Safety and effectiveness of lusutrombopag in patients who have chronic liver disease with thrombocytopenia and undergoing invasive procedures: Real-world post-marketing surveillance in Japan
Abstract
Aim
Lustrombopag has been approved for the treatment of thrombocytopenia in patients with chronic liver diseases who are scheduled to undergo an invasive procedure. Here, we report the final results of a post-marketing surveillance assessing the safety and effectiveness of lusutrombopag in Japan.
Methods
This multicenter, prospective, real-world surveillance collected data from case report forms between October 2016 and May 2021. The observation period was 2 months after the first day of lusutrombopag treatment. Safety and effectiveness (proportion of patients avoiding preoperative platelet transfusion and responders who achieved platelet count increase from baseline) were assessed.
Results
The safety analysis set included 1033 (100.0%), 130 (12.6%), and 14 (1.4%) patients who received one or more, two or more, and three or more treatment cycle(s), and 482 (48.9%), 457 (46.3%), and 43 (4.4%) patients who were Child–Pugh class A, B, and C, respectively. The most common serious adverse drug reactions were portal vein thrombosis, pancytopenia, and white blood cell count decrease, reported in 14 (1.36%), three (0.29%), and two (0.19%) patients, respectively. The incidence of adverse drug reactions was not higher in patients with Child–Pugh class C or those undergoing retreatment cycles compared with other Child–Pugh classes or the first treatment cycle, respectively. During the observation period of the first treatment cycle, 94.7% (889/939) of patients avoided preoperative platelet transfusion and 82.8% (741/895) of the patients met the responder criteria.
Conclusions
This surveillance study further supports the safety and effectiveness of lusutrombopag in a broad range of patients with chronic liver diseases undergoing planned invasive procedures.
Clinical Trial Registration
JapicCTI-163432.
Abbreviations
-
- ADRs
-
- adverse drug reactions
-
- AEs
-
- adverse events
-
- CLD
-
- chronic liver disease
-
- CP
-
- Child–Pugh
-
- CRFs
-
- case report forms
-
- GPSP
-
- Good Post-marketing Study Practice
-
- PMS
-
- post-marketing surveillance
-
- SD
-
- standard deviation
-
- TPORA
-
- thrombopoietin receptor agonist
INTRODUCTION
Thrombocytopenia is one of the common hematological complications in patients with chronic liver disease (CLD).1 Patients with CLD often require invasive procedures for diagnostic or treatment purposes.2
However, bleeding risk is a concern in invasive procedures, particularly in CLD patients with low platelet counts.3-5 Prophylactic platelet transfusion is commonly used to reduce the bleeding risk.1, 6 There are limitations associated with platelet transfusions, such as adverse reactions, short duration of effectiveness, and logistical concerns.3, 7
Lusutrombopag is a second-generation, oral thrombopoietin receptor agonist (TPORA) approved for treatment of thrombocytopenia in patients with CLD before invasive procedures.
Several clinical trials and studies have been conducted to investigate the safety and efficacy of lusutrombopag in CLD patients with thrombocytopenia who have a planned invasive procedure.8-11 Phase III studies have been conducted in Japan (L-PLUS 1 study)10 and outside Japan (L-PLUS 2 study).11
Lusutrombopag was approved in Japan in 2015 for the treatment of thrombocytopenia associated with CLD in patients undergoing planned invasive procedures; however, its use in patients with severe CLD classified as Child–Pugh (CP) class C, has been contraindicated.12 This was because patients with CP class C were excluded in the phase III clinical trials of lusutrombopag.10, 11
The drug was subsequently approved in the USA in 2018 for treatment of thrombocytopenia in adult CLD patients who are scheduled to undergo a procedure, and in the EU in 2019 for treatment of severe thrombocytopenia in adult CLD patients undergoing invasive procedures. The drug was approved in the USA and EU without CP class restrictions.13, 14 Notably, thrombotic/thromboembolic complications are precautions of lusutrombopag treatment, as TPORA could potentially increase the risk for thrombosis by increasing platelet count.15
Interim results of a large post-marketing surveillance (PMS) in Japan suggested the safety and effectiveness of lusutrombopag in clinical practice,16 and here we report the final results, in particular focusing on thrombotic events, and the effectiveness of lusutrombopag in a broad range of CLD patients with thrombocytopenia who underwent an invasive procedure in real-world clinical practice.
METHODS
Study setting and data collection
The surveillance was conducted at 218 sites in Japan between October 2016 and May 2021, and the enrollment period was between October 2016 and September 2020. Patients with CLD and thrombocytopenia undergoing invasive procedure were enrolled centrally within 5 days of starting a treatment cycle with lusutrombopag.
