How corals made rocks through the ages
Abstract
Hard, or stony, corals make rocks that can, on geological time scales, lead to the formation of massive reefs in shallow tropical and subtropical seas. In both historical and contemporary oceans, reef-building corals retain information about the marine environment in their skeletons, which is an organic–inorganic composite material. The elemental and isotopic composition of their skeletons is frequently used to reconstruct the environmental history of Earth's oceans over time, including temperature, pH, and salinity. Interpretation of this information requires knowledge of how the organisms formed their skeletons. The basic mechanism of formation of calcium carbonate skeleton in stony corals has been studied for decades. While some researchers consider coral skeletons as mainly passive recorders of ocean conditions, it has become increasingly clear that biological processes play key roles in the biomineralization mechanism. Understanding the role of the animal in living stony coral biomineralization and how it evolved has profound implications for interpreting environmental signatures in fossil corals to understand past ocean conditions. Here we review historical hypotheses and discuss the present understanding of how corals evolved and how their skeletons changed over geological time. We specifically explain how biological processes, particularly those occurring at the subcellular level, critically control the formation of calcium carbonate structures. We examine the different models that address the current debate including the tissue–skeleton interface, skeletal organic matrix, and biomineralization pathways. Finally, we consider how understanding the biological control of coral biomineralization is critical to informing future models of coral vulnerability to inevitable global change, particularly increasing ocean acidification.
1 INTRODUCTION
Although indigenous peoples have used coral reef resources for tens of thousands of years (Kirch, 2017) and coral fossils were known in Nicolaus Steno's time (the late 17th century; Rosenberg, 2009), coral reefs were first brought to wider European knowledge by accident, at 11 p.m. on 11 June 1770, when Lieutenant James Cook's ship, HM Bark Endeavour, “bumped” and ran aground on the Great Barrier Reef off the northeast coast of Australia (Cook, 1770). The ship was slightly damaged, but to free it, the crew had to throw about 50 tons of cargo, including six cannons, over the side; they subsequently repaired the damage and the ship continued on its voyage. From that time forward, however, most British captains of both naval and merchant ships became extremely cautious about approaching land in uncharted waters of tropical and subtropical seas. Some, who took short cuts, were unlucky, resulting in the multitude of shipwrecks that speak of the dangers of coral reefs, both to the vessels and to the reefs (Work, Aeby, & Maragos, 2008).
In an old-standing reef, the corals, which are so different in kind on different parts of it, are probably all adapted to the stations they occupy, and hold their places, like other organic beings, by a struggle one with another, and with external nature; hence we may infer that their growth would generally be slow, except under peculiarly favourable circumstances. (p. 76)
This was an amazing observation. It was sheer intuitive logic that would later lead to his second treatise, “On the Origins of Species,” first published on 24 November 1859 (Darwin, 1859), the first printing of which sold out within 1 day.
It would take the rest of the 19th century to understand that reef-forming corals are geologically ancient organisms. Their evolutionary origins are still not well constrained. However, reef-building corals have profound influences on the evolution, geology, ecology, and geochemistry of the world's oceans. Here we examine the role of corals from the perspective of biological mineralization and their incredible history of survival, evolution, and adaptability throughout hundreds of millions of years of global climate change.
2 WHAT ARE STONY CORALS?
Together with several anatomically distinct groups, scleractinian corals (commonly called “hard” or “stony” corals; see Cairns, 2007) belong in the class Anthozoa which is in one of the oldest invertebrate phyla, the Cnidaria. Cnidarians are some of the earliest metazoans to possess an organized body structure (Erwin et al., 2011). A characteristic of these marine animals is a radially symmetrical body with only two cell layer types: an ectoderm and an endoderm, separated by the mesoglea, a noncellular gelatinous matrix (Pochon et al., 2010). Their life cycle begins from soft-bodied, planktonic, planula larvae that cannot eat, but rather rely on their yolk for nutrition (Figure 1a). The amount of yolk nutrients determines their ability to survive as plankton without feeding, and therefore, the distance they can travel as plankton from where they were spawned. Upon settlement on a hard substrate, however, the planulae undergo metamorphosis to form polyps with a mouth that allows them to capture zooplankton (Figure 1b,g). For some shallow-water stony corals, this serves to supplement the nutrition they receive from endosymbiotic dinoflagellates (Figure 1e,f), while other species, including deep-water corals, remain asymbiotic or aposymbiotic. Stony corals are sessile and remain at their site of settlement for the rest of their lives.

