30 years of free-air carbon dioxide enrichment (FACE): What have we learned about future crop productivity and its potential for adaptation?
Abstract
Free-air CO2 enrichment (FACE) allows open-air elevation of [CO2] without altering the microclimate. Its scale uniquely supports simultaneous study from physiology and yield to soil processes and disease. In 2005 we summarized results of then 28 published observations by meta-analysis. Subsequent studies have combined FACE with temperature, drought, ozone, and nitrogen treatments. Here, we summarize the results of now almost 250 observations, spanning 14 sites and five continents. Across 186 independent studies of 18 C3 crops, elevation of [CO2] by ca. 200 ppm caused a ca. 18% increase in yield under non-stress conditions. Legumes and root crops showed a greater increase and cereals less. Nitrogen deficiency reduced the average increase to 10%, as did warming by ca. 2°C. Two conclusions of the 2005 analysis were that C4 crops would not be more productive in elevated [CO2], except under drought, and that yield responses of C3 crops were diminished by nitrogen deficiency and wet conditions. Both stand the test of time. Further studies of maize and sorghum showed no yield increase, except in drought, while soybean productivity was negatively affected by early growing season wet conditions. Subsequent study showed reduced levels of nutrients, notably Zn and Fe in most crops, and lower nitrogen and protein in the seeds of non-leguminous crops. Testing across crop germplasm revealed sufficient variation to maintain nutrient content under rising [CO2]. A strong correlation of yield response under elevated [CO2] to genetic yield potential in both rice and soybean was observed. Rice cultivars with the highest yield potential showed a 35% yield increase in elevated [CO2] compared to an average of 14%. Future FACE experiments have the potential to develop cultivars and management strategies for co-promoting sustainability and productivity under future elevated [CO2].
1 INTRODUCTION
Fifteen years ago we analyzed the literature describing crop yield responses to elevated [CO2] in large-scale, replicated FACE experiments (Ainsworth & Long, 2005). At that time, there were only 28 published observations of crop yield responses from large-scale FACE experiments and we used meta-analytic approaches to quantify crop physiological and productivity responses to elevated [CO2]. Meta-analysis is the quantitative synthesis of research results, and we used the approach to broadly generalize crop responses to elevated [CO2] in order to gain a more comprehensive understanding, a common goal for ecological meta-analyses (Gurevitch et al., 2018). This approach has since been applied to quantify responses of individual crops to rising atmospheric [CO2] (e.g., Ainsworth, 2008; Broberg et al., 2017; Challinor et al., 2014; Kimball, 2016; Taub et al., 2008; van der Kooi et al., 2016; Wang et al., 2012, 2015) and to investigate how the CO2 fertilization effect interacts with growing season temperature or water supply (Bishop et al., 2014). However, meta-analytic approaches are not without their limitations, including research and publication bias (Gurevitch et al., 2018; Haworth et al., 2016; Loladze, 2014). To avoid such bias, it is suggested that ecological meta-analyses use open datasets rather than published results (Culina et al., 2018). Unfortunately, open-access databases for FACE experiments are not yet widely available from crop FACE experiments. Moreover, most FACE experiments have been conducted for multiple years on a limited number of sites across the globe, which can limit the generalizability of results (Gurevitch, 2013). It is also the outliers, notably the germplasm that responds far more strongly than average or interactions with environment that nullify or amplify a response, that now need the attention that averaging obscures. While not a formal meta-analysis, in this review we estimate mean yield responses to elevated [CO2] for key crops from over 250 independent estimates in 18 sites where full size replicated FACE plots (≥50 m2) have been used. We summarize mean responses for the different crops and interaction with nested water, nitrogen, and warming treatments, but limit the analysis to increases in [CO2] of no more than 200 ppm to maintain relevance to mid-century (Table 1). FACE experiments now span almost three decades during which ambient [CO2] has risen from <360 ppm to >410 ppm (Figure 1). This review focuses on understanding crop responses to rising [CO2] from FACE experiments, addressing the robustness of our 2005 conclusions and the interactions of rising [CO2] with temperature, drought, nutrient stress, and within-crop genetic variation. In particular we focus not just on the averages, but on the rich information provided, by the apparent outliers or anomalies, and variation between years, that may aid us toward future-proofing our crops to the future higher [CO2] world of mid-century.
| Site | Years of experiment | Crops studied |
Elevated [CO2] ppm |
Additional treatments | Proportionate increase in yield | Source |
|---|---|---|---|---|---|---|
| Maricopa, AZ, USA | 1989–1991 | Cotton | 550 | Water | D 0.35 I 0.38 | Ainsworth and Long (2005) |
| 1993–1994 | Wheat | 550 | Water | D 0.20 I 0.08 | Ainsworth and Long (2005) | |
| 1996–1997 | Wheat | +200 | Nitrogen | L 0.11 H 0.14 | Ainsworth and Long (2005) | |
| 1998–1999 | Sorghum | +200 | Water | D 0.23 W −0.05 | Ainsworth and Long (2005) | |
| Rapolano, Italy | 1995, 1998, 1999 | Potato | +200 | 0.31 | ||
| 1996–1997 | Grape | 550 | 0.46 | Bindi et al. (2001) | ||
| Shizukuishi, Japan | 1998–2000 | Rice | +200 | Nitrogen |
L 0.08 H 0.13 |
Ainsworth and Long (2005) |
| 2003–2004 | Rice | +200 | 4 Cultivars averaged | 0.11 | Shimono et al. (2009) | |
| 2007–2008 | Rice | +200 | 4 Cultivars averaged | 0.17 | Hasegawa et al. (2013) | |
| Braunschweig, Germany |
2000–2003 2001–2004 2002–2005 2007–2008 2014–2015 |
Winter Barley Sugar beet Winter Wheat Maize Winter Wheat |
550 550 550 550 600 |
Nitrogen Nitrogen Nitrogen Water Nitrogen |
L 0.12 H 0.11 L 0.15 H 0.09 L 0.12 H 0.16 D 0.18 I −0.01 L 0.09 H 0.17 |
Weigel and Manderscheid (2012) Weigel and Manderscheid (2012) Manderscheid et al. (2014) Dier et al. (2018) |
| Champaign, IL, USA |
2001–2003 2004–2006 2004–2007 2006 2008 2009–2011 2010 2010 2012–2014 2017 |
Soybean Soybean Soybean Maize Maize Soybean Maize Cassava Soybean Cassava |
550 550 550 550 550 585 585 585 600 600 |
Elevated Ozone 9 Cultivars averaged Nitrogen Warming Warming 8 Cultivars |
0.14 A 0.14 E 0.15 0.12 0.01 L 0.04 H 0.01 C 0.11 W 0.06 C −0.10 W 0.00 0.88 0.20 0.25 |
Morgan et al. (2005) He et al. (2014) Bishop et al. (2015) Leakey et al. (2006) Markelz et al. (2011) Ruiz-Vera et al. (2013) Ruiz-Vera et al. (2015) Rosenthal et al. (2012) Sanz-Saez et al. (2017) Ruiz-Vera et al. (2020) |
| Wuxi, Jiangsu, China |
2001–2003 |
Rice |
+200 |
Nitrogen |
L 0.11 H 0.16 | Yang et al. (2006) |
| Changshu, Jiangsu, China |
2013–2014 2013–2014 |
Rice Wheat |
500 500 |
Warming Warming |
C 0.06 W 0.08 C 0.06 W 0.10 |
Cai et al. (2016) |
| Stuttgart, Germany |
2004–2006 2007 2008 |
Wheat Oilseed Rape Wheat |
+150 500 +150 |
0.01 0.14 0.10 |
Högy et al. (2009) Högy et al. (2010) Högy et al. (2013) |
|
| Yangzhou, Jiangsu, China |
2004–2006 2007–2008 |
Rice Wheat |
+200 +200 |
Nitrogen |
L 0.33 H 0.34 0.15 |
Yang et al. (2006) Zhu et al. (2009) |
| New Dehli, India |
2006–2008 2006–2008 2006–2008 2008–2010 2008–2010 2009–2011 2010–2012 |
Mung bean Mustard Potato Peanut Chickpea Rice Wheat |
550 550 550 550 550 550 550 |
0.20 0.20 0.20 0.21 0.23 0.15 0.15 |
Singh et al. (2013) | |
| Horsham, VA, Australia |
2007–2009 2013–2014 2010–2012 2016 2015–2016 |
Wheat Wheat Field pea Faba bean Lentil |
550 550 550 550 550 |
Water 2 cultivars avg Heat waves 5 cultivars avg Water Heat waves 2 cultivars |
D 0.12 I 0.37 C 0.39 HW 0.35 0.28 D 0.24 I 0.59 C 0.64 HW 0.88 |
Fitzgerald et al. (2016) Macabuhay et al. (2018) Bourgault et al. (2016) Parvin et al. (2019) Bourgault et al. (2018) |
| Walpeup, VA, Australia | 2008–2009 | Wheat | 550 | 0.56 | Fitzgerald et al. (2016) | |
| Tsukubamirai City, Japan |
2010–2011 2010–2012 2012–2014 |
Rice Rice Rice |
+200 +200 +200 |
5 cultivars averaged Warming Nitrogen (2 cultivars) |
0.17 C 0.15 H 0.13 L 0.05 H 0.09 |
Sakai et al. (2019) Usui et al. (2016) Hasegawa et al. (2019) |
| Jaguariúna, Brazil | 2011–2015 | Coffee | +200 | 2 cultivars averaged | 0.13 | Ghini et al. (2015) |
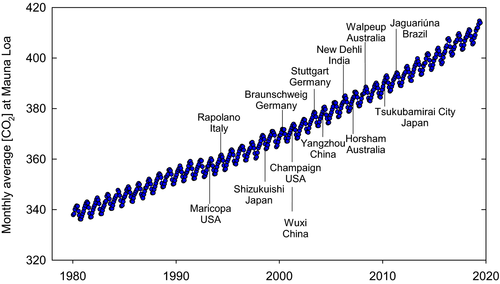
Why focus on FACE, given that there are less costly ways to elevated CO2 around crops, for example in greenhouses, controlled environment chambers, and open top chambers (OTCs)? Controlled environments are important in providing repeatable environments, given that no one crop field season is the same as the next. These are also important in hypothesis generation and in testing the effects of individual environmental variables, induced from field situations. However, wide differences between findings in these environments and open field situations are commonplace. A meta-analysis across a wide range of studies showed a correlation coefficient between controlled environment and field phenotypic data for key traits, from photosynthesis to productivity, of just 0.26 (Poorter et al., 2016). OTCs offer a means to assess responses in partially protected environments in field settings. However, even here, meta-analyses have revealed highly significant differences in response of plants to elevated [CO2] in FACE versus OTC experiments (de Graaff et al., 2006) with yield responses much greater in OTCs compared to FACE for the same elevation of [CO2] (Ainsworth et al., 2008). These findings emphasize the risk of extrapolating future crop performance under rising [CO2] without testing performance under open-air conditions. Previously, we enumerated the multiple differences between the effects of elevation of [CO2] in protected environments versus open fields that could lead to these differences (Ainsworth & Long, 2005; McLeod & Long, 1999). The much greater crop area enclosed in a FACE plot than in OTCs, typically >2 orders of magnitude, has other key advantages. For example, simultaneous studies of many biological scales and processes, from soil microbial community function, to molecular and physiological dissection of physiological and yield changes are possible. Additionally, the large size of the FACE rings enables nesting of open-air drought, temperature, and ozone treatments, as well as the free movement of pests and pathogens, and testing of a range of crop genotypes (e.g., Bourgault et al., 2016, 2017; Gray et al., 2016; Hasegawa et al., 2013; He et al., 2014; Köhler et al., 2019; Ruiz-Vera et al., 2013).