The recommended dosage of lusutrombopag is 3 mg orally once daily for 7 days. It is recommended to begin lusutrombopag dosing 8–13 days before a scheduled procedure. Treatment decisions were made at the attending clinician's discretion, according to local prescribing information; that is, for patients with a high risk of bleeding during an invasive procedure according to the patient's clinical signs and laboratory test results including platelet count.12 It is also noted that the patient's platelet count should be monitored at least once around 5 days after the first day of lusutrombopag treatment. When the platelet count increases by ≥20 × 109/L before the treatment and achieves ≥50 × 109/L, appropriate measures, such as discontinuation of lusutrombopag, should be taken.12
Patients were observed for 2 months following the first day of lusutrombopag treatment (first treatment period). When additional treatment cycle(s) were required within 6 months after the first day of treatment, the patients were observed for 2 months following the first day of each additional treatment cycle(s) (retreatment period[s]).
Participating physicians were asked to complete case report forms with patient data. If re-administration was carried out, the physicians completed another survey form for the retreatment. The collected data included baseline characteristics, clinical data of CLD, comorbidities (including thrombosis or thromboembolism), use of lusutrombopag (including dosage and duration), planned invasive procedures, presence/absence of concomitant drugs, platelet transfusion during the observation period, concomitant therapy during the survey period, and examinations of portal vein thrombosis (PVT) and flow direction, clinical laboratory tests (including platelet counts), and adverse events (AEs).
This surveillance was carried out in accordance with the Good Post-marketing Study Practice (GPSP) for medicinal products in Japan and was registered with the Japan Pharmaceutical Information Center (identifier: JapicCTI-163432). According to exemptions under the GPSP Ordinance No. 171, 2004 by the Ministry of Health, Labor, and Welfare, ethics approval by the institutional review board and informed consent were not required.
Outcome measurements
Safety measurements included the types and frequency of AEs observed during the study period (including abnormal fluctuations of the laboratory test results), the seriousness of the AEs, and the causal relationship between lusutrombopag treatment and the AEs. Serious AEs were defined as events causing death, those that were life-threatening, requiring hospitalization or prolonged hospitalization for treatment, and those resulting in permanent or significant disability/incapacity. Other important medical events might be considered serious AEs as well if a patient's health was at risk and intervention needed to be performed to prevent an above-mentioned outcome.
Adverse drug reactions (ADRs) were defined as AEs for which a causal relationship to lusutrombopag could not be ruled out, as deemed by each physician. A causal relationship and the seriousness of AEs were assessed by both physicians at study sites and those employed by the sponsor company. An AE was reported as an ADR if either of the physicians (study site or sponsor) deemed it to be related to lusutrombopag. Adverse events were coded with the Medical Dictionary for Regulatory Activities Japanese version 24.1 by system organ class and preferred term.
Owing to the potential risk for thrombosis by TPORA treatment, platelet monitoring is recommended during lusutrombopag treatment. Thus, thrombosis and thromboembolism were AEs of special interest in this surveillance, and platelet monitoring status in patients who reported thrombotic events was assessed. Furthermore, data relating to the presence and history of thrombosis/thromboembolism were also collected (such patients were excluded in the previous phase III trials10, 11).
Change in platelet count from baseline was assessed in patients stratified by baseline platelet count, CP class, and the number of treatment cycles. Patients whose platelet count increased by ≥20 × 109/L from baseline and achieved ≥50 × 109/L were considered as responders. Effectiveness measurements included the proportion of patients who did not require preoperative platelet transfusion and the proportion of responders. In the efficacy analysis set, patients who were contraindicated for receiving platelet transfusion and/or who did not have an invasive procedure were excluded from the transfusion avoidance rate analysis. Furthermore, patients who received platelet transfusion during the observation period and/or those without data for platelet counts before and after treatment were excluded from the analysis for responder rate.
Sample size
We aimed to enroll 1000 patients on the basis of an estimate that at least 10.8% would have a hepatofugal blood flow or presence/history of thrombosis or thromboembolism.17 A total of 969 patients were calculated to be required to detect a more than threefold increase of thrombosis-related AEs in such patients taking lusutrombopag compared with previous clinical trial data, with a one-sided significance level of 2.5% and ≥80% power.
Statistical analysis
The safety analysis set consisted of all patients with data-fixed initial or retreatment administration surveillance form, except those considered unsuitable for the safety evaluation, such as registration violation, data duplication, not receiving the drug, not being assessed for safety, and institution refused to publish the data. Of those included in the safety analysis set, patients with off-label use, unapproved dosage, or classified as CP class C (contraindication) were excluded from the efficacy analysis set.
The mean and standard deviation (SD) were used to summarize continuous variables, and numbers and percentages were used for categorical variables. In patients assessed for platelet counts, patients were stratified according to baseline platelet counts (<30, 30–<50, and ≥50 × 109/L), the treatment cycle (first, second, third), and CP class (A, B, C). The statistical software used was SAS ver. 9.4 or later (SAS Institute, Cary, NC, USA).