Scleractinian corals (Figure 2a), which can form reefs in shallow tropical and subtropical seas, are the only extant anthozoans in which settlement and tissue reorganization during metamorphosis leads to deposition of an external mineral skeleton comprised of calcium carbonate. During metamorphosis, the aboral ectoderm of the planula transforms from a columnar epithelium into squamous cells called calicoblasts (the tissue layer composed of such cells is called the calicoblastic cell layer or calicoblastic epithelium [Von Heider, 1881]). The calicoblastic epithelium is in contact with the skeleton (Tambutté et al., 2011) and is mechanically anchored to it by specialized cells (desmocytes), which leave attachment scars on the skeleton (Muscatine, Tambutté, & Allemand, 1997; Figure 1c,l). The first mineral deposits of the initial polyp form a circular plate that shortly is supplemented by vertical blades known as septa and structures forming a cylindrical or cup-like wall (or theca; Figure 1d,h,i). In effect, the coral animals are a thin “glove” of a living organism on a biomineral skeleton of their making and that continuously grows as long as the animals live (Figure 2a). In colonial taxa, these polyps can ultimately form large reefs visible from space (Figure 2a,b).

3 EVOLUTIONARY HISTORY OF REEF-FORMING CORALS
The transition from abiotic calcium carbonate deposition on microbial surfaces to biomolecule-mediated skeletal calcite and aragonite formation by eukaryotes is one of the most dramatic transitions in the evolution of life in the oceans. In the early Archean eon, 4,000 to 2,500 Ma (millions of years before present), prokaryotic photosynthetic microorganisms, such as cyanobacteria, formed vast deposits of calcium carbonate around their sheaths (Allwood, Walter, Kamber, Marshall, & Burch, 2006; Riding, 2006). This process, which occurs on some eukaryotic algae today, is primarily a result of elevated pH on the cell walls. In Archean time, photosynthetic microbes made layer upon layer of carbonates, forming vast stretches along the coastlines of primordial continental landmasses. These ancient reef-like structures, called stromatolites, record some of the earliest evolution of life on Earth (Awramik, 1984). The transition, 2,000 Ma later, to the Phanerozoic eon (“visible life,” 541 Ma to present) was marked by the rapid appearance and evolution of basal metazoa, including those that calcify, ending stromatolite dominance, and kick-starting multicellular animal biomineralization in the oceans (Valentine, 2002).
Prior to the Cambrian “explosion” of animal life, the Ediacaran Period (635–541 Ma) marks a brief intermezzo (on geological timescales) with an abundance of enigmatic, soft-bodied organisms called the “Ediacaran Biota.” The Ediacarans represent an early stage in multicellular animal evolution; however, their relationships with marine animal phyla that subsequently emerged in the Cambrian (and represent the majority of extant phyla) remain enigmatic (Xiao & Laflamme, 2009). Most of the soft-bodied Ediacaran organisms went extinct at the Ediacaran–Cambrian boundary, but some probably continued to diversify (Cai, Xiao, Li, & Hua, 2019; Wood et al., 2019) and several are now considered to be basal Cnidaria (Gehling, 1988; Ivantsov & Fedonkin, 2002). Indeed, molecular clock analysis suggests that Cnidaria originated about 740 Ma, prior to the Ediacaran Period (Park et al., 2012). Cnidaria, therefore, rank among the most basal metazoan phyla dating back to the Neoproterozoic. Within Cnidaria is the class Anthozoa (Figure 3), a group that includes Hexacorallia (Scleractinia, Corallimopharia, Anthipatharia, Actiniaria, Zoanthiniaria), Ceriantharia, and Octocorallia and is thought to be a sister group to all other cnidarians, including Cubozoa (box jellyfish), Hydrozoa (jellyfish and hydroids), and Scyphozoa (true jellyfish; Collins, 2002; Kayal et al., 2018). All cnidarians share cnidae, or nematocytes; basically cellular “harpoons” that spear and poison zooplanktonic prey. Furthermore, the anthozoan life cycle is characterized by a planula larval stage that (Figure 1a), after settlement, develops into a sessile polyp (Figure 1d).
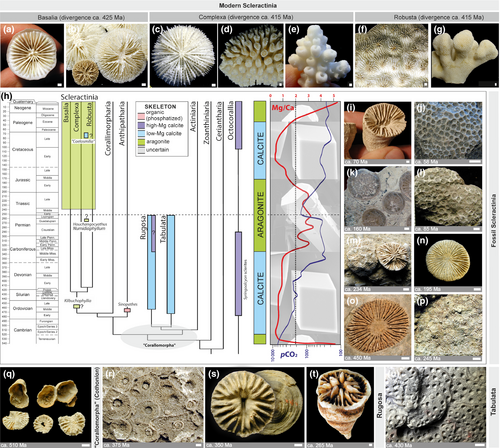
The fossil record during the early Cambrian period is poor (Savarese, Mount, Sorauf, & Bucklin, 1993). Many early anthozoans appear to have been soft-bodied, or used chitin-like structures to build their skeletons (Baliński, Sun, & Dzik, 2012). However, some exceptionally well-preserved soft-bodied fossils provide glimpses of early anthozoan evolution. Tiny fossils of soft-bodied sea anemones have been described from the Lower Cambrian of China (Han et al., 2010). These fossils, from 538 Ma, share characteristics exhibited by all modern anthozoans, and are thus likely stem group representatives (Figure 3h).
