A and ×1 Luminal B. Non-carriers, three of 16 (19%) with basal phenotype, two of 16 (12%) Her-2-positive, six of 16 (38%) Luminal A, five of 16 (31%) Luminal B.
- § ×1 participant at diagnosis was stage T2 N0 M0 had curative surgery but never received chemotherapy, another participant was stage T1 Nx Mx at diagnosis and never received chemotherapy.
- ¶ ×2 participants with stage 4 at diagnosis never received chemotherapy.
- ** ×2 participants received neoadjuvant AC-T, ×1 participant FEC, ×1 docetaxel.
- †† ×6 participants received adjuvant AC-T, ×2 FEC.
- ‡‡ Gleason score at time of diagnosis from the biopsy.
Forty-six of 50 (92%) participants had had one primary cancer ever diagnosed. Pathology review of all cases indicated that all died of metastatic disease linked to their corresponding primary diagnosis and were correctly allocated into their tumour stream for analysis. Two BRCA1 carriers with ovarian cancer had a primary breast cancer diagnosed at ages 43 and 42 years before their diagnosis of primary ovarian cancer, both at age 62 years. One BRCA2 carrier with ovarian cancer had a diagnosis of melanoma at age 68 years, 3 years before her diagnosis of ovarian cancer. One BRCA2 carrier had a diagnosis of breast cancer at age 78 years, 7 years after his diagnosis of prostate cancer, which was the cause of death.
Patient consent statement
All potential participants are given the Participants Information and Consent Form, which informs them about the research project and explains the procedures that are involved. Signed consent by the participant and next-of-kin must be obtained before involvement in the CASCADE programme.
Statistical analysis
Fisher's exact test was used to compare the date of diagnosis and death between BRCA1/2 carriers and non-carriers (Table 2) and the locoregional compared with distant disease across the three tumour streams. A permutation simulation incorporating the number of participants in each tumour group calculated the significance of difference in the number of shared metastatic sites between the BRCA1/2 carrier and non-carrier groups (Table 3).
| Central nervous system | Musculoskeletal | Reproductive | Urinary | Endocrine | Reticuloendothelial | Gastrointestinal | Respiratory | Cardiovascular | Digestive | P * | |
|---|---|---|---|---|---|---|---|---|---|---|---|
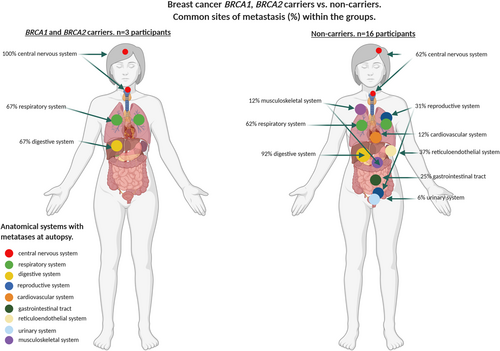
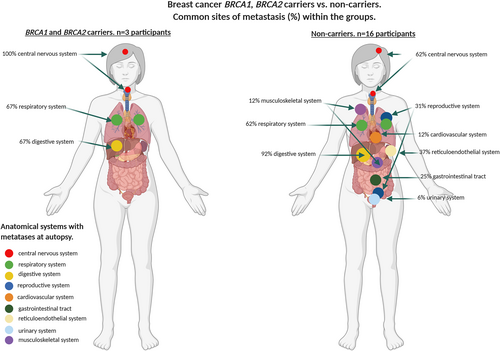
| Breast cancer BRCA1/2 carriers n = 3 | 3/3 (100%) | – | – | – | – | – | – | 2/3 (67%) | – | 2/3 (67%) | 0.0339 |
| Breast cancer non-carriers n = 16 | 10/16 (62%) | 2/16 (12%) | 5/16 (31%) | 1/16 (6%) | – | 6/16 (37%) | 4/16 (25%) | 10/16 (62%) | 2/16 (12%) | 15/16 (92%) | |
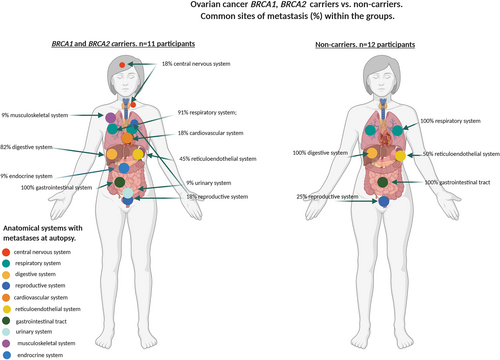
| Ovarian cancer BRCA1/2 carriers n = 11 | 2/11 (18%) | 1/11 (9%) | 3/11 (18%) | 1/11 (9%) | 1/11 (9%) | 5/11 (45%) | 11/11 (100%) | 10/11 (91%) | 2/11 (18%) | 9/11 (82%) | < 0.001 |