RESULTS
Patients
Between October 2016 and September 2020, data were collected from 1057 patients. Of these, 24 patients were excluded due to registration violation (n = 6), data duplication (n = 7), not receiving lusutrombopag (n = 1), not being assessed for safety (n = 1), and institution refused to publish results (n = 10); therefore, a total of 1033 patients were eligible for safety assessment. Of these, 51 patients were excluded due to off-label use (n = 1), unapproved dosage/administration (n = 7), or contraindication with CP class C (n = 43); thus, 982 patients were eligible for effectiveness assessment (Figure 1).
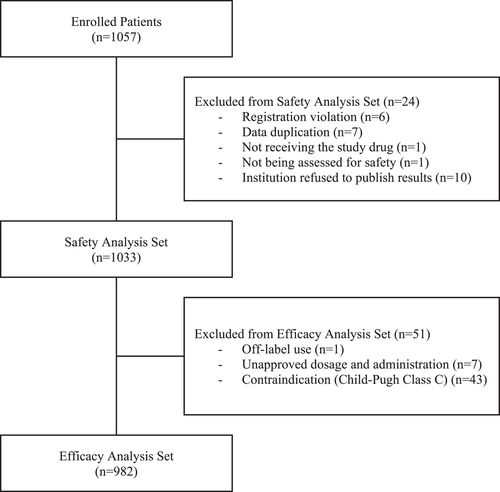
Patient disposition. Of the efficacy analysis set, 939 patients were included in the analysis for avoidance rate of platelet transfusion, and 43 patients were excluded due to contraindication for receiving platelet transfusion and/or not undergoing an invasive procedure. In the responder analysis, 895 patients were included, and 87 patients were excluded due to receiving platelet transfusion during the observation period and having inadequate data of the platelet counts at baseline and after the treatment.
In the safety analysis set, 63.9% (660/1033) of patients were men, and the mean (SD) age was 67.8 (9.8) years (Table 1). The mean (SD) baseline platelet count was 48.7 (15.6) × 109/L (median 47.0 × 109/L, n = 1023). The proportions of patients with baseline platelet counts of <30, 30–<50, and ≥50 × 109/L were 6.7% (69/1033), 52.4% (541/1033), and 40.0% (413/1033), respectively; platelet count was unknown in 10 patients (1.0%).
| Safety analysis set | |
|---|---|
| n = 1033 | |
| Sex, n (%) | |
| Male | 660 (63.9%) |
| Female | 373 (36.1%) |
| Age (years), mean (SD) | 67.8 (9.8) |
| Visit, n (%) | |
| Inpatient | 122 (11.8%) |
| Outpatient | 911 (88.2%) |
| Baseline platelet count, n (%) | |
| <30 × 109/L | 69 (6.7%) |
| 30−<50 × 109/L | 541 (52.4%) |
| ≥50 × 109/L | 413 (40.0%) |
| Unknown | 10 (1.0%) |
| Baseline platelet count (× 109/L), mean (SD)a | 48.7 (15.6) |
| Baseline platelet count (× 109/L), median (range)a | 47.0 (9–147) |
| Chronic liver disease, n (%) | |
| Chronic hepatitis | 61 (5.9%) |
| Cirrhosis | 986 (95.5%) |
| Unknown | 1 (0.1%) |
| Cirrhosis with ascites, n (%)b | |
| Yes | 261 (26.5%) |
| No | 725 (73.5%) |
| Cirrhosis with hepatic encephalopathy, n (%)b | |
| Yes | 74 (7.5%) |
| No | 912 (92.5%) |
| Child–Pugh class for cirrhosis, n (%)b | |
| A | 482 (48.9%) |
| B | 457 (46.3%) |
| C | 43 (4.4%) |
| Unknown | 4 (0.4%) |
| Complications other than impairment of hepatic function, n (%) | |
| Yes | 872 (84.4%) |
| No | 158 (15.3%) |
| Unknown | 3 (0.3%) |
| History of splenectomy, n (%) | |
| Yes | 7 (0.7%) |
| No | 1026 (99.3%) |
| Platelet transfusion refractoriness, n (%) | |
| Yes | 13 (1.3%) |
| No | 171 (16.6%) |
| Unknown | 849 (82.2%) |
| Current or history of thrombosis or thromboembolism, n (%) | |
| Yes | 106 (10.3%) |
| No | 926 (89.6%) |
| Unknown | 1 (0.1%) |
| No. treatment cycles, n (%) | |
| ≥1 | 1033 (100.0%) |
| ≥2 | 130 (12.6%) |
| ≥3 | 14 (1.4%) |
| ≥4 | 0 (0.0%) |
| Time to the invasive procedure since the first administration, mean(days) | 12.6 |
| No. invasive procedures performed after the initial treatment cycle, n (%) | |
| Total | 1174 (100.0%) |
| <9 days | 153 (13.0%) |
| 9–<12 days | 379 (32.3%) |
| 12–<15 days | 348 (29.6%) |
| ≥15 days | 294 (25.0%) |
- Abbreviation: SD, standard deviation.