Two groups of now extinct calcifying anthozoans emerged in the Ordovician (Figures 3 and 4). Tabulata, exclusively colonial corals, appeared in the early Ordovician (485 to 445 Ma). The first Tabulata were encrusting and small in size (Scrutton, 1999), and the group represented a first attempt of biomineralizing cnidarians to contribute to the construction of reef belts in the paleotropical seas (Copper, 2001; Webby, 1992). The Rugosa emerged during the middle Ordovician around 460 Ma (Baars, Ghobadi Pour, & Atwood, 2012). Although colonial forms of rugose corals occur in the fossil record, the overwhelming majority were solitary and composed of calcite, the more stable of the two major polymorphs of calcium carbonate. Unlike modern scleractinian corals, which are radially symmetric with septa inserted cyclically, rugose corals typically exhibit strong bilateral symmetry with septa inserted in a tetraradial fashion. Most likely, rugosans were monophyletic and evolved independently from soft-bodied anthozoans (Baars et al., 2012).

Overall, during Paleozoic time, tabulate and rugose corals built reefs and increased in size, diversity, and geographic distribution, peaking during the Devonian. The end-Devonian crisis, marked by the collapse of reef environments, led to a significant taxonomic decline of tabulates, which suffered a major extinction and did not fully recover during the Carboniferous. In contrast, Rugosa were less affected and diversity remained high until the end of the Permian (250 Ma), at which time the largest extinction event in the Phanerozoic occurred (Raup & John Sepkoski, 1982).
Molecular phylogenies (Kitahara, Cairns, Stolarski, Blair, & Miller, 2010; Stolarski et al., 2011) suggest the emergence of the predecessors of modern reef-forming scleractinian corals in the early Ordovician (ca. 445 Ma). The oldest scleractinian lineages were almost certainly solitary and asymbiotic. Such an ancient origin of scleractinian corals is supported by the occurrence of solitary, conical-to-discoidal corals with septal insertion patterns identical to modern scleractinian corals (Figure 3o). Surprisingly, these fossil corals are limited to the Ordovician period and, thus far have only been found in southern Scotland and Northern Ireland (Scrutton, 1998; Scrutton & Clarkson, 1991). Regardless, fossils of scleractinian corals reappear after the end-Permian extinction, in the mid-Triassic (ca. 245 Ma), and became highly diverse (Stanley & Fautin, 2001). These mid-Triassic ancestors exhibited all growth forms found in modern reef-building corals (Figure 4).
3.1 Extinction and biomineralization of reef-forming corals
Although reef-forming corals were present throughout most of the Phanerozoic period, they underwent several mass extinction events, sometimes followed by so-called “reef gaps” of millions of years, during which time they were absent in the fossil record (Stanley, 1988, 2003). For example, the end of the Cambrian is marked by an extinction and led to a period of nearly 30 Ma without significant reef formation by metazoans (Copper, 2001). The end-Devonian extinction affected mainly colonial tabulate corals wiping out 80%–90% of taxa (Zapalski & Berkowski, 2012), but solitary Rugosa, living in deeper waters, were less affected. Following the complete extinction of all Rugosa and Tabulata during the end-Permian extinction, there was a second “reef gap” during the first few million years of the Triassic. During this period, Scleractinia rose to prominence and became prolific reef builders along the margins of the Tethys Sea (Flugel & Senowbari-Daryan, 2001), an environment similar to many modern, tropical marine regions (Wang et al., 2016). The fossil remnants of these reefs can be seen in the Alpine mountain range in Austria and Italy (Bosellini, Gianolla, & Stefani, 2003; Figure 5). The end-Permian extinction also marked a prominent shift in the mineralogy of coral biomineralization: while Tabulata and Rugosa generated calcite, scleractinian corals began to produce aragonite which they continue to do today. Fine-scale skeletal features and stable isotope skeletal signatures, such as δ15N, suggest that symbiosis with photosynthetic dinoflagellates was a key driver of their evolutionary success (Frankowiak et al., 2016; Muscatine et al., 2005; Figure 1e,f). This intracellular symbiotic association with photosynthetic algae marked the beginning of modern-type scleractinian coral reefs.

Perhaps the most famous, but not the most extreme, of all Phanerozoic extinctions at the end of the Cretaceous (66 Ma), led to the demise of sauropod dinosaurs as well as extensive ocean acidification (Henehan et al., 2019). However, this event only resulted in a moderate loss of scleractinians; approximately 45% of all taxa went extinct (Kiessling & Baron-Szabo, 2004). The extinction was more severe for highly integrated zooxanthellate corals, but these also recovered quite rapidly on geologic time scales (Kiessling & Baron-Szabo, 2004).