2 EARLY LESSONS FROM FACE EXPERIMENTS
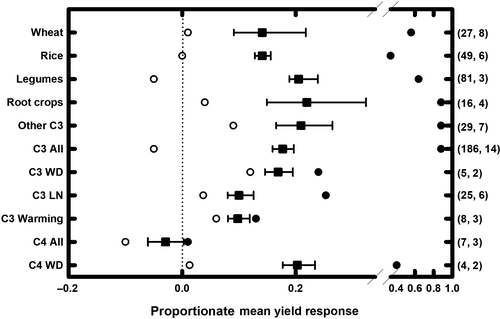
Our previous analysis drew a number of conclusions about crop productivity responses to elevated [CO2]. First, woody species were more responsive to elevated [CO2] than C3 herbaceous crops. Second, C4 crops would not be more productive in elevated [CO2] in the absence of drought stress. Third, crop yield responses to elevated [CO2] varied significantly with different environmental conditions. In the absence of additional stresses, crop yield increased by ~40% (based on five observations); however, low N stress and wet growing conditions counteracted benefits of elevated [CO2] to crop yield (Ainsworth & Long, 2005). Since 2005, there have been many additional studies of crop responses to elevated [CO2] in FACE experiments, along with more quantitative syntheses. While important food and commodity crops including rice, wheat, soybean, and maize have continued to be widely examined for [CO2] response in FACE experiments (see Ainsworth, 2008; Broberg et al., 2019; Leakey et al., 2009; Wang et al., 2015 for reviews), coffee, cassava, pea, maize, mustard, rape seed, sugar beet, potato, peanut, mung bean, chickpea, and barley have also been studied (Figure 1; Table 1; Kimball, 2016). These additional studies have challenged some of our original conclusions, supported others, and illuminated the difficulties in drawing general conclusions across disparate studies and genotypes within a crop. On average across 186 independent studies of 18 C3 crops, elevation of [CO2] by ca. 200 ppm caused a ca. 18% increase in yield with adequate water and nutrients, so far lower than the ca. 40% of our 2015 review. Within these, legumes (21%) and root crops (22%) showed a greater increase than the C3 average while rice (14%) and wheat (14%) were below (Figure 2).
Our first conclusion, that woody crops were more responsive to elevated [CO2] than herbaceous crops was based on a limited comparison of cotton and grape with herbaceous crops. Since then, Ghini et al. (2015) have investigated coffee responses to elevated [CO2] (550 μmol/mol) and reported increased coffee yields of two commercial varieties by 12%–14.6%. This is much less than the reported ~40% increase in cotton yield from early FACE experiments in Arizona (Mauney et al., 1994) and ~40%–45% increase in grape yield from FACE experiment in Italy in the late 1990s (Bindi et al., 2001). The studies were done 2 decades apart during which time ambient [CO2] increased from ~353 to 390 μmol/mol (Figure 1), yet the elevated [CO2] treatment remained the same (~550 μmol/mol; Table 1), and so the treatment differential was smaller. Therefore, drawing firm conclusions about the strength of woody crop responses to elevated [CO2] from these very different studies is not possible.
The second conclusion was that in the absence of drought stress, C4 crops, which concentrate CO2 in bundle sheath cells and do not generally show a direct stimulation of photosynthesis with increased atmospheric [CO2] from 400 to 550 μmol/mol (reviewed in Pignon & Long, 2020), would not show yield gains at elevated [CO2]. This conclusion was based on experiments with sorghum grown in ambient and elevated [CO2], with and without drought stress in Maricopa, AZ, USA (Ottman et al., 2001). In that experiment, growth at elevated [CO2] stimulated sorghum biomass and yield when plants were grown with a severe drought treatment (<50% of potential evapotranspiration), but not under well-watered conditions (Ottman et al., 2001). This interaction of elevated [CO2] and drought is supported by the additional FACE experiments on C4 crops since 2005 (Figure 2; Leakey, 2009; Manderscheid et al., 2014). Experiments in one of the world's major maize growing regions in Champaign, IL, USA showed across three growing seasons, and with different nitrogen and heating treatments, elevated [CO2] did not significantly increase maize yields (Leakey et al., 2004, 2006; Markelz et al., 2011; Ruiz-Vera et al., 2015). Across multiple growing seasons in the temperate, highly productive Midwest agroecosystem, maize photosynthesis was stimulated during the periods of water stress while stomatal conductance was consistently lower in maize grown at elevated [CO2] (Leakey et al., 2004, 2006; Markelz et al., 2011; Ruiz-Vera et al., 2015). Elevated [CO2] increased maize canopy temperature, reduced canopy evapotranspiration by 9% on average (Hussain et al., 2013), and increased soil moisture content (Leakey et al., 2006; Markelz et al., 2011). Yet these improvements to water supply did not have a significant impact on maize yields. Experiments which combined rain shelters and FACE in Germany tested maize responses to elevated [CO2] in multiple growing seasons, with and without drought stress (Manderscheid et al., 2014). These experiments showed that rising [CO2] only stimulated maize yields under prolonged drought conditions. The average yield change in maize and sorghum in response to elevated [CO2] in FACE across seven independent studies and three locations was −3.0% and not significantly different from zero (n = 7), but a significant +20% (n = 4) under conditions of water deficit (Figure 2).
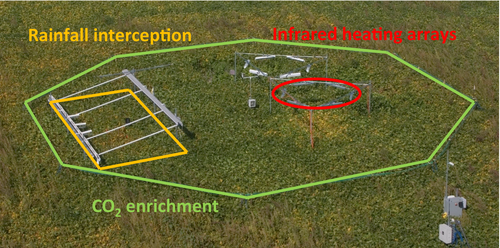
Our third conclusion was that crop yield responses to elevated [CO2] significantly varied with other environmental conditions, and that low N would limit crop yield enhancement at elevated [CO2] (Ainsworth & Long, 2005). Addressing the interaction of elevated [CO2] with warming temperatures, altered water availability, nitrogen, and tropospheric ozone pollution has been a major feature of FACE experiments over the past 15 years (Table 1). FACE technology was adapted to enrich ozone concentrations within plots (Morgan et al., 2004; Tang et al., 2011), and soybeans were exposed to both elevated [CO2] and [O3] in combination and separately at the SoyFACE experiment (Bernacchi et al., 2006; Dermody et al., 2008; Gillespie et al., 2012; He et al., 2014). The large size of FACE rings enabled the nesting of heating arrays for season-long warming (Cai et al., 2016; Ruiz-Vera et al., 2013, 2015), physical structures to capture rainfall (Erbs et al., 2015; Gray et al., 2016), and split plot designs for variable nutrient treatments, water amendments, and/or staggered planting dates (Bourgault et al., 2016; Fitzgerald et al., 2016; Manderscheid et al., 2016; Markelz et al., 2011; Mauney et al., 1994; Table 1; Figure 3). Additionally, the long-term investment in FACE experiments enabled investigation of the effects of elevated [CO2] under natural droughts, heat waves, and other variable growing season conditions (Dermody et al., 2008; Gray et al., 2016; Hasegawa et al., 2013). Investigations of the interactive effects of rising [CO2] with temperature, nutrient availability, water stress, and ozone pollution using manipulative treatments and interannual variation in growing seasons have supported some theoretical predictions, and at other times challenged those predictions, as detailed in the sections below.
