Microbial carbon limitation: The need for integrating microorganisms into our understanding of ecosystem carbon cycling
Abstract
Numerous studies have demonstrated that fertilization with nutrients such as nitrogen, phosphorus, and potassium increases plant productivity in both natural and managed ecosystems, demonstrating that primary productivity is nutrient limited in most terrestrial ecosystems. In contrast, it has been demonstrated that heterotrophic microbial communities in soil are primarily limited by organic carbon or energy. While this concept of contrasting limitations, that is, microbial carbon and plant nutrient limitation, is based on strong evidence that we review in this paper, it is often ignored in discussions of ecosystem response to global environment changes. The plant-centric perspective has equated plant nutrient limitations with those of whole ecosystems, thereby ignoring the important role of the heterotrophs responsible for soil decomposition in driving ecosystem carbon storage. To truly integrate carbon and nutrient cycles in ecosystem science, we must account for the fact that while plant productivity may be nutrient limited, the secondary productivity by heterotrophic communities is inherently carbon limited. Ecosystem carbon cycling integrates the independent physiological responses of its individual components, as well as tightly coupled exchanges between autotrophs and heterotrophs. To the extent that the interacting autotrophic and heterotrophic processes are controlled by organisms that are limited by nutrient versus carbon accessibility, respectively, we propose that ecosystems by definition cannot be ‘limited’ by nutrients or carbon alone. Here, we outline how models aimed at predicting non-steady state ecosystem responses over time can benefit from dissecting ecosystems into the organismal components and their inherent limitations to better represent plant–microbe interactions in coupled carbon and nutrient models.
1 INTRODUCTION
Industrialization, land use changes, and intensive agriculture have led to globally elevated atmospheric CO2 levels and to greater availability of nitrogen (N) in many areas, altering the stoichiometry and functioning of natural ecosystems (Peñuelas et al., 2013; Peñuelas, Sardans, Rivas-ubach, & Janssens, 2012). Currently, terrestrial ecosystems take up more CO2 from the atmosphere through photosynthesis, than is respired back to the atmosphere by autotrophs and heterotrophs. Terrestrial ecosystems globally sequester the equivalent of roughly 30% of the CO2 that humans emit to the atmosphere (Le Quéré et al., 2017) and thereby mitigate climate warming, yet the future sequestration potential of land is uncertain (Liu et al., 2019; Penuelas et al., 2017). Environmental stoichiometry can be used to explain the differences in carbon (C) and nutrient demands of plants and microorganisms in the soil, rhizosphere, and litter layer and meet the grand challenges of the 21st century to resolve uncertainty in ecosystem responses to non-steady state conditions (UN, 2019). For this to happen, we must recognize the basic concept that microbial C limitation in the soil feeds back to plant nutrient demands from the soil to explain whole ecosystem responses to non-steady state conditions such as elevated CO2 and N enrichment.
One characteristic of ecosystems that is rarely ever embedded in earth system or land surface models, yet may be crucial for predicting ecosystem responses to climate change, is the role of nutrient and C limitation of plants and soil microorganisms in controlling biogeochemical cycles. Our understanding of nutrient limitations to plant growth is well established after centuries of agricultural fertilization experiments focused on increasing crop yields. Recent advances in methods to measure microbial growth now provide better evidence that soil heterotrophic microorganisms are primarily limited by C, and only secondarily by nutrients. Plants depend on the activity of heterotrophic soil organisms for their nutrient supply and can stimulate heterotrophic decomposition of dead organic matter by providing decomposers with energy-rich substrates (i.e. priming). Heterotrophs, in turn, require plant-derived organic compounds for energy and enhance plant productivity by making nutrients available for uptake. Thus, within natural ecosystems, plants will essentially be nutrient limited, while decomposers in the soil will be C limited, and ecosystems as a whole are limited by neither.
This concept of simultaneous plant nutrient limitation and microbial C (energy) limitation is contradicting any “ecosystem limitation” by nutrients, as it is currently found in many textbooks. First, ecosystems are not organisms and thus cannot be limited themselves. Second, ecosystems must be composed of autotrophic and heterotrophic organisms, and because autotrophs and heterotrophs are inherently limited by different factors, a limitation of an ecosystem per se is not possible. Reports on N- or phosphorus (P)-limited ecosystems in the scientific literature usually refer to ecosystems in which primary production is either N or P limited; such studies thus ignore that heterotrophic organisms play essential roles in nutrient and C cycling.