The causes that led to coral extinction throughout the Phanerozoic are likely varied. Ocean acidification, due to strong increases in atmospheric CO2, has been suggested (Greene et al., 2012), but the patterns shown in the fossil record of corals are more complex. Development of extensive ice caps, decreases in global temperature, and sea-level fluctuations were likely responsible for end-Ordovician coral extinctions that could also have led to their disappearance from the fossil record and potentially led to their migration into deep waters. The extinction of the Tabulata at the end of the Devonian and reef decline several million years prior to the end-Permian extinction suggest other factors. Suessialean dinoflagellates, which includes the family Symbiodiniaceae (LaJeunesse et al., 2018), the main symbiont of extant corals, almost completely disappeared at the end of the Triassic (Stanley & van de Schootbrugge, 2009). A breakdown in symbiotic relationships could have led to preferential extinction of colonial corals during several mass extinction events. In turn, the rise of scleractinians during the Triassic was likely linked to the evolution of photosymbiosis (Frankowiak et al., 2016; Kiessling, 2010).
Changes in atmospheric pCO2 may have indirectly shaped the trajectories of corals and other calcifying organisms through changes in temperature. This is perhaps most clearly illustrated by the large carbon cycle perturbation that occurred 55 Ma, at the boundary between the Paleocene and Eocene periods, and which serves as a deep time analog for current anthropogenic carbon emissions. The Paleocene–Eocene Thermal Maximum (PETM) was marked by the rapid release (<20,000 years) of 1,000s Pg of carbon, likely through the destabilization of seafloor gas hydrates or volcanism, driving an increase in global temperature of 5–8°C (Gutjahr et al., 2017; Sluijs et al., 2006; Zachos et al., 2005, 2003; Zeebe, Zachos, & Dickens, 2009). Surprisingly, most shallow-water marine calcifiers, including scleractinian corals, were not severely affected (Gibbs, Stoll, Bown, & Bralower, 2010).
4 SKELETAL STRUCTURE AND FORMATION
The principal crystalline “building block” of the approximately 1,500 listed species of modern Scleractinia (Cairns, 2007; Veron, 2000) consists almost exclusively of calcium carbonate crystals in the form of aragonite (e.g., Clode, Lema, Saunders, & Weiner, 2011; Foster & Clode, 2016; Von Euw et al., 2017). However, the presence of extracrystalline phases coexisting with aragonite has sometimes been reported. These include not only calcite (Frankowiak, Mazur, Gothmann, & Stolarski, 2013; Gladfelter, 1983; Houck, Buddemeier, & Chave, 1975; Lazareth et al., 2016; Wainwright, 1963) but also other noncarbonate minerals such as brucite (Mg(OH)2; Nothdurft et al., 2005). Nonetheless, these extracrystalline phases are not considered as the direct result of the coral biomineralization process since their presence could have different origins such as a mineral deposition due to the activity of boring organisms present within the skeleton (Macintyre & Towe, 1976), diagenetic alteration of the primary skeletal deposits (Frankowiak et al., 2013), secondary deposition of calcareous matter (Cusack et al., 2008; Dalbeck et al., 2011; Enmar et al., 2000), or even the result of an inorganic precipitation upon drying.
4.1 Contrasting the geological and biological model of biomineralization in corals
Although seawater is supersaturated with respect to calcium and bicarbonate ions, carbonates do not form spontaneously in seawater. However, they do form in a reliable, species-specific manner between coral tissue and the substrate. To examine this paradox, geochemists have investigated the chemistry of coral calcifying fluid, biologists have widely examined the skeletal organic matrix (SOM), while materials scientists have used various physical techniques to characterize the skeletal inorganic material. However, there is ongoing debate on the degree to which the biomineralization process in corals is directly related to seawater carbonate chemistry versus biological control.