3 UNDERSTANDING INTERACTIONS: NUTRIENTS AND ELEVATED [CO2]
The prediction that growth under elevated [CO2] and low N supply would limit C3 crop responses to elevated [CO2] has been supported by some additional studies, but not all (Table 1; Figure 2). Twenty-five studies at six FACE sites around the world have grown C3 crops under deficient N and elevated [CO2], and on average the mean yield response of crops is lower with low N supply compared to adequate N supply (8% lower, Figure 2). However, individual studies often fail to find evidence for a CO2 x N interaction (Weigel & Manderscheid, 2012; Yang et al., 2006). When a typical crop rotation of winter barley, ryegrass, sugar beet, and winter wheat was grown at elevated [CO2] and two N fertilizer treatments (adequate and 50% adequate) in Germany, only wheat yields showed an interaction of CO2 and N (Weigel & Mandersheid, 2012). Further analysis of wheat grown at ambient and elevated [CO2] and three levels of calcium ammonium nitrate fertilizer (40, 180, and 320 kg N/ha) at the same site showed that the lowest N treatment prevented the stimulation of wheat aboveground biomass at elevated [CO2], but stimulation of biomass was similar in the moderate and high N treatments (Dier et al., 2018). Hybrid rice also showed similar yield responses to elevated [CO2] under high and low N treatments (Yang et al., 2006). N uptake was increased by 10% in elevated [CO2] under both N treatments, consistently during vegetative and reproductive growth, which was hypothesized to explain the large stimulation of yield at elevated [CO2] (Yang et al., 2006). One reason for the inconsistent results may be that quantifying “low N” or “adequate N” depends upon soil type, environmental conditions, crop management, and other factors. This makes standardizing comparisons of N treatments and their interactions with elevated [CO2] very difficult.
Nearly two decades ago, Loladze (2002) proposed that elevated [CO2] would alter the stoichiometric balance of nutrients in plants, as growth at elevated [CO2] increases photosynthesis and carbohydrate content, but not mineral elements, with significant implications for human nutrition. Since that time, there has been growing concern that human nutrition will suffer with increasing atmospheric [CO2] (Loladze, 2014; Myers et al., 2014; Zhu et al., 2018). Many, but not all, studies have supported the notion that crop growth at elevated [CO2] leads to dilution of N and protein concentration in plant tissues from increased carbohydrate content (Taub et al., 2008; Taub & Wang, 2008). A meta-analysis showed a 6% decrease in protein in wheat and 8% in rice in FACE experiments, but no significant decrease in soybean or peas (Myers et al., 2014). These results may reflect the ability of leguminous crops to trade some of their extra carbon for N fixation. Nodule mass of soybean increased 28% under elevated [CO2] in the SoyFACE experiment (Ort et al., 2006). Increased nodulation or nodule activity would explain the lack of an effect on protein content in the leguminous crops. Consistent with this suggestion is the recent analysis of six lentil cultivars in FACE, where no decrease in seed N content was found in elevated [CO2], despite large yield increases (Bourgault et al., 2017). Phosphorus content also was not significantly different under elevated [CO2] in rice, pea, and soybean (Myers et al., 2014). Arbuscular mycorrhizal associations are considered to play a key role in the acquisition of nutrients, and in particular phosphorus (Smith & Smith, 2012). A recent meta-analysis of mycorrhizal response to plant growth in elevated [CO2] identified a significant 7% increase in AM biomass under elevated [CO2] (Dong et al., 2018). Mycorrhizal symbiosis was a key step in allowing plants to colonize land in the high [CO2] atmosphere of the Ordovician (Strullu-Derrien et al., 2018). While ability to associate with AM fungi is now recognized as a very early trait in the evolution of land plants, a few groups—notably the Brassicaceae—have lost this capacity (Keymer et al., 2017). As such, crops in these clades lacking capacity to form AM associations may be particularly disadvantaged under rising [CO2]. To date, there have been few experiments of Brassica responses to elevated [CO2]. In an un-replicated experiment in India, mustard (Brassica juncea) yields were stimulated by 20% at elevated [CO2], and in Germany, oilseed rape yields were increased by 18% (Högy, Franzaring et al., 2010; Högy, Keck, et al., 2010; Singh et al., 2013), which is more than observed for rice and wheat, and similar to the average response observed for all C3 crops (Figure 2). More fully replicated experiments with Brassica crops on phosphorus-deficient soils are needed to test if they are less responsive to elevated [CO2] than crops able to form mycorrhizal associations.
Simple nutrient dilution does not fully explain the response of protein and N to elevated [CO2]. Slower decomposition of litter with lower N, N immobilization by soil microbes, and/or inhibited shoot nitrate assimilation have been proposed to contribute to lower N content at elevated [CO2] (Uddling et al., 2018). In our 2005 review we noted that most of the decrease in plant N content was accounted for by a lower investment in Rubisco, a finding that is supported by subsequent FACE studies (Ainsworth & Long, 2005; Wang et al., 2020). With higher efficiency in elevated [CO2] and a shift from Rubisco to RubP regeneration limitation, less Rubisco is needed as predicted both by metabolic modeling and least-cost optimality theory (Long et al., 2004; Smith & Keenan, 2020). However, lower N uptake is not found universally. Dier et al. (2019) showed that elevated [CO2] improved N uptake efficiency and remobilization in wheat over a wide range of N fertilization levels, resulting in 8%–12% greater N yields with only a small change (ca. 3%) in grain N concentration. Based on controlled environment, hydroponic studies, it was hypothesized that under elevated [CO2], where nitrate is the source of N, its assimilation would be inhibited since CO2 and nitrate could be competing for reductant (Bloom et al., 2010). This effect would be absent when the source of N is already in the reduced form of ammonium. In the Arizona wheat FACE experiment the ratio of nitrate to total nitrogen in wheat was increased under elevated [CO2], consistent with the hypothesis (Bloom et al., 2014). However, this did not address whether assimilation of N would be greater when provided with ammonium. A test was provided by a further wheat FACE study with split plots nested within the CO2 treatments. Here one split plot was provided with N in the form of ammonium and urea, with nitrification inhibitors that maintained the soil N predominantly as ammonium. The other was provided with N, predominantly as nitrate. Aboveground productivity was the same in the two N treatments, but N acquisition in elevated [CO2] was significantly greater when plants were provided with N in the form of nitrate versus ammonium. This showed that inhibition of nitrate assimilation was not a cause of lower plant N concentration in elevated [CO2] (Dier et al., 2018).
McGrath and Lobell (2013) described additional mechanisms potentially contributing to decreases in other nutrients in crops grown at elevated [CO2], including altered partitioning among tissues at elevated [CO2] and decreased transpirational flow of minerals in the xylem stream. When Myers et al. (2014) sampled grains from C3 and C4 crops grown in FACE experiments on three continents, Zn content was reduced by 3.3%–9.3% and Fe by 4.1%–5.1% across wheat, rice, peas, and soybean. Maize showed a 5.2% decrease in Zn and 5.8% decrease in Fe even though photosynthesis and yield were not increased in these same plants under elevated [CO2] (Myers et al., 2014). In the SoyFACE experiment, the decrease in soybean season-long evapotranspiration was 8.6%, but the decrease in Fe was 4.1% (Bernacchi et al., 2007; Myers et al., 2014). Similarly, evapotranspiration declined by 9% in maize, yet Zn content was reduced only 5.1% (Hussain et al., 2013; Myers et al., 2014). These results suggest some compensatory effect or only a partial dependence on transpiration for the changes in nutrient content. Support for a role for transpiration comes from the observation that elevated temperature, which would drive increased transpiration, in combination with elevated [CO2], averted the decreases in nutrients otherwise observed in elevated [CO2] (Köhler et al., 2019). However, FACE studies in Germany did not support a role of transpiration causing lower nutrient content. Investigation of maize showed that although evapotranspiration was significantly lower (Manderscheid et al., 2014, 2016), there was no significant effect of elevated [CO2] on nutrient content (Erbs et al., 2015). At the same site, wheat Zn and Fe content were not significantly affected by elevated [CO2] (Dier et al., 2020) despite significant decreases in seasonal evapotranspiration (Manderscheid et al., 2018). A recent analysis of wheat in FACE under elevated [CO2] in Australia showed an increase in the ratio of nutrient uptake per unit of transpired water, for Ca, Mg, and Mn, again supporting a compensatory mechanism, albeit insufficient to prevent some decline in nutrient contents (Houshmandfar et al., 2018). Additional experiments are needed to understand the mechanisms and environmental conditions that result in lower nutrient content in elevated [CO2].