- a n = 1023.
- b n = 986.
The proportion of patients who had a current or history of thrombosis/thromboembolism was 10.3% (106/1033). The numbers (%) of patients who received one or more, two or more, or three or more treatment cycle(s), were 1033 (100.0%), 130 (12.6%), and 14 (1.4%), respectively; no patients had more than three treatment cycles. Of 986 patients with cirrhosis, the numbers (%) of patients with CP class A, B, and C were 482 (48.9%), 457 (46.3%), and 43 (4.4%), respectively; CP class was unknown in four (0.4%) patients. The median (range) point of CP class A, B, and C were 6 (5–6), 8 (7–9), and 10 (10–13), respectively.
After the first treatment cycle, a total of 1174 invasive procedures were performed among patients in the safety analysis set (some patients underwent multiple procedures). The mean time from the first administration of lusutrombopag to the planned invasive procedure was 12.6 days. Of the 1174 planned invasive procedures, 32.3% and 29.6% were performed 9–<12 days and 12–<15 days after the first administration (Table 1). The most frequent procedures were radiofrequency ablation (28.3%), endoscopic injection sclerotherapy (14.9%), and transcatheter arterial chemoembolization (14.6%; Table 2).
| Invasive procedure | Child–Pugh A | Child–Pugh B | Child–Pugh C | Total | ||||
|---|---|---|---|---|---|---|---|---|
| N | Component ratio (%) | N | Component ratio (%) | N | Component ratio (%) | N | Component ratio (%) | |
| Radiofrequency ablation (RFA) | 161 | 28.5 | 147 | 28.4 | 6 | 15.4 | 332 | 28.3 |
| Endoscopic injection sclerotherapy (EIS) | 96 | 17.0 | 73 | 14.1 | 2 | 5.1 | 175 | 14.9 |
| Transcatheter arterial chemoembolization (TACE) | 74 | 13.1 | 91 | 17.6 | 6 | 15.4 | 171 | 14.6 |
| Endoscopic variceal ligation (EVL) | 53 | 9.4 | 47 | 9.1 | 2 | 5.1 | 102 | 8.7 |
| Percutaneous needle biopsy | 32 | 5.7 | 19 | 3.7 | 5 | 12.8 | 71 | 6.0 |
| Transcatheter arterial embolization (TAE) | 21 | 3.7 | 23 | 4.4 | 3 | 7.7 | 47 | 4.0 |
| Tooth extraction | 15 | 2.7 | 13 | 2.5 | 4 | 10.3 | 34 | 2.9 |
| Lipiodol-transcatheter arterial infusion (Lip-TAI) | 10 | 1.8 | 10 | 1.9 | 0 | 0.0 | 21 | 1.8 |
| Endoscopic mucosal resection (EMR) | 12 | 2.1 | 5 | 1.0 | 1 | 2.6 | 21 | 1.8 |
| Endoscopic submucosal dissection (ESD) | 12 | 2.1 | 8 | 1.5 | 0 | 0.0 | 20 | 1.7 |
| Microwave coagulation therapy (MCT) | 9 | 1.6 | 8 | 1.5 | 0 | 0.0 | 18 | 1.5 |
| Endoscopic polypectomy | 6 | 1.1 | 5 | 1.0 | 0 | 0.0 | 12 | 1.0 |
| Percutaneous ethanol injection therapy (PEIT) | 3 | 0.5 | 9 | 1.7 | 0 | 0.0 | 12 | 1.0 |
| Argon plasma coagulation (APC) | 4 | 0.7 | 4 | 0.8 | 0 | 0.0 | 8 | 0.7 |
| Laparoscopy and measurements | 5 | 0.9 | 1 | 0.2 | 0 | 0.0 | 6 | 0.5 |
| Various paracentesis procedures (including abscess paracentesis) | 0 | 0.0 | 1 | 0.2 | 1 | 2.6 | 4 | 0.3 |
| Other procedures | 51 | 9.0 | 54 | 10.4 | 9 | 23.1 | 120 | 10.2 |
| Total | 564 | 100.0 | 518 | 100.0 | 39 | 100.0 | 1174 | 100.0 |
- Note: Component ratio (%) = number of invasive procedures / total number of invasive procedures × 100.
- Abbreviation: N, Number of invasive procedures.
Overall safety
In total, 479 AEs were reported in 22.36% (231/1033) of patients during the overall survey period, and 151 serious AEs were reported in 104 patients (10.07%). Of these, 16 patients (1.55%) had fatal outcomes; however, no deaths were considered related to lusutrombopag.