| Ovarian cancer non-carriers n = 12 | – | – | 3/12 (25%) | – | – | 6/12 (50%) | 12/12(100%) | 12/12 (100%) | – | 12/12 (100%) | |
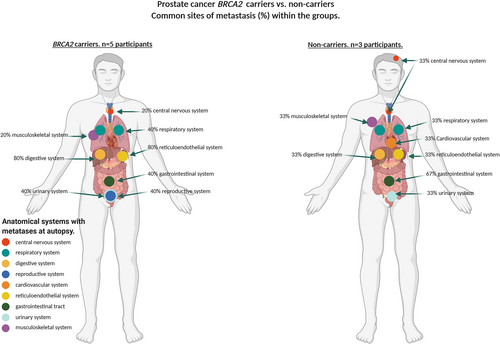
| Prostate cancer BRCA1/2 carriers n = 5 | 1/5 (20%) | 1/5 (20%) | 2/5 (40%) | 2/5 (40%) | – | 4/5 (80%) | 2/5 (40%) | 2/5 (40%) | – | 4/5 (80%) | 1 |
| Prostate cancer non-carriers n = 3 | 1/3 (33%) | 1/3 (33%) | – | 1/3 (33%) | – | 1/3(33%) | 2/3 (67%) | 1/3 (33%) | 1/3 (33%) | 1/3 (33%) |
- * The P-values indicate the significance of the difference in the number of MET sites between carriers and non-carriers. They are derived using a permutation simulation incorporating the number of participants in each group.
Ethics approval statement
The CASCADE ‘Tissue donation after death to improve our understanding of the progression from primary stage cancer to metastatic disease; incorporating the CAncer tissue Collection After DEath (CASCADE)’ is approved by the Peter MacCallum Cancer Centre, HREC 11/102.
Results
Locoregional versus distant disease in carriers and non-carriers
Metastatic distant disease in all participants was recorded and collected at autopsy and observed at a significantly higher frequency (93.5%) compared with locoregional disease (6.5%) for carriers and non-carriers across the three tumour streams (P < 0.001): breast 64 versus three sites, ovarian 84 versus seven sites and prostate 25 versus two sites, respectively. There was more locoregional disease recorded (4.6%) in the non-carriers with breast cancer than BRCA1/2 carriers (0%), whereas there was more locoregional disease in carriers with prostate cancer (8%) compared with non-carriers (0%) (P = 0.54); there was no difference in locoregional disease in carriers with ovarian carcinoma (8.7%) compared with non-carriers (6.7%) (P = 1).
Metastatic dissemination in carriers and non-carriers
Metastases in all participants were most common in the digestive system (liver) (82%), respiratory (76%), gastrointestinal (65%) and reticuloendothelial (42%) systems. BRCA1/2 mutation carriers with breast cancer overall had significantly fewer systems involved by metastases (median n = 2, range = 1–3) compared with non-carriers (median n = 4, range = 1–7) (P = 0.03). In contrast, carriers with ovarian cancer had significantly more affected systems (median n = 3, range = 3–8), compared with non-carriers (median n = 4, range = 3–7) (P < 0.001). There was no statistically significant difference in the number of sites in BRCA2 carriers with prostate cancer (median n = 3, range = 2–6) compared with non-carriers (median n = 2, range = 1–6). These findings were confirmed by a permutation simulation that incorporates the number of participants in each tumour stream and indicates the difference in the number of metastatic sites between the BRCA1/2 carriers versus non-carrier groups (Table 3 and supporting information online, Table S1).