4 UNDERSTANDING INTERACTIONS: TEMPERATURE AND ELEVATED [CO2]
In warm climates, rising temperature and increased incidence of heat waves will have well-documented impacts on crop production (Bita & Gerats, 2013; Schlenker & Roberts, 2009; Zhao et al., 2017). Far less is known about how simultaneous increases in [CO2] and temperature will interact. From theory, two effects may be expected. First, since a near universal effect of [CO2] increase is a decrease in stomatal conductance, resulting in decreased evapotranspiration and less cooling of the canopy, elevation of temperature at the crop level will be exacerbated. Second, the temperature optimum of C3 photosynthesis is determined primarily by the ratio of oxygenations to carboxylations at Ribulose-1:5-bisphosphate (RuBP) carboxylase/oxygenase (Rubisco), which increases with temperature and decreases with [CO2], due to the competitive inhibition of oxygenation by CO2. At suboptimal temperatures, therefore, a positive interaction between rising [CO2] and rising temperature will be expected. From theory for a typical C3 Rubisco, the temperature optimum will increase as CO2 increases to ~28°C assuming a mid-century [CO2] of 550 ppm. In this temperature range, an increase in temperature today would lower photosynthesis, but at this future [CO2], it would increase photosynthesis; that is, the interaction would reverse the response to rising temperature (Long, 1991). At all temperatures, elevated [CO2] would increase photosynthesis. Whether this results in more production with rising temperature depends on what other effects higher temperatures have downstream of photosynthesis, for example on translocation and partitioning of assimilates, on respiration, and most importantly seed set and development. In cereals, elevated temperature speeds developmental stages, including grain filling, thereby shortening the period of grain filling. In wheat the number of kernels set decreases by 4% for each additional 1°C above the optimum during the 30 d prior to anthesis, which has been related to the decreased carbohydrate availability during the shortened vegetative phase (Barnabas et al., 2008). Temperatures above 30°C during floret formation cause complete sterility. In rice heat stress results in stamen and pistil abnormalities. Heat stress impacts are therefore a combination of effects on development and decreased carbohydrate supply (Barnabas et al., 2008). Increased temperature is commonly confounded with increased evaporative demand. Saturation water vapor pressure deficit (VPD) rises exponentially with temperature. For example, assuming a dew point temperature of 20°C, an increase in air temperature from 26°C to 28°C would increase the water VPD from 1.02 to 1.44 kPa, in effect increasing the evaporative demand of the atmosphere by 41%. Therefore we have to assume that direct effects of elevated temperature are commonly confounded with some degree of water stress.
What is clear from analysis of yields with current weather data is that in higher temperature growing regions, a 1–2°C increase above the current annual mean can decrease the yields of maize in Africa and wheat in Australia, by over 50% (Asseng et al., 2011; Lobell et al., 2011). This may be lessened where germplasm more tolerant of such temperature increase is identified and utilized. Looking to the middle of this century, will the simultaneous increase in [CO2] exacerbate or ameliorate the impacts of temperature rise? The advent of open-air feedback controlled radiative heating systems that provide a constant elevation of crop canopy temperature in combination with FACE will be key in answering this. This combination provides a means to address this question under open-air situations within crop fields (Kimball, 2016). Although, as noted above at the physiological level this will not allow the separation of direct temperature effects from increased water stress due to elevated plant to atmosphere VPD. This could be overcome if water vapor was also injected with temperature elevation to maintain a VPD in the elevated temperature subplots equal to that of the ambient. In the case of FACE experiments with rice, a different experimental approach was taken given the high thermal mass of water, which was a constant elevation of soil and paddy water temperature (Tokida et al., 2010). This approach also raised the humidity within the crop boundary layer and so avoided the VPD effect of other systems. In both cases the interactive effects have been determined by adding heated subplots within FACE and control rings. Most experiments have fallen into two groups, a constant season-long elevation of temperature or an elevation over a few days to represent heat waves at different developmental stages (Table 1).
Soybean grown in FACE with season-long elevation of [CO2] to 200 ppm above ambient showed an increase in yield, averaged over 3 years, of 13% relative to ambient [CO2]. When canopy temperature was elevated by an average of 2.7°C with infrared heaters, seed yield was depressed by 25% in ambient [CO2] and 17% in elevated [CO2], showing some but not complete protection by elevated [CO2]. In both ambient and elevated [CO2], warming decreased both aboveground biomass and harvest index (Ruiz-Vera et al., 2013). Transgenic addition of a cyanobacterial gene to upregulate fructose-1,6-bisphosphatase and sedoheptulose-1,7-bisphosphatase (FBP/SBPase) activity increased leaf photosynthesis relative to controls at the combined elevation of [CO2] and temperature. The yield of these plants under combined elevation of [CO2] and temperature equaled that of untransformed plants under current conditions, suggesting a means to protect against the yield loss otherwise seen under the combined temperature and [CO2] increase (Köhler et al., 2017). Season-long elevation of the canopy temperature of a modern maize hybrid by 2.7°C at the same site resulted in a 14% decrease in seed yield. This was attributed to a season-long decrease in photosynthetic capacity coupled with a decrease in harvest index. These measures were affected by elevated temperature, similarly, regardless of whether in current ambient or elevated [CO2] (Ruiz-Vera et al., 2015). When water and soil temperature were elevated by 2°C season-long within a rice paddy FACE system at Tsukuba, Japan, grain yield at both ambient and ambient +180 ppm [CO2] was not affected significantly over 3 years. Elevated [CO2] increased the yield by 14% averaged over the experiment (Usui et al., 2016). The lack of a temperature effect might reflect the fact that the site, with an average annual temperature of 13°C, is relatively cool for rice and different effects might be expected at tropical sites, where elevation of temperature above the average clearly impacts rice yield (Lesk et al., 2016). Across eight independent studies at three FACE sites, yields of C3 crops were increased 10% by elevated [CO2] when grown at a ca. 2°C temperature elevation, compared to an average of 18% at ambient temperature (Figure 2).
A more critical long-term effect of combined elevation of [CO2] and temperature might be loss of soil C. Increased supply of labile C to the soil, as can occur in elevated [CO2], through increased root exudates and litter incorporation, accelerates the decomposition of stored soil organic C (SOC), a response termed the priming effect (Hill et al., 2015; Vestergard et al., 2016; Zhu et al., 2014). Year-long elevation of temperature by ca. 2°C and growing season elevation of [CO2] by 200 ppm increased microbial respiration and decreased soil carbon in a soybean–maize rotation (Black et al., 2017). Black et al. (2017) hypothesized that this resulted from the priming effect, with increased C inputs under elevated [CO2] priming a loss of old soil carbon at the elevated temperatures. Application of these findings to the widely used Century model of soil C dynamics suggested this interaction would lower soil carbon by 15% over 100 years (Black et al., 2017). Given the central importance of soil C to soil fertility and water holding capacity, this suggests a major long-term indirect effect of rising temperature and [CO2] on crop productivity.
A widely predicted effect of global change is increased incidence of brief periods of temperatures elevated well above average, that is, heat waves. If these occur during the reproductive phase of crops, significant yield losses can occur (Gourdji et al., 2013; Lesk et al., 2016). Lentil grown at ambient and [CO2] elevated to +150 ppm at the AGFACE facility at Horsham, Victoria was subjected to a 3-day heat wave (38–40°C) during the 6 middle hours of the day during early pod development. This resulted in a 30% loss in yield relative to controls, regardless of [CO2]. The loss was attributed to a small decrease in aboveground biomass and a large decrease in harvest index due to both fewer pods per unit land area and fewer seeds per pod. Elevated [CO2] in the absence of the heat wave treatment increased yield by 34%, a gain that was eliminated by the heat wave (Bourgault et al., 2018). While this experimental heat wave was well above the average temperature for this grain filling period, a natural heat wave event reaching 37°C was recorded during the same period, suggesting the treatment was not unrealistic of the future.