A total of 57 ADRs were reported in 40 (3.87%) patients. Of these, 29 ADRs were serious and reported in 27 patients (2.61%; Table 3). The most common serious ADRs observed in patients were PVT, pancytopenia, and white blood cell count decrease; 14 (1.36%), three (0.29%), and two (0.19%) patients, respectively (Table 4). One patient reported myelodysplastic syndrome and pancytopenia, and another patient reported platelet count decrease and hematemesis.
| No. events | No. patients, n (%) | |
|---|---|---|
| N = 1033 (100.00%) | ||
| Total AEs | 479 | 231 (22.36%) |
| Total serious AEs | 151 | 104 (10.07%) |
| AEs leading to death | 18 | 16 (1.55%) |
| AEs leading to discontinuation | 9 (0.87%) | |
| Thrombotic AEs | 21 | 20 (1.94%) |
| Thrombotic AEs led to discontinuation | 0 | 0 (0.00%) |
| Bleeding-related AEs | 25 | 23 (2.23%) |
| AEs by CP class | ||
| A | 99/482 (20.54%) | |
| B | 113/457 (24.73%) | |
| C | 13/43 (30.23%) | |
| Unknown | 1/4 (25.00%) | |
| Total ADRs | 57 | 40 (3.87%) |
| Total serious ADRs | 29 | 27 (2.61%) |
| ADRs leading to death | 0 | 0 (0.0%) |
| Thrombotic ADRs | 15 | 15 (1.45%) |
| Bleeding-related ADRs | 4 | 4 (0.39%) |
| ADRs by CP class | ||
| A | 15/482 (3.11%) | |
| B | 22/457 (4.81%) | |
| C | 1/43 (2.33%) | |
| Unknown | 1/4 (25.00%) | |
| ADRs in each treatment period | ||
| 1st treatment period | 36/1033 (3.48%) | |
| 2nd treatment period | 4/130 (3.08%) | |
| 3rd treatment period | 0/14 (0.00%) | |
- Abbreviations: ADR, adverse drug reaction, AE, adverse event; CP, Child–Pugh.
| No. events | No. patients, n (%) | |
|---|---|---|
| N = 1033 (100.00%) | ||
| Most common serious ADRs (>1) | ||
| Portal vein thrombosis | 14 | 14 (1.36%) |
| Pancytopenia | 3 | 3 (0.29%) |
| White blood cell count decreased | 2 | 2 (0.19%) |
| Bleeding-related serious ADRs | ||
| Hemothorax | 1 | 1 (0.10%) |
| Hematemesis | 1 | 1 (0.10%) |
| Esophageal varices hemorrhage | 1 | 1 (0.10%) |
| Intra-abdominal hemorrhage | 1 | 1 (0.10%) |
| Other serious ADRs | ||
| Mesenteric vein thrombosis | 1 | 1 (0.10%) |
| Myelodysplastic syndrome | 1 | 1 (0.10%) |
| Renal impairment | 1 | 1 (0.10%) |
| Pyrexia | 1 | 1 (0.10%) |
| Neutrophil count decreased | 1 | 1 (0.10%) |
| Platelet count decreased | 1 | 1 (0.10%) |
- Abbreviations: ADR, adverse drug reaction.
The patients with the baseline platelet count of ≥50 × 109/L had a similar magnitude and timing of change in platelet count with the groups with the baseline platelet count of <30 or 30–<50 × 109/L (Figures 2a and b).

(a) Median platelet count and (b) median change in platelet count stratified by the baseline platelet counts during the initial treatment period. Error bars represent the 25th and 75th percentiles.
Thrombotic events
A total of 21 thrombotic events were reported in 20 (1.94%) patients during the overall study period; of these, 15 events in 15 patients (1.45%) were drug-related (Table 3). Portal vein thrombosis and mesenteric vein thrombosis were considered serious ADRs, and were reported in 14 (1.36%) patients and one (0.10%) patient, respectively (Table 4). In the first treatment period, 17 thrombotic events in 16 patients were reported. Most thrombotic AEs and ADRs occurred between 8 and 14 days after the first administration of lusutrombopag in the first treatment period (Figure 3a) and after the procedure (Figure 3b).
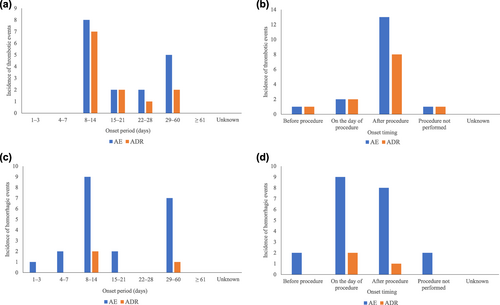
The onset of thrombotic events by (a) onset period and (b) by onset timing, and hemorrhagic events by (c) onset period and by (d) onset timing during the initial treatment period. ADR, adverse drug reaction; AE, adverse event.
A total of 20 patients reported thrombotic events and had platelet count monitoring between 4 and 7 days after the first administration. Of these, 16 and four patients reported thrombotic events in the initial and the second treatment periods, respectively. Two patients who underwent partial splenic embolization reported an increase in platelet count (>200 × 109/L) during the first treatment period. However, no such increase in platelet count was observed in the remaining 18 patients (data not shown). No patients discontinued lusutrombopag treatment due to thrombotic events.