Here, we argue that understanding the interaction of heterotrophic and autotrophic communities within ecosystems and its implication for the regulation of ecosystem functioning and C cycling is key to accurately project ecosystem C balance in response to nutrient availability and increasing atmospheric CO2 concentrations. First, we define ‘limitation’ at the organismal level and provide evidence for microbial C limitation. Then, we describe the empirical methods for determining microbial C limitation and how microbial C limitation can help to explain certain ecological phenomena. Finally, we discuss ways of integrating microbial C limitation into ecosystem models to improve predictions of ecosystem responses to global change drivers.
2 CONCEPTS OF LIMITATION
While the concept of limitation is a key concept in ecology, it remains poorly defined in many studies, especially in the context of global change. While the C contained in an ecosystem at any single point in time is measured by component pool sizes, the cycling of C into and out of terrestrial ecosystems is determined by the rates of processes such as photosynthesis, respiration, and growth, which may be sensitive to environmental change. To examine how these processes are affected by global change conditions, we invoke the concept of ‘Blackman's Limitation’, which defines the rate of a process as limited by the pace of the slowest factor, that is, nutrient or C uptake (Blackman, 1905). This is in contrast with ‘Liebig's Law of the Minimum’, which states that biomass production is determined by the availability of the scarcest, or most limiting, resource (von Liebig, 1840). Leibig's model is based on centuries of agricultural research on N, P, and potassium fertilizations to increase crop yield. The Leibig concept of limitation has crossed over into ecological theory of how the availability of nutrients in ecosystems limits net primary production, yet Blackman's limitation is more fitting for process rates such as photosynthesis and biomass growth, which are often not correlated with standing biomass, or yield.
An alternative to single nutrient limitation models is the ‘Multiple Limitation Hypothesis’ (Gleeson & Tilman, 1992; Sperfeld, Martin-Creuzburg, & Wacker, 2012), which suggests that nutrient demands of organisms or populations can be adjusted so that nutrients become co-limiting. This can occur for various reasons, such as physiological interactions within an organism (mostly between different resources, such as CO2 and nutrients), the acquisition of one nutrient being dependent on the availability of another (e.g. N fixation depending on sufficient P supply), or uneven distribution of nutrients between species within a given population/community. Thus, additions of multiple nutrients at once can lead to an increase in community biomass because species with different nutrient demands respond to different nutrients in the mix (Saito, Goepfert, & Ritt, 2008; Vitousek, Porder, Houlton, & Chadwick, 2010).

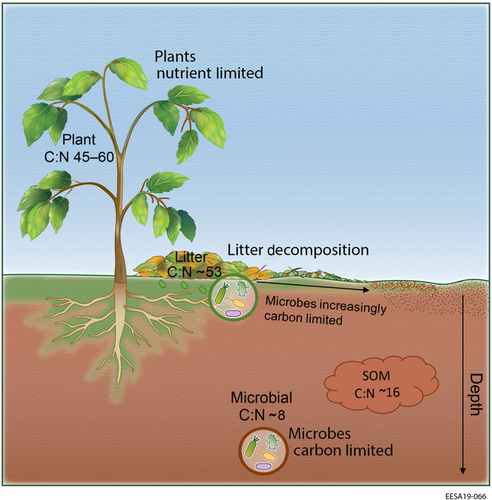
Soil microorganisms need C to satisfy their energy demands for maintenance (i.e. respiration costs) and for the synthesis of structural molecules to build biomass. However, catabolic and anabolic pathways have divergent stoichiometric demands. For example, while C is the main fuel for the energy costs of microbial maintenance, biomass growth has relatively higher nutrient demands due to the synthesis of structural molecules (e.g. N for protein and enzyme synthesis, P for DNA and RNA synthesis and for energy storage). Soil microorganisms may therefore modulate their metabolic pathways according to the stoichiometry of substrates available in soil, leading to shifts in CUE. This could provide a powerful approach for integrating shifts in microbial metabolic pathways into models of ecosystem C and nutrient exchange.