5 UNDERSTANDING INTERACTIONS: DROUGHT × ELEVATED [CO2]
Two fundamental responses of leaves of C3 crops to rising atmospheric [CO2] are increased photosynthetic carbon assimilation and reduced stomatal conductance (Ainsworth & Rogers, 2007; Long et al., 2004). Both of these direct responses have the potential to influence crop yield responses to the interaction of elevated [CO2] and drought through stimulating canopy growth and leaf area and/or reducing whole canopy water use (Bernacchi & VanLoocke, 2015). Our original meta-analysis reported that crop yield responses under wet conditions were not as strong as under drought conditions, where elevated [CO2] increased yield by 28% on average (Ainsworth & Long, 2005). At that time, those conclusions were based on the FACE experiment in Maricopa, Arizona where cotton, wheat, and sorghum were exposed to both elevated [CO2] and different levels of drought stress (Mauney et al., 1994; Kimball et al., 1995; Ottman et al., 2001). While cotton showed a very significant increase in yield at elevated [CO2] (~43%) in both well irrigated and slightly drought-stressed plots (with 67%–75% of the irrigation applied to well-watered plots; Mauney et al., 1994), the increase in wheat yields at elevated [CO2] was substantially greater under dry conditions (50% of full irrigation) compared to wet conditions (Kimball et al., 1995). Sorghum only showed greater yields at elevated [CO2] under dry conditions (Ottman et al., 2001). These early experiments provided evidence for the hypothesis that under drought, the benefits of elevated [CO2] would be greater because lower stomatal conductance and canopy evapotranspiration would benefit soil water content, such that crops could sustain growth in a drought cycle for a longer period of time (Kimball, 2016; Leakey et al., 2009; Tausz-Posch et al., 2019). With more CO2 x water treatments in recent FACE experiments, Figure 2 shows a different picture for C3 crops, with an apparently similar average response to elevated [CO2] with and without water deficit. The experiments at Maricopa in Arizona, summarized in our 2005 review, simulated drought by providing 50%–75% of potential evapotranspiration, depending on the crop, throughout the growth cycle. This constant deficit might not be a good proxy for rainfed conditions where soil moisture can fluctuate greatly between periods of heavy rain and periods of prolonged drought.
More recent FACE experiments with drought treatments or dry growing seasons have shown very different interactions of soil moisture and elevated [CO2]. Stimulation of wheat, soybean, and lentil yields were greater in wet growing seasons compared to dry growing seasons (Fitzgerald et al., 2016; Gray et al., 2016; Houshmandfar et al., 2016; Parvin et al., 2018). Wheat and lentil experiments were conducted at the Australian Grains FACE experiments in Horsham and Walpeup in semiarid to arid environments, while the soybean experiments were conducted in Champaign, Illinois, USA, in a temperate, humid, continental environment. In these experiments, in contrasting growing environments, early season biomass accumulation and increased shoot:root ratios at elevated [CO2] had negative consequences when late season, severe drought stress occurred. In the case of wheat, stimulation of aboveground biomass at elevated [CO2] led to decreased tiller survival and inability to complete grain filling under dry, late-season conditions (Houshmandfar et al., 2016). Similarly, larger early season soybean and lentil crop canopies more than offset the effect of lower stomatal conductance resulting in more water extracted from the soil in the elevated [CO2] treatment under the subsequent dry conditions, whereas soil moisture content was greater under elevated [CO2] in wet years (Gray et al., 2016; Parvin et al., 2018). Sufficient soil moisture was necessary for maintaining N2 fixation in legumes during the grain filling period in order to stimulate yields at elevated [CO2] (Parvin et al., 2018) and maintain leaf and seed protein content (Gray et al., 2013). Together, these more recent FACE experiments suggest that as climate change intensifies drought stress in agricultural regions, the benefits of elevated [CO2] on C3 crop yields will not be realized without additional irrigation (Jin et al., 2017).
6 UNDERSTANDING INTERACTIONS: OZONE × ELEVATED [CO2]
Tropospheric ozone (O3) is a secondary pollutant formed as a by-product of the photochemical oxidation of nitrogen oxides in the presence of carbon monoxide and hydrocarbons (Myhre et al., 2013). Both the global CO2 and O3 cycles have greatly intensified over the past 100 years with opposing impacts on plant productivity (Ainsworth et al., 2019). While CO2 fertilization over the past 50 years has increased crop yields by an estimated 9%–14% (for maize and soybean, respectively; McGrath & Lobell, 2011), O3 pollution has decreased yields by 5%–10% (McGrath et al., 2015). Free-air CO2 enrichment facilities have been modified to enable O3 enrichment across large plots (e.g., Feng et al., 2011; Morgan et al., 2004; Shi et al., 2009; Tang et al., 2011), but only the SoyFACE experiment in Champaign, IL, USA combined CO2 and O3 fumigation across replicated plots (Bernacchi et al., 2006; Dermody et al., 2008; Gillespie et al., 2012).
The combined experiments with elevated [CO2] and [O3] on soybean showed that elevated [CO2] offered some protection from O3 damage. Dermody et al. (2008) reported the effects of elevated [CO2] and [O3] on soybean aboveground productivity and season-long canopy light and conversion efficiencies. Greater total biomass in elevated [CO2] was driven by greater radiation conversion efficiency, while lower biomass in elevated [O3] was driven by accelerated canopy senescence and lower conversion efficiency (Dermody et al., 2008). When elevated [CO2] and [O3] were combined, the negative effects of O3 on conversion efficiency and leaf CO2 uptake were partially counteracted by elevated [CO2] (Bernacchi et al., 2006; Dermody et al., 2008). Gillespie et al. (2012) also found that the transcriptional response of soybean to elevated [O3] was dampened at elevated [CO2] compared to ambient [CO2]. While many of the same genes involved in antioxidant and respiratory pathways responded significantly to elevated [O3] in plants grown at both ambient and elevated [CO2], the magnitude of the response was lower at elevated [CO2] and associated with a lower flux of O3 into the leaf due to the lower stomatal conductance at elevated [CO2] (Gillespie et al., 2012). These studies of a single crop species in one location offer only a limited view of potential crop responses to rising [CO2] and [O3], and more experimentation is needed in other locations and on different crops to fully understand how rising [CO2] and [O3] will combine to impact future crop productivity.
7 THE INFLUENCE OF PESTS, DISEASE, AND SOIL MICROBES IN ELEVATED [CO2]
7.1 Pests
When our original review of FACE studies was published in 2005, almost all extant analyses of the effects of elevated [CO2] on pests, diseases, and soil microbes were based on pot or small plot experiments in protected environments, that is, greenhouses and chambers. Beside the environmental modifications these impose, an obvious limitation is that pests and microbe spores cannot move freely, as they do in the field. Experiments therefore had to rely on their artificial introductions. Early work with elevated [CO2] in protected environments showed what are now widely observed changes in the chemistry of leaves and the plant. Typically these were reduced N concentrations, including total protein, and greatly increased total non-structural carbohydrates; changes that were also observed in FACE studies (Ainsworth & Long, 2005). Such findings led to the projections that insect herbivores would be less successful in a future elevated [CO2] world. This was because leaf and stem-mining guilds and leaf chewers would need to consume more material to obtain sufficient N for their growth and maintenance, and similarly sucking insects would need to consume more sap, so expend more energy, to gain needed nutrients (Lincoln et al., 1986; Schädler et al., 2007; Zavala et al., 2013). Early studies in protected environments partially confirmed these expectations (Whittaker, 1999). Consumption increased significantly across all feeding guilds, while development took longer and fecundity declined. As such, pests would be less fit when feeding on elevated [CO2]-grown material. Phloem feeders were an exception in showing growth stimulation, while seed eaters appeared unaffected (Bezemer & Jones, 1998). In subsequent years it became possible to examine these expectations under open-air conditions of elevated [CO2] where insects could move freely into the crop. In contrast to expectations, a major pest of soybean, the leaf-chewing Japanese Beetle (Popillia japonica), along with other leaf-chewing insects, were found in greater numbers and producing greater leaf damage in elevated [CO2] at the SoyFACE experiment (DeLucia et al., 2012). One possible explanation was that the increased numbers were an artifact of insects being attracted into the FACE plots by the elevated [CO2], given that some insects use [CO2] sensing to detect food sources (Jones, 2013). To test this, a replicated laboratory experiment fed Japanese Beetles on soybean leaves grown either under elevated [CO2] or grown under the ambient conditions at SoyFACE. Those feeding on leaves grown under elevated [CO2] lived ca. 16% longer and showed double the fecundity of those fed the leaves from the ambient plots (O’Neill et al., 2008). To test if this was a result of the higher carbohydrate content, a third cohort was fed ambient leaves that had been petiole-fed soluble sugars to achieve a similar soluble carbohydrate composition to the elevated [CO2]-grown leaves. This had no effect on fecundity or longevity, indicating some other effect of growth under elevated [CO2] (O'Neill et al., 2008). Subsequent analysis of the leaf transcriptome revealed downregulated expression of the defense signaling genes: lipoxygenase 7 (lox7), lipoxygenase 8 (lox8), and 1-aminocyclopropane-1-carboxylate synthase (acc-s) in soybean leaves growing under elevated [CO2]. This downregulation reduced the production of cysteine proteinase inhibitors (CystPIs), which deter coleopteran herbivores by inhibiting their ability to digest soybean leaf material. Confirming this, gut cysteine proteinase activity was significantly higher in beetles consuming leaves in the elevated [CO2] plots than in ambient (Zavala et al., 2008, 2009). This would also explain the increased fecundity under elevated [CO2] of the western corn rootworm rotation-resistant variant (Diabrotica virgifera), which feeds on soybean late in the growing season and then lays its eggs for the rotational maize crop of the following growing season (Schroeder et al., 2006). A further factor could be the increases in emissions of some of the volatile organic compounds, known to attract insect herbivores, that were observed under elevated [CO2] in this FACE experiment (O'Neill et al., 2010). Populations of the soybean aphid (Aphis glycines) were also monitored in this experiment and were greater in the elevated [CO2] plots (O'Neill et al., 2010). However, here a different cause was identified. Because elevated [CO2] partially suppresses stomatal opening and transpiration, the foliage is warmer by 1–2°C in the middle of sunny days (Bernacchi et al., 2007). By artificially lowering the temperature to that of ambient-grown leaves, it was shown that increased aphid performance under elevated [CO2] resulted from the temperature increase, and was not a direct result of tissue composition or emission changes resulting from elevated [CO2] (O'Neill et al., 2011). This is an effect unlikely to be observed in protected environments, where altered plant-atmosphere coupling would alter the temperature increase occurring under natural open-air conditions (McLeod & Long, 1999). Other crops grown under elevated [CO2] have been less extensively analyzed than soybean. However, consistent with trends seen in soybean, significantly increased populations of the cereal leaf beetle (Oulema melanopus) at the critical early grain fill stage of wheat grown at elevated [CO2] were observed in a 4-year experiment at Stuttgart in Germany. Increases in thrip (Thripidae) populations were also observed in the stem elongation phase (Oehme et al., 2013). In the long-term rice–wheat rotation FACE experiment in Jiangsu Province, China, a significant ca. 60% increase in herbivorous root nematode populations was observed at the critical grain filling stage of rice under elevated [CO2] (Hu et al., 2017). This unpredictable and unexpected doubling of crop damage by pests, as observed in some of these experiments, speaks to the need for FACE experiments with different crops and locations, if we are to understand the future of global food supply under rising [CO2].