Affected systems by tumour type and carrier status
There were differences in the affected systems in the breast, ovarian and prostate cancers and within each of the tumour types by carrier status. The frequency of CNS involvement was significantly higher in carriers with breast cancer (100%) compared with non-carriers (62%), and there was also a difference in the frequency of involvement in the digestive system between carriers (67%) and non-carriers (92%) (P = 0.0339). There was a higher frequency of involvement in the digestive system of prostate cancer carriers (80%) compared with non-carriers (33%) that was not significant. There was no significant difference in the frequency of metastatic involvement in the respiratory and digestive systems of carriers and non-carriers with ovarian carcinoma (91% carriers; 100% non-carriers; 82% carriers; 100% non-carriers, respectively); however, there was a significant difference in CNS metastatic involvement in patients with ovarian cancer between carriers (18%) and non-carriers (0%) (P = 0.001) (Table 3 and supporting information online, Table S1, Figures 1-3).
Tumour phenotype and site of metastases in patients with breast cancer
Table 2 details the tumour phenotype of carrier and non-carrier breast cancer patients. While the frequency of CNS metastases was lower in non-carriers compared with carriers, no discernible difference in phenotype was identified in those participants with CNS metastases. Possibly by virtue of numbers in the carrier compared with the non-carrier group (three versus 16), there were no primary HER-2-positive breast cancers in the carrier group. The single BRCA1 breast carrier with CNS involvement had a triple-negative basal phenotype in the primary biopsy prior to neoadjuvant therapy, but subsequently presented with HER-2-positive disease in the CNS metastasis with unequivocal high-level amplification by in-situ hybridisation.23
Comparison of the frequency and pattern of metastatic spread in carriers and non-carriers identified with imaging
One hundred and eighty-one of 185 (97%) systems with metastatic deposits collected were identified by the diagnostic imaging (MRI-CT-PET) scans performed prior to the autopsy. The four metastatic sites not identified on imaging, but identified at autopsy in three patients with ovarian cancer, were due to no imaging of the CNS (n = 1), a breast metastasis (n = 1) and an endocrine tumour (n = 1) that were unlikely to be present at the last diagnostic scan performed 12 months prior to autopsy. The other missed lesion was a metastatic node at the base of the neck in a breast non-carrier who had had a CT scan 4 months prior to death.
Discussion
This is the first prospective study, to our knowledge, that has compared the pattern of metastatic disease from rapid autopsies of patients with breast, ovarian and prostate cancer with and without germline BRCA1/2 mutations.
As no other studies have documented the pattern of metastatic disease at autopsy in a similar cohort it is not possible to relate this study directly to others, which have relied mainly upon diagnostic imaging or medical records for frequency and patterns of common sites of metastatic spread. In this study we have identified widespread metastases among multiple body systems, with the CNS, digestive, respiratory, gastrointestinal and reticuloendothelial systems being most affected.11-14, 17 We also identified striking differences in the pattern and frequency of metastases between breast and ovarian cancer mutation carrier and non-carriers. Specifically, BRCA1/2 mutation carriers with breast cancer had significantly fewer systems involved with metastases compared with non-carriers. Although the numbers are small, the systems and cancer subtypes that were involved in carriers and non-carriers differed substantially; for example, 100% of breast cancer carriers had CNS involvement whereas only 62% of non-carriers involved this system, the BRCA1 carrier having a basal phenotype and BRCA2 carriers and non-carriers having mixed phenotypes. Although the primary had a basal phenotype, the metastasis was HER2-amplified in the BRCA1 carrier with a CNS metastasis. This is likely to have occurred through clonal HER-2 expansion, with HER-2-positive disease having a predilection for CNS metastases.23, 24 These findings are supported by a review of MRIs from now-deceased kConFab BRCA1/2 carriers (n = 9) with breast cancer not part of the CASCADE programme. The number of metastatic sites in carriers is low: one to four versus one to three in the autopsy series.