During the first treatment period, the incidence rates of thrombotic AEs were comparable between patients who were monitored for platelet count from 4 to 7 days after the administration of lusutrombopag (1.9% [10/519] of the patient) and those who were not (1.2% [6/514] of the patients). Among those monitored, four patients met the predefined criteria to consider discontinuing the administration (i.e., platelet count increased by ≥20 × 109/L from baseline and achieved ≥50 × 109/L), and the administration was continued in three and discontinued in one patient, respectively, by the decision of the physicians.
Bleeding events
During the overall study period, 25 bleeding events were reported in 23 (2.23%) patients, and 21 serious bleeding events were reported in 20 (1.94%) patients. Of these, four events (hemothorax, hematemesis, esophageal varices hemorrhage, intra-abdominal hemorrhage) were drug-related and were reported in four (0.39%) patients (Table 4). In the first treatment period, 21 events in 19 patients were reported. The onset of bleeding AEs and ADRs during the first treatment period is shown in Figure 3c. A total of two and one bleeding ADRs occurred on and after the day of the invasive procedures, respectively (Figure 3d).
Safety in patients with Child–Pugh class C
The safety analysis set included 43 patients with CP class C. Of these, 13 of 43 (30.23%) of patients reported 38 AEs during the overall treatment period. However, no event was deemed as drug-related during the first treatment period. Although a serious ADR, PVT was reported in one of 43 (2.33%) of patients with CP class C in the second treatment period, the patient experienced no exceeding increase in platelet count, and the company physician suspected the invasive procedure or underlying diseases as the possible cause. The incidence of ADRs in patients with CP class C (1/43 patients, 2.33%) was not higher than that of CP class A (15/482 patients, 3.11%) or B (22/457 patients, 4.81%; Table 3). The trajectory of the measurement value and median change of platelet count of CP class C were not over those of CP class A or B (Figures 4a and b). Although the median of the maximum amount of change was highest in CP class C, the measurement value was below 200 × 109/L and comparable with CP class A or B (Appendix 1).
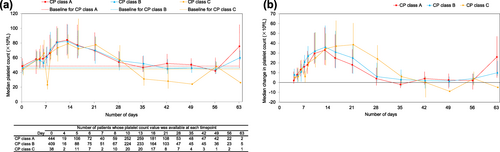
(a) Median platelet count and (b) median change in platelet count stratified by Child–Pugh (CP) class during the initial treatment period. Error bars represent the 25th and 75th percentiles.
Safety in patients with retreatment cycle(s)
A total of 130 patients who received retreatment cycles were included in the safety analysis set. Of these, four of 130 (3.08%) patients who received retreatment cycles reported four ADRs: PVT and esophageal varices hemorrhage were reported in three patients and one patient, respectively. No patients (0/14) who received three treatment cycles reported ADRs.
The absolute and median platelet count change of the retreatment cycles did not overly exceed the first treatment cycle (Figures 5a and b). The incidence of ADRs in patients with a retreatment cycle was comparable with those without (Table 3).
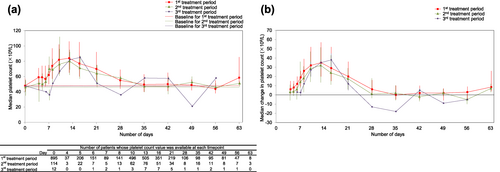
(a) Median platelet count and (b) median change in platelet count in patients who received retreatment cycle(s). Error bars represent the 25th and 75th percentiles.
Effectiveness during the first treatment period
In the efficacy analysis set, 889/939 (94.7%) patients were able to avoid preoperative platelet transfusion and 741/895 (82.8%) patients met the responder criteria during the first treatment period. The mean (SD) maximum platelet count was 98.2 (47.4) × 109/L (median, 88.0 × 109/L; range 25–496 × 109/L) and was observed at a mean (SD) of 12.8 (5.0) days (median, 12 days; range 3–61 days) after the first administration.
Effectiveness during the retreatment period
Of the 122 patients who received at least two treatment cycles and who were eligible for the analysis of the platelet transfusion, 116 (95.1%) patients did not require preoperative platelet transfusion prior to/at the second planned invasive procedure. Of the 114 patients who received at least two treatment cycles and who were eligible for responder analysis, 85 (74.6%) patients met the responder criteria.
Of the 14 patients who received three treatment cycles, 13 (92.9%) patients did not require preoperative platelet transfusion prior to/at the third planned invasive procedure. Of the 12 patients who received three treatment cycles, 11 (91.7%) patients met the responder criteria.
DISCUSSION
This surveillance reports the final results of an evaluation of safety and effectiveness of lusutrombopag in CLD patients with thrombocytopenia undergoing planned invasive procedures in clinical practice in Japan. The safety and effectiveness profiles were generally similar to those of the interim results.16 The interim and final results of this surveillance reflect the use of lusutrombopag in clinical practice.