7.2 Diseases
As with pests, increased leaf C/N in elevated [CO2] might similarly be expected to slow pathogen growth. Additionally, elevated [CO2] reduces stomatal conductance in plants, mostly through reduced aperture, but in some cases through reduced stomatal numbers (Ainsworth & Rogers, 2007). Fewer stomata and smaller apertures would impede the entry of many pathogens (Eastburn et al., 2011). Downy mildew (Peronospora manshurica), brown spot (Septoria glycines), and sudden death syndrome (SDS; Fusarium virguliforme) incidence on soybean at the SoyFACE facility in Illinois were monitored over 3 years. In line with expectation, incidence of downy mildew was significantly decreased by over 60% at elevated [CO2] in all 3 years, while brown spot and SDS incidence were unaffected (Eastburn et al., 2010). Experience with other major crops should however cause concern. In a 3-year study at the Japan Rice FACE facility in Shizukuishi, the number of leaf lesions caused by the rice blast fungus (Pyricularia oryzae) were significantly higher under elevated [CO2], with 65% more lesions in the worst year at the critical stage of panicle emergence. Inoculation of the plants with the pathogen spores showed the plants grown in elevated [CO2] were more susceptible. As in the case of soybean pest damage, it appears a secondary effect was responsible for this increased susceptibility, that is a lower leaf silicate content under the elevated [CO2] treatment, which can be related to decreased transpiration (Kobayashi et al., 2006). Silicate accumulation in rice leaves is closely associated with resistance to rice blast (Kim et al., 2002; Rodrigues et al., 2004). In a 2-year study of wheat in the AGFACE facility at Horsham VA, Australia, the amount of crown rot (Fusarium pseudograminearum) was significantly increased by fourfold in the most susceptible cultivar under elevated [CO2] with a concomitant increase in stem browning in the first year, but not in the second (Melloy et al., 2010). The variation between years and with water supply emphasizes the need for much greater understanding of the interactions of elevated [CO2] with other environmental variables in disease progression. In a separate study in the same facility over 3 years in which barley yellow dwarf virus (BYDV) had a high occurrence, incidence of disease was significantly (14% - 34%) higher in the elevated [CO2] plots. This may reflect increased feeding and incidence of the aphids that transmit BYDV (Trebicki et al., 2017).
7.3 Soil microbes
The greatly increased levels of non-structural carbohydrates observed in C3 crops grown at elevated [CO2] across the FACE experiments suggest a greater availability of carbohydrates for soil exudation and for symbioses with soil microbes. In the Swiss Pasture FACE experiment, elevated [CO2] resulted in a ca. 36% increase in diurnal CO2 uptake (Ainsworth et al., 2003), but soil carbon efflux, based on 13C pulse labeling was doubled (Staddon et al., 2004). Labeling showed this was associated with greater utilization of plant C by soil gram negative bacteria, saprotrophic fungi, and arbuscular mycorrhizal fungi (AMF). Notably the flux of C into AMF was faster and larger than in ambient [CO2]. As noted in the section on nutrients and elevated [CO2], microbial associations may play a key part in offsetting decreases in key nutrients (Staddon et al., 2004). This provides strong direct evidence of an increased proportion of photosynthate allocated to the soil microbiota under elevated [CO2]. In SoyFACE, after observing a lower C/N during early soybean growth at elevated [CO2], the ratio returned to the levels of ambient-grown plants in later growth despite a significant productivity increase. This suggested that nodulation and/or nodule activity increased with elevated [CO2] (Rogers et al., 2006). This was consistent with subsequent observations of a large increase in nodule numbers and mass in elevated [CO2] (Gray et al., 2013; Ort et al., 2006). A significant difference in microbial species diversity in the rhizosphere was also detected under elevated [CO2], suggesting that soybean might adapt to its increased C supply by recruiting more effective rhizobia to meet its increased N demand (Wang et al., 2017).
Mycorrhizal associations are considered important in providing a range of nutrients, but in particular phosphorus (Smith et al., 2003). Despite considerable increases in soybean yield under elevated [CO2], the concentration of phytate, as the principal storage form of phosphorus was unchanged (Myers et al., 2014), suggesting that uptake of phosphorus increased in proportion with rising plant productivity. Consistent with this, substantial changes in the mycorrhizal community were observed under elevated [CO2] (Cotton et al., 2015) together with increased soil concentrations of the microbial RNAs coding for exopolyphosphatase (Ppx) and polyphosphate kinase (Ppk; He et al., 2014). Similarly, in the Swiss Pasture FACE experiment after 7 years of elevated [CO2] treatment, significant increases in root colonization by AMF of Lolium perenne and Trifolium repens were observed together with a marginally significant increase in arbuscules (Gamper et al., 2004).
These limited observations of interactions with the soil microbiota in FACE suggest that crops under elevated [CO2] may, as previously postulated from studies in protected environments (Treseder, 2004), trade some of the additional C gained through increased photosynthesis for other elements, notably N and P, to offset dilution by the additional C. The degree to which this is possible appears to vary across crops. Despite a 14% increase in soybean yield under elevated [CO2], phytate and protein concentrations were unchanged, suggesting that N and P supply rose in concert. In wheat, however, a 13% yield increase under elevated [CO2] corresponded with significant decreases in phytate (4%) and protein (6%). While soybean was grown without the addition of fertilizer, wheat was grown with a high level on N fertilization (Long et al., 2006; Myers et al., 2014).
8 FACEing ADAPTATION
Rising [CO2] has the potential to increase photosynthetic efficiency and productivity. However, feedbacks at the individual plant level in terms of sink limitation and at the system level in terms of nutrient limitation lessen the actual increases achieved. Further, the ancestors of our major food crops evolved in a [CO2] that averaged 220 ppm, about half that of today's atmospheric concentration. Almost half of the increase in atmospheric [CO2] has occurred in just the last 40 years (Figure 1). This gives very little time in which evolution or breeder selection could adjust to this change. Indeed, several aspects of the photosynthetic apparatus of C3 crops appear optimized to a much lower [CO2] than that even of today (Srinivasan et al., 2017; Zhu et al., 2004, 2007). The following sections examine how FACE might be used to achieve adaptation so that in addressing global food supply the increased potential productivity resulting from elevated [CO2] might be realized in the context of the multiple detrimental effects of climate change.