One of the motivations to understand biomineralization in stony corals is that their skeletons incorporate atoms of elements other than calcium, carbon, and oxygen, often in relation to various environmental parameters such as pH (e.g., Hönisch et al., 2004; McCulloch et al., 2018), temperature (e.g., Dunbar, Wellington, Colgan, & Glynn, 1994; Mitsuguchi, Matsumoto, Abe, Uchida, & Isdale, 1996; Weber, 1973), and salinity (e.g., Giri, Swart, & Devlin, 2018; review by Corrège, 2006). Thus, through so-called geochemical “proxies,” the chemistry of coral skeletons can potentially allow reconstruction of past ocean conditions. However, with very few exceptions, trace element and stable isotope incorporation in coral skeletons is not in thermodynamic equilibrium with abiotically precipitated aragonite. The offsets are termed “vital effects” (e.g.; Adkins, Boyle, Curry, & Lutringer, 2003; Erez, 1978; Hönisch et al., 2004), a phenomenon that is often viewed as a geochemical process that has been slightly modified by the living coral. Such modifications include increased concentrations of Ca2+ (Al-Horani, Al-Moghrabi, & De Beer, 2003; Ohno et al., 2017; Sevilgen et al., 2019; Taubner, Hu, Eisenhauer, & Bleich, 2019) and 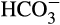 (Chen, Gagnon, & Adkins, 2018; Comeau et al., 2017; McCulloch et al., 2018; Sevilgen et al., 2019; Zoccola et al., 2015, among others). These are then reflected in altered ratios of element/Ca and offsets in the isotopic ratio of elements such as C, O, and B as compared to seawater. These vital effects arise from the biomineralization process.
(Chen, Gagnon, & Adkins, 2018; Comeau et al., 2017; McCulloch et al., 2018; Sevilgen et al., 2019; Zoccola et al., 2015, among others). These are then reflected in altered ratios of element/Ca and offsets in the isotopic ratio of elements such as C, O, and B as compared to seawater. These vital effects arise from the biomineralization process.
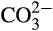 dissolved in seawater to their equilibrium concentrations as (Zeebe & Wolf-Gladrow, 2001):
dissolved in seawater to their equilibrium concentrations as (Zeebe & Wolf-Gladrow, 2001):
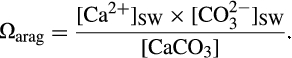
Therefore, geochemists usually consider the chemical composition of the calcifying fluid with respect to Ωarag, and hence the ability of calcium carbonate to precipitate abiotically (e.g., Cohen & Holcomb, 2009; Figure 6a). In this context, decreased pH due to the release of anthropogenic CO2 to the atmosphere leads to lower Ωarag and therefore should, logically, result in decreased calcification rates (e.g., Doney, Fabry, Feely, & Kleypas, 2009; Erez, Reynaud, Silverman, Schneider, & Allemand, 2011; Kleypas et al., 1999; Silverman, Lazar, Cao, Caldeira, & Erez, 2009). Although, there is evidence that this simplified model can explain calcification in some coral species in response to decreased pH (e.g., Anthony, Kline, Diaz-Pulido, Dove, & Hoegh-Guldberg, 2008; Drenkard et al., 2013; Strahl et al., 2015), in many others there appear to be mechanisms to resist this stressor to some extent (e.g., Comeau, Edmunds, Spindel, & Carpenter, 2013; Crook, Potts, Rebolledo-Vieyra, Hernandez, & Paytan, 2012; Fabricius et al., 2011; Ries, Cohen, & McCorkle, 2009; Shamberger et al., 2014; Strahl et al., 2015).
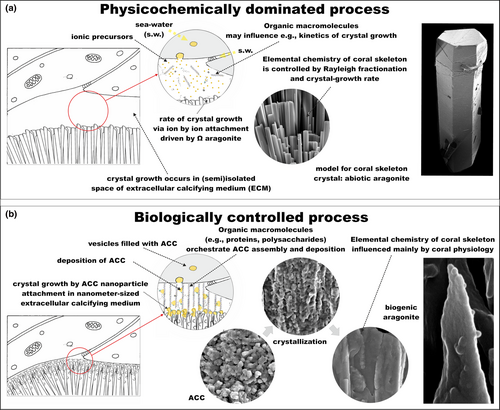
Preparation of cross sections of coral branches or nubbins in which the tissue can be preserved using a chemical fixative have allowed for increased understanding of how the calcifying milieu is established (e.g., Tambutté et al., 2007; Vandermeulen, 1975). This and other approaches focusing on the skeleton allow for investigation of the skeletal surface and/or internal regions (Benzerara et al., 2011; Cuif & Dauphin, 2005b; Cuif, Dauphin, Doucet, Salome, & Susini, 2003; DeVol et al., 2015; Gutner-Hoch et al., 2016; Isa, 1986; Nothdurft & Webb, 2007; Stolarski, 2003; van de Locht et al., 2013; Von Euw et al., 2017), along with the putative “calcification site” on the skeleton's surface or possibly within the animal tissue. Sites of biomineralization are any locations in which nucleation of the solid mineral phase occurs, including vesicles present within the calicoblastic cells (Hayes & Goreau, 1977; Mass, Drake, Heddleston, & Falkowski, 2017; Neder et al., 2019) or in the subectodermal space potentially present at the interface between the calicoblastic cells and the established skeleton (Clode & Marshall, 2002, 2003a, 2003b).