A direct comparison between our study and the breast cancer study of Cummings et al.25 is not possible, as their patients were not assessed on family history or mutation status. Nevertheless, they reported 945 metastases and provided a population-based list of metastatic sites; the most common involved the lung-pleura (80%) and uncommon sites, kidney, gastrointestinal tract, pituitary, which harboured metastases in 11.7, 10.7 and 8.6% of patients, respectively. Both studies recorded a higher percentage of liver metastases in patients diagnosed with breast cancer ≤ 49 years (Cummings 71.6% versus 67% in BRCA1/2 carriers, 92% in non-carriers).
In contrast to other groups26 who have shown that MRI is not a good surrogate for assessing the degree of tumour dissemination, we have shown that a full-body CT-MRI-PET scan performed close to death provides an accurate assessment for detecting even low-volume metastatic disease. This might be due to the highly specialised radiology performed in our cancer centre where the patients were treated, rather than community-based radiology.
With regard to the higher incidence of CNS disease, we observed that BRCA1 mutation carriers compared with non-carriers correlated with the registry approaches reported by Song et al.26 and Albiges et al.27 that used imaging. Our observed higher incidence of CNS involvement compared to these other studies is due probably to their use of older cohorts (1981–2014 and 1969–2004, respectively), when patients did not have routine brain imaging. Our findings suggest that the predilection for the CNS might be dependent upon carrier status, the corollary of which is that different mechanisms of dissemination are being utilised.
In contrast to breast cancer, we observed that patients with ovarian cancer had a low frequency of CNS involvement, similar to findings reported by others in high-risk and general populations of patients with ovarian cancer.12, 28 We also observed that, whereas no non-carriers with ovarian carcinoma had CNS involvement identified at autopsy, carriers with ovarian carcinoma had a one-in-five prevalence of developing cerebral metastases. BRCA1/2 mutation carriers with ovarian cancer had greater tumour dissemination with twice the number of organ systems involved by metastases compared with non-carriers, which could have implications for surveillance strategies.
There are limited data from non-autopsy confirmed studies on the frequency and pattern of metastatic spread in BRCA1/2 carriers with breast and ovarian cancer, and no data available on the rate and pattern of metastatic spread in prostate cancer patients with germline mutations. Although there was no difference in the overall number of systems with metastasis, the reticuloendothelial and digestive systems were significantly more frequently involved in the BRCA2 carriers compared with non-carriers with prostate cancer. This observation of a predilection for certain organ systems in carriers with specific tumour subtypes, in addition to the hypothesis of the microenvironment supporting a shorter time for metastatic tumour growth in some organs, as described in brain metastases,29, 30 highlights the opportunity to define these underlying mechanisms. Further interrogation of the autopsy samples together with the differences in the ‘soil’ of the sites will help to identify the key processes that drive the observed differential tumour metastases and help in the recognition of potential new pathways for treatments. The major limitation of this study is that our numbers are small, particularly when looking at tumour types separately highlighting the logistical challenge of conducting such studies.
While patients close to death do not typically have any imaging unless clinically indicated, this study highlights that full-body imaging close to death provides an accurate recording of metastatic sites and could help to determine appropriate treatment options to improve outcome in these generally young patients. Understanding the differences in metastatic spread in carriers and non-carriers within the different tumour types has the potential to refine and augment personalised treatment development between BRCA1/2 carrier and non-carrier groups.
Acknowledgements
The CASCADE programme is funded by a grant from the Peter MacCallum Cancer Foundation. The CASCADE investigators would like to acknowledge the support and guidance of the CASCADE Management Committee, including the contributions of our community representatives, Ms Shirley Carvosso and Ms Heather Watson and Colleen Niland, Metro North HHS, Pathology Queensland and Newhaven Funerals for their contributions. We sincerely thank the efforts of hospital and hospice staff involved in the care of CASCADE participants, the staff at Tobin Brothers Funerals, Melbourne and the Victorian Institute of Forensic Medicine, Melbourne and the participants and their families. Open access publishing facilitated by The University of Melbourne, as part of the Wiley - The University of Melbourne agreement via the Council of Australian University Librarians.
Conflicts of interest
None to declare by any of the authors.
Open Research
Data availability statement
Researchers are granted access to the collected CASCADE biospecimens samples and data after they receive project approval by a Human Research Ethics Committee followed by a formal review and approval by the CASCADE access committee.