The stoichiometric argument highlights the fact that heterotrophic C consumption by decomposers is fundamentally different from light-driven photosynthetic reactions that drive autotrophic acquisition of C from atmospheric CO2. Nutrient limitations of whole ecosystems do not exist due to the fact that ecosystems are comprised of many organisms with varying physiological constraints and stoichiometric demands (Peñuelas et al., 2019; Sardans, Rivas-Ubach, & Penuelas, 2012; Turner, Brenes-Arguedas, & Condit, 2018). The direct effect of a nutrient addition on increasing autotrophic growth can, however, indirectly impact heterotrophs that feed on the products of autotrophic activity, although it may not directly affect the heterotrophs. As decomposers degrade soil organic matter and utilize it for their growth, surplus nutrients not needed for microbial growth are mineralized and made available for plant uptake while mineralized C is respired to the atmosphere as CO2 (Hodge, Robinson, & Fitter, 2000; Mooshammer, Wanek, Hämmerle, et al., 2014; Spohn & Kuzyakov, 2013). This excess nutrient release by microorganisms is fundamental to ecosystem functioning (Capek et al., 2018). The fact that plants release an organic C surplus for soil microorganisms, and microorganisms provide a nutrient surplus to plants, is a cornerstone property of ecosystem functioning (Figure 1).
3 EMPIRICAL METHODS OF DETERMINING MICROBIAL CARBON LIMITATION
Measuring soil microbial growth responses to C and nutrient additions is not straightforward. Traditionally, elemental limitation has been estimated for plant communities directly by measuring net primary productivity or aboveground plant biomass (LeBauer & Treseder, 2008) responses to changing nutrient availability, or indirectly by measurements of leaf stoichiometry (Hou, Chen, McGroddy, & Wen, 2012) or comparisons across ecosystems (Vitousek & Farrington, 1997). For soil heterotrophs, resource limitations have typically been estimated by measuring a net change in microbial biomass (standing stock) or a change in respiration (interpreted as microbial activity) after substrate amendment. Measurements of net biomass changes are typically done by chloroform fumigation extraction (Vance, Brookes, & Jenkinson, 1987), direct cell counts (Alexander, 1982), membrane lipid concentrations (Balkwill, Leach, Wilson, McNabb, & White, 1988), or substrate induced respiration methods (Anderson & Domsch, 1978). Standing biomass itself is dynamic because it depends on the occurrence and activity of predators and viruses (Fierer, 2017), and thus is not adequate at addressing substrate limitations to microbial growth.
Growth limitation of microbial communities has traditionally been measured by changes in soil respiration in response to added substrates and nutrients, as a proxy for growth. However, microbial respiration is composed of respiration for maintenance, growth, enzyme production, and overflow as well as waste metabolism to overcome stoichiometric imbalances (Manzoni, Taylor, Richter, Porporato, & Agren, 2012). An increase in respiration with nutrient or C additions can also be due to the revitalization of otherwise dormant microorganisms (Blagodatskaya & Kuzyakov, 2013), stimulation of a selected portion of the microbial population (Cleveland & Liptzin, 2007; Mori, Lu, Aoyagi, & Mo, 2018), or priming of native soil organic matter decomposition (Kuzyakov, Friedel, & Stahr, 2000). More generally, respiration is an estimate for catabolic reactions, while growth should be estimated by a measure for anabolic reaction. Therefore, respiration per definition is not an adequate metric of the nutrient or C limitation of microbial growth (Mori et al., 2018). Some methods measure growth rates of microbial communities by the incorporation of radiolabeled substrates such as 14C-acetate, 14C-leucine, or 3H-thymidine in their respective biopolymers (ergosterol, proteins, or nucleic acids, respectively; Rousk & Bååth, 2011). However, since these substrates contain C and in part N, those methods need to be treated with care, when they are used to assess C and nutrient limitations.