4.2 Composition of the coral SOM
There is significant organic matter in coral skeletons. Indeed, the organic portion of the coral skeleton has been studied for over 150 years. Silliman described the skeletal composition of 30 species in the mid-1840s (Silliman, 1846) and calicoblastic cells were recognized as being responsible for skeleton formation as early as 1881 (Von Heider, 1881; reviewed in Bourne, 1899). Furthermore, Duerden describes the remaining “colloidal material” following skeleton decalcification as bearing a resemblance to the mesoglea (Duerden, 1905), but that the appearance of this matrix becomes finer in older portions of the skeleton (Duerden, 1904). Mucopolysaccharides including chitin (Goreau, 1956, 1959; Wainwright, 1963), proteins (Johnston, 1980; Mitterer, 1978; Young, 1971; Young, O'Connor, & Muscatine, 1971), and lipids (Johnston, 1980; Young et al., 1971) were subsequently extracted from coral skeleton and described, with symbiont-derived photosynthate as one ultimate source of this fixed carbon (Muscatine & Cernichiari, 1969; Young, 1973; Young et al., 1971).
The most extensively studied component of the coral SOM is the protein complex retained in the inorganic aragonite skeleton even after the overlying animal dies. These proteins are secreted by the calicoblastic ectoderm (Puverel, Tambutté, Zoccola, et al., 2005; Yamashiro & Samata, 1996), serve to connect that cell layer to the pre-existing skeleton (Clode & Marshall, 2002, 2003a), and pattern both the physical and chemical environment of the calcifying medium (Chen et al., 2018; Clode & Marshall, 2003a, 2003b; Drake et al., 2018). The SOM allows coral aragonite precipitation even under calcite-promoting conditions (Higuchi et al., 2014; Higuchi, Shirai, Mezaki, & Yuyama, 2017; Yuyama & Higuchi, 2019). The amino acid composition of the SOM proteins across Scleractinia is heavily biased toward aspartic and glutamic acids (Mass et al., 2012; Mitterer, 1978; Young, 1971), a phenomenon not observed for tissue protein complexes (Yamashiro & Samata, 1996). Due to the proliferation of sequenced coral genomes and proteomes, the identities of approximately 30 skeleton proteins have been determined and functions of several of these proteins have been established.
The most interesting of the coral SOM proteins are the coral acid-rich proteins (CARPs), or alternatively called skeletal aspartic acid-rich proteins (SAARPs; Drake et al., 2013; Mass et al., 2013; Ramos-Silva et al., 2013; Takeuchi, Yamada, Shinzato, Sawada, & Satoh, 2016). To dispel confusion introduced by using two different names for some members of this class of proteins, CAPR4 and SAARP1 are homologs, CARP5 and SAARP2 are homologs, and a previously described partial protein sequence, P27 (Drake et al., 2013) and SAARP3 are homologs. Together, these proteins group as a coral-specific clade exhibiting an expansion of two polyaspartic acid domains (Drake et al., 2013), part of which was originally sequenced by Edman degradation (Puverel, Tambutté, Periera-Mouries, et al., 2005). CARPs have pH values between ca. 3 and 4.5, lead to calcium carbonate precipitation when added to unamended seawater at physiologically relevant concentrations (Mass et al., 2013), and are upregulated upon settling of larvae as spat on hard substrate (Akiva et al., 2018; Mass et al., 2016). The discovery of these proteins strongly suggests that the control of carbonate precipitation within the calicoblastic space is not a simple function of Ωarag. There is a distinct spatial patterning of the CARPs in the skeleton (Mass, Drake, Peters, Jiang, & Falkowski, 2014) and on growing aragonite crystals in coral cell cultures (Mass, Drake, et al., 2017), suggesting specific roles for each of the CARPs in coral biomineralization. An additional group of acidic coral skeletal proteins about which little is known are the skeletal acidic proteins (SAPs), several of which appear to be restricted to the family Acroporidae (Ramos-Silva et al., 2013; Shinzato et al., 2011; Takeuchi et al., 2016). CARPs and other highly acidic proteins such as the SAPs may also function to transport concentrated Ca2+ from intracellular pools to the extracellular calcifying medium (Mass, Drake, et al., 2017).