8.1 Exploitation of genetic variability within crop germplasm
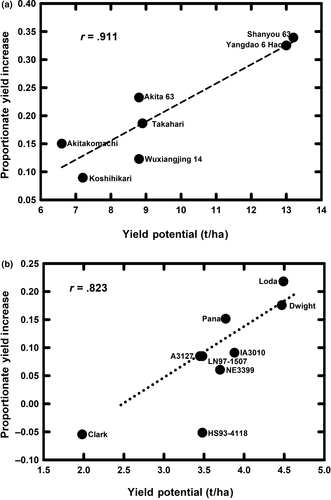
Genetic variation in the response of crops to elevated [CO2] is essential for efforts to breed for greater productivity and quality in the future (Ziska et al., 2012). Thus, one of the most promising results from FACE experiments around the world is the presence of significant genetic variation in crop productivity and quality responses to elevated [CO2] (Bishop et al., 2015; Hasegawa et al., 2013; Myers et al., 2014). The scale of FACE plots (ca. 300 m2) has only allowed the analysis of a limited range of crop germplasm (Bourgault et al., 2017; Hasegawa et al., 2013; Nakano et al., 2017; Sanz-Saez et al., 2017; Tausz et al., 2013; Tausz-Posch et al., 2015). As such, quantitative assessments of intraspecific variation in crop response to elevated [CO2] and the physiological and molecular drivers of variation are only beginning to emerge. Still, significant variation in grain yield stimulation at elevated [CO2] has been reported, ranging from 3% to 36% for eight rice cultivars (Hasegawa et al., 2013) and from 0% to 24% for nine soybean genotypes (Bishop et al., 2015; Figure 4). Variation of this level, especially if it is consistent across the years of study, should be more than sufficient for breeding selection (Tausz et al., 2013). Sink strength is one such trait, and for rice, the degree to which seed yield was stimulated at elevated [CO2] was correlated with panicle size and density, spikelet number per panicle, and seed size. The product of spikelet number per unit land area and individual seed mass as a measure of yield potential was strongly and positively correlated with yield increase under elevation of [CO2] by 200 ppm (Figure 4a; Hasegawa et al., 2013; Liu et al., 2008; Nakano et al., 2017; Yang et al., 2006). Although the compilation (Figure 4a) includes japonica, indica, and japonica x indica cultivars at sites in Japan and China, an earlier analysis confined to japonica cultivars at two sites in Japan showed a similar dependence of yield response to elevated [CO2] on sink capacity (Hasegawa et al., 2013). Soybean cultivar yields under elevated [CO2] and otherwise near-optimum growing conditions as a proxy of yield potential were also strongly and positively correlated with yield increase under elevation of [CO2] by 200 ppm (Figure 4b, Bishop et al., 2015). Both compilations suggest that sink capacity in these seed crops is a key limitation to yield responsiveness to elevated [CO2] in the field. The proportion of filled spikelets was ca. 60% in the most responsive rice cultivars, but ca. 90% in the least (Hasegawa et al., 2013; Liu et al., 2008; Nakano et al., 2017; Yang et al., 2006). This suggests that the lower response observed in earlier analyses of these crops (Long et al. 2006) resulted from inadequate sink strength in utilizing the additional photosynthetic potential under elevated [CO2]. In wheat, stimulation of grain yield at elevated [CO2] in cultivars with contrasting source–sink relationships was similar, but the mechanisms driving greater yields at elevated [CO2] were different (Tausz-Posch et al., 2015). An increase in fertile tiller number drove yield gains in a freely tillering cultivar at elevated [CO2], while yield stimulation was associated with greater kernel weight in cultivars with restricted tillering capacity (Tausz-Posch et al., 2015). Additional comparisons of wheat varieties with different transpiration efficiencies at the AgFACE experiment in Horsham, Australia showed greater [CO2] response in a line selected for high transpiration efficiency (Tausz-Posch et al., 2012). While not all crops have shown significant genetic variation in yield response to elevated [CO2] in FACE experiments (Bourgault et al., 2017; Parvin et al., 2018), a very limited number of cultivars have been studied in most crops. Greater efforts to screen the tremendous genetic variation in important crops will almost undoubtedly uncover meaningful differences in [CO2] response, which may be exploited in breeding. Achieving the theoretical yield response that rising [CO2] could deliver may be critical in addressing the predicted shortfall in supply versus demand as this century progresses (Ray et al., 2013).
Although many of the nutritional changes in crops to elevated [CO2] were consistent across the small set of cultivars tested by Myers et al. (2014), there were significant differences in rice varieties in zinc and iron responses to elevated [CO2] (Myers et al., 2014). This implies that selection for varieties that achieve necessary levels of these micronutrients under future elevated [CO2] is possible. In a further study of 18 rice cultivars grown in FACE experiments in Japan and China, Zhu et al. (2018) were less optimistic about the potential to select for genetic variation in nutritional responses to elevated [CO2]. They reported consistent decreases in protein, zinc, and iron as well as decreases in N-rich B vitamins. Zhu et al. (2018) suggested that many more genotypes would need to be screened in order to identify sufficient genetic variation for selection. To add to this challenge, the presence of other stresses associated with climate change alter grain quality responses to elevated [CO2] (Köhler et al., 2019). Again, this speaks to the need for FACE facilities of adequate scale for breeder selection (Ainsworth et al., 2008).
A trade-off between yield and mineral or nutritional content of crops has been one of the negative side effects of historical selection for increased yields, the so-called breeder's dilemma (Morris & Sands, 2006). The selection for fruit size, yield, and other market characteristics has been associated with the loss of nutritional quality in fruit and vegetables as well as grain crops (Davis, 2009; Davis et al., 2004; Fan, Zhao, et al., 2008; Morris & Sands, 2006). Both historical surveys of nutritional data and side-by-side field comparisons of old and modern cultivars have reported significant declines in nutritional quality of fruit, vegetable, and grain crops of newer cultivars. A comparison of nutritional quality of 43 garden fruit and vegetables reported in the USDA Nutritional Database from 1950 and 1999 revealed that protein, calcium, phosphorus, iron, riboflavin, and ascorbic acid significantly decreased between 1950 and 1999 (Davis et al., 2004). In England, it was found that concentrations of zinc and iron in wheat from the one of the longest running agronomic experiments at Rothamsted, the Broadbalk experiment, were stable from 1845 to the 1960s, but then decreased with the introduction of high-yielding, semi-dwarf varieties (Fan, Li, et al., 2008; Fan, Zhao, et al., 2008). Side-by-side trials of 63 historical and modern wheat varieties in the United States also showed that as grain yield has increased from the late 1800s to modern times, the concentration of copper, iron, magnesium, manganese, phosphorus, selenium, and zinc has declined (Murphy et al., 2008), showing the dominant effect to be increased yields of new varieties and not rising [CO2]. The mineral iron concentration of wheat decreased from 35.7 mg/kg dry weight in historical cultivars to 32.3 mg/kg in a modern line, and zinc concentration decreased from 33.9 mg/kg in historical cultivars to 27.2 mg/kg (Table 2; Murphy et al., 2008). This historical decline in iron and zinc content in wheat is slightly greater than the decline in those elements reported from FACE experiments at elevated [CO2] (Myers et al., 2014). Are these declines in nutrient content with both historical breeding and rising atmospheric [CO2] cause for human nutritional concern? Studies have suggested that the additional carbohydrate in crops due to rising atmospheric [CO2] could increase obesity, while the fall in mineral content may exacerbate malnutrition in areas of the world where people depend on one or a few staple crops (Loladze, 2014; Zhu et al., 2018). However, consideration of natural genetic variation within crops provides a different perspective. Marles (2017) reviewed historical changes in nutrient content, emphasizing the incredibly broad natural range of mineral content across cultivars of grains, fruit, and vegetables. Wheat iron content ranges from 16.3 to 163 mg/kg and zinc content from 15.3 to 100.2 mg/kg dry weight (Table 2; Marles, 2017). Divergent selection of maize has resulted in germplasm lines which span a grain protein content of 5% to 28% (Moose et al., 2004). Thus, the statistically significant and apparently large percentage decreases that accompany historical breeding and rising atmospheric [CO2] are small in proportion to the natural range of the species or the range that may be achieved by divergent selection, suggesting that given a demand there is ample genetic variation to maintain nutritional quality of plants, even as [CO2] increases.
| Mineral | Concentration | Range of mineral content in wheat grain | ||
|---|---|---|---|---|
| Historical lines | Modern lines | % Change | ||
| Calcium (mg/kg) | 421.6 ± 10.9 | 398.5 ± 16.1 | −6NS | 80–800 |
| Copper (mg/kg) | 4.8 ± 0.1 | 4.1 ± 0.1 | −16*** | 1–14 |
| Iron (mg/kg) | 35.7 ± 1.0 | 32.3 ± 1.8 | −11** | 16.3–163 |
| Magnesium (mg/kg) | 1,402.6 ± 21.0 | 1,307.6 ± 25.6 | −7*** | 200–2,200 |
| Manganese (mg/kg) | 50.0 ± 1.2 | 46.8 ± 3.1 | −7* | 10–90 |
| Phosphorus (mg/kg) | 3,797.1 ± 55.7 | 3,492.7 ± 119.3 | −9*** | 2,500–9,100 |
| Selenium (μg/kg) | 16.2 ± 1.7 | 10.8 ± 2.7 | −50* | — |
| Zinc (mg/kg) | 33.9 ± 0.9 | 27.2 ± 1.9 | −25*** | 15.3–100.2 |
- * p < .05, **p < .01, ***p < .001.
Exploiting genetic variation in crop yield responses to elevated [CO2] requires screening of diverse germplasm and structured populations to identify the genomic regions associated with greater yield quantity and quality at elevated [CO2]. Such experiments can be challenging given the size of individual FACE plots, and potential variation between and within FACE plots, but they are possible. A set of 65 chromosome segment substitution lines derived from indica and japonica rice cultivars was screened at the China FACE experiment, and quantitative trait loci for the CO2 response of yield, panicle number per plant, and grain number per panicle were reported (Fan, Li, et al., 2008). Free-air O3 enrichment experiments have investigated over 200 diverse inbred maize lines for O3 response, as well as half-diallel populations and near-isogenic lines (Choquette et al., 2019; Sorgini et al., 2019; Yendrek et al., 2017). These experiments used spatially explicit designs to control for cultivar location within the FACE plots, and established that atmospheric conditions can alter the heritability of key traits. They provide a blueprint for future FACE studies aimed at future-proofing crops. Additionally, experiments have shown that genotypes that respond strongly to low planting density also respond strongly to elevated [CO2] (Shimono, 2011; Shimono et al., 2009, 2014) raising the possibility of prescreening large numbers of genotypes or populations under conditions that serve as a surrogate in predicting likely performance at elevated [CO2] (Shimono et al., 2019). Both approaches, using FACE facilities for germplasm screening and using surrogate environments, have their limitations, but the potential to exploit genetic variation in crop responses to elevated [CO2] is clear and is critical to adapting crops to the future. The existing FACE studies have shown significant genetic variation in ability of our major crops to respond positively to rising [CO2]. However, a breeding program to implement this will require controlled elevation of much larger areas than is possible in current FACE facilities. The technology exists for expansion, but its realization now depends on a political will by the international community to enact it (Ainsworth et al., 2008).