Recent technical developments have now made it possible to measure microbial growth directly without adding C or N containing substrates, using 18O-DNA labeling, finally allowing for a more rigorous exploration of what limits soil microbial growth in ecosystems under change (Geyer, Dijkstra, Sinsabaugh, & Frey, 2019; Spohn, Potsch, et al., 2016). This novel 18O-DNA method estimates microbial growth by measuring the synthesis of DNA by the incorporation of 18O from 18O-enriched water into microbial DNA (Spohn, Klaus, Wanek, & Richter, 2016). This, in contrast to traditional methods, allows for the differentiation between new growth (gross growth rates), microbial biomass changes (net growth rates), or standing microbial biomass stocks, and for the quantification of microbial CUE within a given environment. Using the 18O-DNA method, only investment in new growth (i.e. synthesis of ds-DNA) is assessed, thus investment in other cellular compounds not associated with growth, such as extracellular enzymes or extracellular polymeric substances that are exuded into the environment are not accounted for. Under an assumption of steady state, microbial biomass turnover could be calculated using the 18O-DNA method; however, since the microbial pool is not static, we caution this application. Instead, an independent assessment of microbial turnover is necessary to understand whether controls of biomass turnover rates (e.g. microbial death rates, predation, viral lysis, etc.) are limited by the same elements as growth rate. The ability to quantify new microbial growth directly and independent of substrate addition, rather than net biomass changes, using the 18O-DNA method represents a new advancement in the field of microbial ecology that can be utilized to test the C and nutrient limitation of soil microbial communities.
4 HOW CARBON LIMITATION OF SOIL DECOMPOSERS DRIVES ECOSYSTEM PROCESSES
4.1 Carbon and nutrient mineralization during litter and soil organic matter decomposition
Leaf litter decomposition studies are particularly illustrative of how the limitation of decomposers changes as C-rich plant material is progressively decomposed into lower C:N soil organic matter (Figure 1). During the early, high mass-loss, phase of litter decomposition, excess labile C availability leads to microbial nutrient limitation, and N is translocated from the soil to meet microbial stoichiometric needs as excess C is respired as CO2 or leached out into the soil (Bonan, Hartman, Parton, & Wieder, 2013; Frey, Six, & Elliott, 2003; Soong, Parton, Calderon, Campbell, & Cotrufo, 2015). In later stages of litter decomposition, litter mass loss and microbial activity slow down progressively due to an increasing limitation of easily decomposable organic matter (Cotrufo et al., 2015). As the C:N of decomposing material narrows, and approaches that of the microbial community, decomposers become C limited and N is mineralized (Melillo et al., 1989). The switch from N limitation to C limitation during litter decomposition explains why N additions stimulate the early stages of litter decomposition but in general do not affect longer-term decomposition rates (Knorr, Frey, & Curtis, 2005). The heterogeneous composition of soil often masks microbial C limitation, for example, although N additions can accelerate the decomposition of C-rich plant residues in the light fraction, it does not stimulate lower C:N mineral-associated organic matter or bulk soil decomposition (Neff et al., 2002). Thus, recognition of soil microorganisms as primarily C limited explains the variation in their response to C and N availabilities along the decomposition continuum and across sites with heterogeneous belowground composition.
4.2 Carbon sequestration in deep soils and its vulnerability
The C limitation of microorganisms also helps to explain the increasing residence time and persistence of deep soil C (Fontaine et al., 2007; Torn, Swanston, Castanha, & Trumbore, 2009). The median depth of new C incorporation into the mineral soil is 10 cm, while half of the soil C is located in soil layers deeper than 30 cm (Balesdent et al., 2018). This can be explained in part by the lack of fresh plant inputs, which are concentrated at or near the soil surface, and fuel higher microbial activity in top soil layers (Loeppmann, Blagodatskaya, Pausch, & Kuzyakov, 2016).