Platelet transfusion medicine requires a constant supply of blood donors, because prepared platelets must be used within 4 days. The coronavirus disease 2019 pandemic has negatively impacted the number of blood donors, and a shortage of donor blood poses a risk to patient care.18 Therefore, the need for other treatment options, such as TPORAs, has been rising. A treatment algorithm for thrombocytopenia in patients with CLD undergoing a planned invasive procedure has been recently proposed in Japan.3 This algorithm recommends TPORAs, including lusutrombopag, as the first-line therapy for patients with severe thrombocytopenia (platelet count <50 × 109/L).3 The use of TPORAs may be considered for those with moderate thrombocytopenia (platelet count >50–75 × 109/L) and moderate bleeding risk.3
The data specific to this surveillance were: patients with baseline platelet count ≥50 × 109/L (413 patients, 40.0%), CP class C (43 patients, 4.4%), retreatment cycle(s) (130 patients, 12.6%), a current or history of thrombosis and/or thromboembolism (106 patients, 10.3%), and a history of splenectomy (7 patients, 0.7%). Furthermore, the planned invasive procedures were carried out outside the time window recommended by the package insert; 13.0% of the patients underwent the procedure within 8 days, and 25.0% underwent the procedures 15 days or more after the first administration of lusutrombopag.
Notably, 43 patients received lusutrombopag despite having CP class C, which is contraindicated in Japan. The median (range) point of CP class C was 10 (10–13), relatively mild for CP class C. A previous study demonstrated that 33% of patients with decompensated cirrhosis and CP class C improved to class B at the end of direct-acting antiviral treatment.19 In clinical practice, thus, the CP class may not be constant for each patient.
In clinical practice, individual physicians often bear the responsibility for prescribing lusutrombopag and for determining the timing of the invasive procedure. As a result, physicians have administered lusutrombopag to a broad range of patients from moderate to severe thrombocytopenia (baseline platelet count <50 × 109/L), and a CP class ranging from A, B, and C at clinically appropriate timing.
Overall, the incidence of serious AEs and ADRs reported in this surveillance were similar to the interim analysis, and no additional concerns have been raised.16 The proportions of the patients who reported thrombotic AEs or ADRs were comparable with the interim analysis.16 In this surveillance, the proportion of the patients with thrombotic ADRs relativized to the total ADRs were 37.47% (15/40), and clinically meaningful differences were not observed between the L-PLUS 1 study (24.97%, 1/4) and the L-PLUS 2 study (33.33%, 2/6).10, 11
Of the 20 patients who reported thrombotic events and received platelet monitoring, two patients experienced increased platelet count (>200 × 109/L). These patients underwent partial splenic embolization, a procedure to increase platelet count. The increased platelet count was observed during the initial treatment period, but not during the second treatment period, suggesting this was unlikely related to retreatment with lusutrombopag. The proportions of patients who reported thrombotic events were comparable between the subgroups with and without platelet monitoring; no increased risk for thrombotic events was observed in patients who did not have platelet monitoring. This result is aligned with the findings of the phase IIIb study that platelet monitoring during 7 days of lusutrombopag treatment may not always be necessary.20
The proportion of patients who reported bleeding events was 2.23% (23/1033 patients), and lower than those of two previous studies: (1) a secondary analysis of lusutrombopag studies (phase 2b, L-PLUS 1, and L-PLUS 2) reporting a bleeding-related AEs rate of 11.9% in patients receiving placebo with platelet transfusion and 6.5% in patients receiving lusutrombopag without platelet transfusion21; and (2) a database study in Japan reporting bleeding events in 8.2% of the platelet transfusion group and 3.7% of the lusutrombopag treatment group.22 These results suggested that lusutrombopag is an effective prophylactic treatment to prevent bleeding in CLD patients undergoing planned invasive procedures. Although this surveillance does not have a control arm, the incidence of bleeding events was similar to or lower than the previous studies.