One of the other best-studied nonacidic proteins in coral skeleton is α-carbonic anhydrase (CA), an enzyme responsible for the hydration of CO2 to bicarbonate and a proton (H+), an important process for biomineralization, and the dehydration of 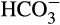 to CO2, which is necessary for aquatic carbon fixation. The function and evolution of CAs across life and their specialization in corals has been well-detailed elsewhere (Bertucci et al., 2013). Metazoan αCAs appear to have undergone multiple duplication events and those involved in biomineralization typically have a low complexity domain in the C-terminal half of the protein (Le Roy, Jackson, Marie, Ramos-Silva, & Marin, 2014). Their total activity in coral tissue has been determined to be sufficient, particularly when highly concentrated in certain areas, to supply CO2 in Symbiodinium-containing cells for photosynthesis and
to CO2, which is necessary for aquatic carbon fixation. The function and evolution of CAs across life and their specialization in corals has been well-detailed elsewhere (Bertucci et al., 2013). Metazoan αCAs appear to have undergone multiple duplication events and those involved in biomineralization typically have a low complexity domain in the C-terminal half of the protein (Le Roy, Jackson, Marie, Ramos-Silva, & Marin, 2014). Their total activity in coral tissue has been determined to be sufficient, particularly when highly concentrated in certain areas, to supply CO2 in Symbiodinium-containing cells for photosynthesis and 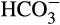 in the calicoblastic space for biomineralization (Hopkinson, Tansik, & Fitt, 2015). Although it was originally proposed to be limited to the cytosol of calicoblastic ectodermal cells (Bertucci, Tambutté, Supuran, Allemand, & Zoccola, 2011), a carbonic anhydrase, STPCA2, has been detected in coral skeleton (Drake et al., 2013; Ramos-Silva et al., 2013). STPCA2 and its scleractinian homologs belong to a clade of secreted αCAs with a cnidarian-specific secondary structure (Le Goff et al., 2016), exhibit significantly increased expression after larval settlement (Akiva et al., 2018; Mass et al., 2016), and are highly active when compared to other known CAs (Bertucci et al., 2011). While STPCA2’s catalytic activity exhibits a persistent decrease under decreased pH conditions, its expression shows a hyperbolic response with increased expression under moderate to low pH (~7.9 and 7.6, respectively; Drake et al., 2018), but greatly reduced expression at pHs corresponding to very high pCO2 (pH = 7.2–7.3, pCO2 = 2,000 ppm CO2; Drake et al., 2018; Zoccola et al., 2016). Although upregulation of STPCA2 under reduced pH conditions may compensate for its lower activity, allowing maintenance of biomineralization under moderately acidified conditions by continuously supplying bicarbonate to the calcifying medium (Chen et al., 2018; reviewed in Bertucci et al., 2013), its role is dramatically diminished under strong ocean acidification conditions (Zoccola et al., 2016).
in the calicoblastic space for biomineralization (Hopkinson, Tansik, & Fitt, 2015). Although it was originally proposed to be limited to the cytosol of calicoblastic ectodermal cells (Bertucci, Tambutté, Supuran, Allemand, & Zoccola, 2011), a carbonic anhydrase, STPCA2, has been detected in coral skeleton (Drake et al., 2013; Ramos-Silva et al., 2013). STPCA2 and its scleractinian homologs belong to a clade of secreted αCAs with a cnidarian-specific secondary structure (Le Goff et al., 2016), exhibit significantly increased expression after larval settlement (Akiva et al., 2018; Mass et al., 2016), and are highly active when compared to other known CAs (Bertucci et al., 2011). While STPCA2’s catalytic activity exhibits a persistent decrease under decreased pH conditions, its expression shows a hyperbolic response with increased expression under moderate to low pH (~7.9 and 7.6, respectively; Drake et al., 2018), but greatly reduced expression at pHs corresponding to very high pCO2 (pH = 7.2–7.3, pCO2 = 2,000 ppm CO2; Drake et al., 2018; Zoccola et al., 2016). Although upregulation of STPCA2 under reduced pH conditions may compensate for its lower activity, allowing maintenance of biomineralization under moderately acidified conditions by continuously supplying bicarbonate to the calcifying medium (Chen et al., 2018; reviewed in Bertucci et al., 2013), its role is dramatically diminished under strong ocean acidification conditions (Zoccola et al., 2016).
Further coral SOM proteins that remain to be functionally characterized include a very large protocadherin, mucin, vitellogenin, and proteins containing MAM and LDL receptor, zona pellucida, and EGF and laminin G domains (Drake et al., 2013; Ramos-Silva et al., 2013; Takeuchi et al., 2016). The function of galaxin, the first fully sequenced coral SOM protein, also remains to be described (Conci, Wörheide, & Vargas, 2019; Fukuda et al., 2003; Reyes-Bermudez, Lin, Hayward, Miller, & Ball, 2009; Watanabe, 2003; Wirshing & Baker, 2014). The shared skeletal proteins comprise a portion of the coral biomineralization protein “toolkit” (Drake, Mass, & Falkowski, 2014; Marin, Bundeleva, Takeuchi, Immel, & Medakovic, 2016; Ramos-Silva et al., 2013).