Fresh C inputs from plants in the form of litter or root exudates provide energy to microorganisms and can lead to the priming of soil organic matter (Bingemann, Varner, & Martin, 1953; Zhu et al., 2014). Input of these C-rich, labile plant materials in shallow soils and the rhizosphere alleviates microbial C limitation and leads to hot spots of microbial activity in the soil (Blagodatskaya & Kuzyakov, 2013; Cheng, Zhang, Coleman, Carroll, & Hoffman, 1996; Kuzyakov & Blagodatskaya, 2015). This can be seen in the linear scaling of the priming affect with microbial biomass along a litter addition gradient (Xiao, Guenet, Zhou, Su, & Janssens, 2015) whereby as litter inputs from steppe vegetation increased, microbial biomass increased, along with the decomposition, or priming, of more nutrient-rich soil organic matter to meet the stoichiometric demands of their greater biomass (Chen et al., 2014). Inclusion of the priming effects on microbial biomass can improve predictions of global soil organic C stocks and predictions of their change due to climate forcing over the 21st century (Guenet et al., 2018). The vulnerability of soil organic matter to increased decomposition with increased plant inputs that alleviate microbial C limitation indicates that deep soil C may be vulnerable to decomposition if elevated CO2 and N enrichment change root exudation by plants (Phillips, Bernhardt, & Schlesinger, 2009; Shahzad et al., 2018).
Although deep soil organic matter may have longer mean residence times in soils, it is as vulnerable to decomposition as shallow soils given a shift in conditions that favor microbial activity, such as warming temperatures (Hicks Pries, Castanha, Porras, & Torn, 2017) or labile C inputs (Fontaine et al., 2007; de Graaff, Jastrow, Gillette, Johns, & Wullschleger, 2014). In an incubation of root litter at several depths along a 1 m soil profile, initially the labile portion of root litter was decomposed at similar rates along the soil profile, but the later stages of decomposition slowed down much more in deep soils (Hicks Pries et al., 2018). This is likely due to the lack of labile C in deeper soils, which is needed to decompose the lower C:N material remaining at the later stages of decomposition (Knorr et al., 2005; Soong et al., 2015). Estimating the C sequestration potential from deeper root-C inputs to the soil due to land-use or climate change, must therefore account for both the direct inputs of root-C to deep soils but also the potential priming effect of root exudates to stimulate microbes to decompose soil organic matter due to their C limitation. This underscores how changes in deep soil C inputs due to land use or climate change could destabilize current C-climate feedbacks in natural ecosystems by alleviating deep soil microorganisms of their C limitations, which currently inhibit the decomposition of soil organic matter and contribute to vast soil C sequestration in deep soils.
4.3 Nutrient fertilization experiments
Nutrient fertilization experiments do not consistently demonstrate a stimulation of soil-C decomposition with nutrient additions because soil microorganisms are primarily C limited. Carbon limitation of microorganisms can explain the lack of latitudinal trends in microbial nutrient responses (Capek et al., 2018; Wild et al., 2015), when aboveground primary productivity generally shifts from N-limitation in high latitudes or young soils to P-limitation in low latitudes and older soils (Vitousek & Farrington, 1997; Vitousek et al., 2010). In the arctic tundra, long-term N fertilization led to a loss of soil C (Mack, Schuur, Bret-Harte, Shaver, & Chapin, 2004); however, it is unclear whether this was caused by N directly stimulating microbial decomposition, or indirectly by shifting vegetation allocation, rooting structure, and inputs (Mack et al., 2004; Sistla et al., 2013; Weintraub & Schimel, 2003). In the Gigante fertilization experiment in the Panamanian tropics, even clear evidence of decreased phosphatase enzyme activity and microbial biomass after 8 years of P fertilization (Turner & Wright, 2014) cannot rule out the possibility of increased C inputs from higher plant productivity (Wright et al., 2011) as a co-explanatory factor of the microbial responses (Mori et al., 2018). A review of over 20 experiments from tropical forests did not find evidence of P additions significantly affecting decomposition and microbial respiration (Camenzind, Hattenschwiler, Treseder, Lehmann, & Rillig, 2018). Phosphorus additions can lead to desorption of organic compounds, alleviating the C limitation of microorganisms and an increase in respiration as an indirect response to P additions (Spohn & Schleuss, 2019).
It is difficult to partition direct microbial responses to nutrient additions from indirect responses mediated by altered plant C inputs in situ. Results from laboratory soil incubations in the absence of plants demonstrate the primary limitation of microorganisms by C, and secondarily by nutrients across ecosystems from soils from the arctic (Jonasson, Michelsen, Schmidt, Nielsen, & Callaghan, 1996; Wild et al., 2014), sub-arctic grasslands (Marañón-Jiménez et al., 2019), mangroves (Keuskamp, Schmitt, Laanbroek, Verhoeven, & Hefting, 2012), and tropical forests (Duah-Yentumi, Rønn, & Christensen, 1998; Soong et al., 2018).