Although the number of CP class C patients was small, safety concerns, such as the platelet count increase and the incidence of ADRs, were comparable among CP classes, and the safety profile was in accordance with the previous report.23
The previous phase IIIb trial revealed no meaningful differences in efficacy of lusutrombopag between naïve and non-naïve patients.20 In this surveillance, 130 patients received lusutrombopag for at least two cycles, and favorable safety and effectiveness profiles were observed. These results suggest that retreatment with lusutrombopag may be safe and effective in lusutrombopag-experienced patients. The responder rates in both first and retreatment periods were high (approximately 80%), and comparable between lusutrombopag-naïve and experienced patients. Thus, lusutrombopag may be effective in both lusutrombopag-naïve and experienced patients, as the previous trial data suggested.20
The proportions of the patients who did not require platelet transfusion were also high (approximately 95%) in both groups. These proportions were higher than the primary endpoints in the treatment group of the previous L-PLUS 1 (79.2%)10 and L-PLUS 2 (63.0%) studies, which was the proportion of patients who met both avoidance of pre-procedure platelet transfusion and avoidance of rescue therapy for bleeding.11 This discrepancy is likely caused by the difference in the protocols, where the platelet transfusions were performed for patients whose platelet count was <50 × 109/L in the phase III trials; however, the decision on whether to implement platelet transfusion was determined by the physicians in this surveillance. The responder rates defined by the platelet counts were comparable between this surveillance and the L-PLUS 1 study,10 as well as the L-PLUS 2 study.11
The mean (SD) time to reach the maximum platelet count was comparable between this surveillance (12.8 [5.0]), the L-PLUS 1 study (13.4 [3.8] days),10 and the L-PLUS 2 study (vs. 12.4 [4.7] days).11 This result suggests that invasive procedures may be performed between 12 and 14 days after the first administration of lusutrombopag to achieve a clinically relevant platelet count.
One of the limitations of the present study was because of the observational study design; this was a single-arm study, and data collection points were not specified. The surveillance only included patients from pre-specified institutions in Japan, thus, the data generalizability is limited. Furthermore, some clinical factors, such as administration of antithrombin III for increased risk of thrombotic events, were not collected.
There were some differences in the results between this surveillance and the L-PLUS 2 study, which are most likely attributable to differences in the study designs. Further research may be beneficial to validate the safety and effectiveness of lusutrombopag in non-Japanese populations. Nevertheless, this surveillance reported the safety profile on a larger number of patients compared with previous studies.
A recent meta-analysis, indirectly comparing the effectiveness and safety of five different TPORAs, suggested that lusutrombopag was the treatment with the best balance between high short-term efficacy and safety, in terms of platelet response, platelet count, risk of bleeding, and AEs.24 In line with the findings of this meta-analysis, our surveillance demonstrated low incidence rates of bleeding events, thrombotic events, and ADRs. Furthermore, a responder rate and an avoidance rate of platelet transfusion were both high in CLD patients with thrombocytopenia in clinical practice in Japan.
CONCLUSIONS
The safety and effectiveness profiles of lusutrombopag in this real-world surveillance were similar to those of the previous phase III trials. This surveillance further validates the safety and effectiveness of lusutrombopag in a broad range of CLD patients with thrombocytopenia undergoing planned invasive procedures, including lusutrombopag-naïve and experienced patients. No safety concerns were associated with the severity of CP class, baseline platelet counts, or the timing or number of administrations of lusutrombopag.
ACKNOWLEDGMENTS
The authors thank all participating physicians and patients for their cooperation in this PMS. The authors also acknowledge Dr. Michio Imawari (Institute for Gastrointestinal and Liver diseases, Shin-Yurigaoka General Hospital, Kanagawa, Japan) for his expertise and assistance on the study planning. The authors thank Mie Yamamoto, Ph.D., and Andrew Jackson, Ph.D., at MIMS Co. Ltd. for providing medical writing support in accordance with the Good Publications Practice (GPP 2022) guidelines (https://www.ismpp.org/gpp-2022), which was funded by Shionogi & Co., Ltd. The authors also thank Ikumi Nishi, an employee of Shionogi & Co., Ltd., for providing medical writing and editorial assistance.
CONFLICT OF INTEREST STATEMENT
M.K. and H.Y. are Editor-in-Chief and Deputy Editors-in-Chief of the journal, respectively, and co-authors of this article. They were excluded from the peer review process, and all editorial decisions related to the acceptance and publication of this article. Peer review was handled independently by Dr. Naoya Sakamoto to minimize bias. H.Y. received honoraria from Otsuka, ASKA, Gilead, and AbbVie, and received research funding from Otsuka, ASKA, and AbbVie. M.K. received honoraria from Eisai, AbbVie, Gilead, Chugai, Incyte, and Eli Lilly. J.S., M.I., E.T., and M.M. are employees of the study sponsor (Shionogi and Shionogi Group Companies). M.I. owns stock of Shionogi.
ETHICS STATEMENTS
This surveillance was carried out in accordance with the GPSP for medicinal products in Japan. According to exemptions under the GPSP Ordinance No. 171, 2004 by the Ministry of Health, Labor, and Welfare, ethics approval by the institutional review board and informed consent were not required.
Approval of the research protocol: N/A
Informed Consent: N/A
Registry and the Registration No. of the study/trial: This surveillance was registered with the Japan Pharmaceutical Information Center (identifier: JapicCTI-163432).
Animal Studies: N/A
Research involving recombinant DNA: N/A
Open Research
DATA AVAILABILITY STATEMENT
Data used in this surveillance may be publicly available after 2024 or 2025, following the reassessment of lusutrombopag by Japan Pharmaceuticals and Medical Devices Agency.