8.2 Genetic engineering
As noted above there may be sufficient genetic variability in the major crops to breed for improved sink strength and nutrient content to offset some of the feedbacks on increased photosynthetic potential under elevated [CO2]. However, the failure of conventional breeding to achieve any significant increases in photosynthetic efficiency over the past 60 years suggests limited variability in photosynthesis within germplasm for breeding to act upon (Evans, 1997; Koester et al., 2016; Long et al., 2015). As noted previously, the photosynthetic apparatus of our crops appears optimized for a much lower [CO2] than today, and even less optimal for the future. Therefore, genetic engineering may be necessary to optimize the photosynthetic system to gain better efficiency under future conditions and to achieve sustainable yield increases by enhancing microbial interactions to provide the additional nutrients needed to balance the additional C.
All autotrophic assimilation of CO2 in eukaryotes occurs through the enzyme Rubisco. Since Rubisco is not saturated by the current, or projected future, atmospheric [CO2], the first-order effects of rising [CO2] are to increase the velocity of carboxylation while also competitively inhibiting the oxygenation reaction that leads to photorespiration (Long et al., 2004). However, a commonly observed response of C3 plants to growth in elevated [CO2] is a decrease in Rubisco content, which offsets part of the gain in photosynthesis that would otherwise result from rising [CO2]. Across the FACE experiments surveyed previously, leaf Rubisco content was decreased with growth under elevated [CO2] by ca. 20%, accounting for most of the observed decrease in leaf [N] (Ainsworth & Long, 2005). Could this reduction be reversed? A recent transgenic study upregulated Rubisco content in rice, resulting in significant increases in photosynthesis and yield in replicated field trials in the current atmosphere (Yoon et al., 2020). This technology is therefore a means that could be applied to offset the negative feedback on Rubisco content observed under elevated [CO2]. Two competing properties of Rubisco are key to optimizing the enzyme to a given [CO2]. At low [CO2] the specificity of the enzyme for CO2 versus O2 (τ) is critical to assimilation rate, while at high [CO2] the catalytic rate of carboxylation (kcat) dominates. Increased specificity requires tighter binding of CO2, which would be expected to slow catalysis, resulting in a trade-off between τ and kcat. Consistent with this expectation, photosynthetic organisms from near anaerobic environments show a high kcat and low τ, and vice versa for those in the current ambient atmosphere of high [O2]/[CO2], including our C3 food crops. However, analysis of the properties of C3 crop Rubisco in the context of canopy photosynthesis over a diurnal course indicated that kcat/τ was optimal for an atmospheric [CO2] of 220 ppm, but suboptimal for the 417 ppm of 2020. The study indicated that a Rubisco with a higher kcat even at the expense of τ could result in a 10% increase in daily carbon assimilation for the same amount of Rubisco (Zhu & Long, 2009; Zhu et al., 2004). As [CO2] rises, even larger gains might result from further increase in kcat/τ. While comparisons across the full range of photosynthetic organisms suggest a strong inverse relationship between kcat and τ, the dependency is much weaker when constrained to the angiosperms, raising the possibility of raising kcat without the loss of τ (Bouvier et al., 2020; Orr et al., 2016). This would be more efficient and require less [N] than upregulation of Rubisco content. Two possible means might achieve a more effective enzyme. Rubisco in C4 leaves operates in the elevated [CO2] environment of the leaf bundle sheath. In some species this appears to have resulted in the evolution of forms with a higher kcat and lower τ, which might coincidentally be optimal for C3 crops in a future elevated [CO2] world (Sharwood et al., 2016; Zhu & Long, 2009; Zhu et al., 2004). Significant progress has been made in transferring forms of Rubisco between plants, although it remains limited to relatively few recipient species (Whitney et al., 2015). An alternative is the use of Escherichia coli expressing functional crop Rubisco as a platform for directed evolution or other forms of enzyme optimization (Aigner et al., 2017; Conlan & Whitney, 2018; Lin et al., 2020; Wilson & Whitney, 2015). As the residue differences that provide improved kcat are unraveled, then change could be achieved by gene editing.
As both [CO2] and temperature rise, control of the rate of CO2 assimilation shifts progressively from Rubisco to RuBP regeneration. RuBP regeneration is affected by the rates of whole chain and cyclic phosphorylation, by activity of Calvin cycle enzymes and by capacity to balance consumption of sugar phosphates exported from the chloroplast with the rate of their production (Long et al., 2004). As noted in the above section on the interaction of [CO2] and temperature, the chloroplastic form of SBPase has been identified as a key control point in RuBP regeneration. Transgenic overexpression of SBPase in soybean was shown to protect against temperature-induced yield loss under elevated [CO2] at the SoyFACE facility (Köhler et al., 2017). Upregulation of the Calvin cycle enzyme FbP aldolase, as well as the Rieske Fe-S protein of electron transport and the H-protein of the leaf mitochondrial glycine cleavage system, plus introduction of Cytochrome C6, have all been found to increase RuBP regeneration rates, leaf photosynthesis, and productivity (Lopez-Calcagno et al., 2019, 2020; Simkin, Lopez-Calcagno, et al., 2017; Simkin, McAusland, et al., 2017). This suggests these would be further routes to maximizing photosynthesis under conditions of elevated [CO2].
As noted in the section on soil microbial interactions, soybean and possibly other legumes appear capable of adjusting their interactions with symbionts to trade C for nutrients, such that tissue nutrient content remains unchanged as productivity rises in elevated [CO2], while other crops do not. Given the long-term need for sustainable crop yield increases and in many areas of the globe an economic or logistic inability to obtain sufficient fertilizer, utilizing some of the additional carbohydrate that may be gained under elevated [CO2] to fix nitrogen and mine phosphorus would be beneficial. This could be realized if N2 fixation could be engineered into cereals and other nonleguminous crops, along with increased capacity to form mycorrhizal associations. Once far-fetched, recent rapid advances in understanding of the molecular biology of these related plant–microbe interactions show that these goals could be achieved in the not-too-distant future (Bailey-Serres et al., 2019; Feike et al., 2019; Feng et al., 2019; Mus et al., 2016; Zipfel & Oldroyd, 2017).
9 FUTURE PERSPECTIVE
Anthropogenic CO2 emissions have intensified over the past 60 years, with average emissions tripling between the 1960s and the 2010s (Le Quéré et al., 2018). Increased atmospheric [CO2] has impacted every corner of the planet, yet complex interactive effects of rising [CO2] with temperature, drought stress, nutrient variability, pests, and diseases result in regional variability in the “CO2 fertilization” effect on crops (McGrath & Lobell, 2013). Unfortunately, regional disparities in crop production will be amplified by global climate change. Recent and long-term FACE experiments have begun to provide better understanding of the interactions of rising [CO2] with temperature and drought. They have revealed many unexpected and unpredicted responses, which would undermine any ability to predict crop production and future food supply under atmospheric change. Much more work is needed across growing regions to accurately model and project these interactions to enable accurate projections of future food production and FACE studies are urgently needed in tropical regions. Studies also need to identify which management strategies will be most effective in co-promoting sustainability and productivity with rising [CO2]. Agricultural management practices and soils have potential to sequester C (Bernacchi et al., 2005; Lal, 2018), potentially offsetting more CO2 emissions. However, these practices have not been tested in FACE experiments, and limited data suggest agricultural C sequestration might be less likely under elevated [CO2] (Black et al., 2017). FACE experiments in agricultural systems are needed to test management and adaptation strategies, including identifying mechanisms for genetic variation in response to rising CO2 and testing transgenic strategies to improve yields and sustainability in anticipated future atmospheres. Thus, there is considerable opportunity to incorporate creative management approaches along with promising crop genotypes into FACE experiments to fully test future adaptation strategies that will minimize the environmental burden of agriculture and exploit technologies to optimize crop yields. Testing management strategies alongside genetic strategies for optimizing yields in elevated [CO2] has the best potential to offer timely solutions that can be implemented by farmers.
ACKNOWLEDGEMENTS
We thank the USDA ARS for long-term support of the Soybean free-air CO2 enrichment (SoyFACE) facility.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in “Data used to construct Table 1 and Figs. 2 and 4 in Ainsworth & Long (2020) 30 Years of Free-Air Carbon Dioxide Enrichment (FACE): What Have We Learned About Future Crop Productivity and the Potential for Adaptation? Global Change Biology” at https://doi.org/10.13012/B2IDB-2432396_V1.