4.4 Water limitations
The stoichiometric explanation that soil microbial growth is primarily limited by C availability and plant growth is primarily limited by nutrient availability does not account for other environmental limitations, such as water availability. Under arid and semi-arid conditions, plants may restrict their photosynthetic capacity, limiting their C uptake to minimize water loss from open stomata (Peters et al., 2018). Reduced plant C uptake and allocation belowground, along with increased organo-mineral stabilization, can exacerbate soil microbial C limitation under dry conditions (Huang & Hall, 2017). Plant microorganism, C-nutrient, mutualistic interactions could breakdown further under water-limited conditions if resources are invested in osmotic adjustment or osmoregulation, rather than growth, and loss of water films inhibits microbial access to C-rich substrates in the soil.
5 INTEGRATING CARBON AND NUTRIENT LIMITATIONS OF ORGANISMS INTO CONCEPTUAL AND NUMERICAL MODELS
We must move beyond the concept of ecosystem limitations as a whole and move away from plant-centric ecosystem thinking to recognize how the limitations of individual heterotrophic and autotrophic organisms balance one another out to maintain ecosystem functioning. Recognition of how C limitation of soil decomposers drives the ecosystem processes outlined here can help to resolve the heterogeneous belowground responses to non-steady state conditions. New molecular techniques are now allowing for better measurements of growth responses of microbial communities, or even of specific microbial taxa, which allow for the limitations of decomposers to be better tested and quantified (Geyer et al., 2019; Hungate et al., 2015; Spohn, Potsch, et al., 2016).
Since the byproduct of microbial growth, microbial necromass, is essentially the building block of stable soil organic matter, microbial growth and CUE are important parameters to measure the impact microbial decomposition on an ecosystem's C balance. In plants, shifts in CUE have been observed: managed trees growing on fertile soils allocated a greater fraction of their gross primary productivity to growth and thus exhibit higher CUE than trees on infertile soils (Campioli et al., 2015; Vicca et al., 2012). By measuring microbial growth responses directly, we should now explore whether microbial C- or nutrient-use efficiencies respond similarly to environmental change. Quantification of C- and nutrient-use efficiencies of organisms in relation to available resources in space and time is a promising tool to fully integrate the C and nutrient limitations of soil microorganisms and plants into models of ecosystem C exchange (Huang et al., 2018; Tang & Riley, 2013; Wang et al., 2015; Wieder, Grandy, Kallenbach, Taylor, & Bonan, 2015). If microbial CUE responds to changing environmental conditions, for example, then models could alter CUE parameters to estimate microbial growth and respiration under future scenarios.
Ecosystem models must continue to improve their representation of ecosystem responses to changing environmental conditions over time to better inform land use and climate-based decision-making. The feedbacks and interactive effects among nutrient ratios, climate, and the capacity of ecosystems to store and release CO2 have only recently begun to be studied in experiments and by introducing N and P cycles into C and climatic models (Fleischer et al., 2019; Goll et al., 2017; Peñuelas et al., 2013; Wang et al., 2018). Recent advances in our ability to quantify the energy and nutrient limitations of heterotrophs and autotrophs within ecosystems provides a powerful tool for improving predictions of the ecosystem C balance in response to nutrient availability and increasing atmospheric CO2 concentrations. The interaction between nutrient and C demands of plants and microorganisms represents an exciting new frontier in biogeochemistry that will allow for the integration of soil microbial communities, and their decisive role in nutrient recycling and ecosystem C storage, into models of ecosystems undergoing changes in resource availability.
ACKNOWLEDGEMENTS
This work was funded by the European Research Council Synergy grant Imbalance-P #610028, and by the Terrestrial Ecosystem Science Program by the Office of Science, Office of Biological and Environmental Research, of the US Department of Energy under contract DE-AD01-05CH11231. Illustration by Diana Swantek, Earth and Environmental Sciences Area, Lawrence Berkeley National Laboratory, using Adobe Illustrator.