In addition to the acidic amino acids, coral SOM acidity is increased bythe presence of polysaccharides and the sulfation and glycosylation of SOM proteins. Staining has revealed that epidermally derived mucus contains polysaccharides (Goreau, 1956), as does the extracellular matrix secreted by coral cell cultures (Helman et al., 2008). Saccharide moieties similar to the polysaccharides, hyaluronan (Goldberg, 2001a) and chitin (Adamiano et al., 2014), and a variety of monosaccharides (Naggi et al., 2018; Ramos-Silva et al., 2014; Takeuchi et al., 2018), as well as modifications by sulfation (Cuif & Dauphin, 1998; Cuif et al., 2003; Dauphin & Cuif, 1997; Goldberg, 2001a) have been observed in coral SOM.
While the coral SOM lipid fraction has not been well characterized, lipids may make up a significant fraction of the organic component of skeleton (Adamiano et al., 2014; Falini et al., 2013; Farre, Cuif, & Dauphin, 2010; Goffredo et al., 2011). Phospholipids likely function in the vesicular transport of mineral precursors to the calcifying space (Akiva et al., 2018; Goffredo et al., 2011) and may stabilize amorphous calcium carbonate (Goffredo et al., 2011). It is also established that some skeletal phospholipids bind Ca2+ directly (Isa & Okazaki, 1987).
When added to a solution containing CaCl2 and bicarbonate or unamended seawater, macromolecules of the SOM complex interact with the growing mineral, often in a species-specific manner (e.g., Falini et al., 2013; Goffredo et al., 2011; Mass et al., 2013; Sancho-Tomás et al., 2014). In vitro experiments have highlighted the effects of the SOM complex as combining with the aragonite saturation state of the calcifying milieu to distinguish various growth patterns such as early mineralization zones (Njegic et al., 2019).
4.3 Cellular biochemical processes in coral skeleton formation
Coral skeletal aragonite is produced within a semienclosed extracellular compartment, termed the extracellular calcifying medium (ECM), proposed to be of a few nano- to micrometer thickness between the skeleton and the calicoblastic epithelium (Allemand, Tambutté, Zoccola, & Tambutté, 2011; Mass, Giuffre, et al., 2017; Sevilgen et al., 2019; Tambutté et al., 2007; Venn, Tambutté, Holcomb, Allemand, & Tambutté, 2011; Figure 6). Together with the three overlaying tissue layers, the calicoblastic epithelium spatially separates the ECM from direct contact with the surrounding seawater, which is the main source of ions to the ECM (reviewed in Allemand et al., 2011). Therefore, in order to precipitate CaCO3 the coral must transport Ca2+ and dissolved inorganic carbon (DIC) from the seawater or intracellular fluid, respectively, to the ECM. Furthermore, in addition to the import of necessary ions, there are suggestions that corals must use additional means to raise the aragonite saturation state of the calcifying fluid to obtain rapid rates of calcification (Cohen & McConnaughey, 2003).
Ca2+ can be transported from seawater to the calcifying space through transcellular (i.e., diffusion or active transport out of the calicoblastic ectodermal cells; Cai et al., 2016; Sevilgen et al., 2019) or paracellular (i.e., between cells at junctions) processes (Allemand et al., 2011), or a combination of the two (Allemand et al., 2011; Ohno et al., 2017; Tambutté et al., 2011, 2012). A plasma membrane calcium pump (PMCA) in corals functions in the same manner as is known in mammals, where Ca2+ ATPases transport calicoblastic ectodermal cytosolic Ca2+ to the calcifying space (Zoccola et al., 2004). stpPMCA immunolocalizes to the calicoblastic ectoderm (Barott, Perez, Linsmayer, & Tresguerres, 2015; Zoccola et al., 2004) and is upregulated in coral nubbins grown at very low pH (7.2; Vidal-Dupiol et al., 2013). Combined with its added function of exchanging H+ and Ca2+ across the calicoblastic ectodermal membrane (Salvador, Inesi, Rigaud, & Mata, 1998), this upregulation of the gene likely allows corals to increase the saturation state of the calcifying fluid to maintain biomineralization under suboptimal ocean conditions. Additional Ca2+ transport from the calicoblastic ectodermal cells to the calcifying space can be achieved by voltage-dependent Ca2+ channels (Tambutté, Allemand, Mueller, & Jaubert, 1996), one of which has been identified and localized to the calicoblastic ectoderm in S. pistillata (Zoccola et al., 1999).
It has been suggested that corals biologically control the composition of the ECM by increasing pH and DIC concentrations above that of the surrounding seawater (Al-Horani et al., 2003; Allison, Cohen, Finch, Erez, & Tudhope, 2014; Cai et al., 2016; McCulloch, Falter, Trotter, & Montagna, 2012; Venn et al., 2011). This allows the formation of aragonite, which is thought to constitute a crucial step in the coral biomineralization process (Lowenstam, 1981). Recent studies conducted using microscope-guided microsensors, B stable isotopes, and B/Ca ratios to measure or calculate pH, [Ca2+], [CO32−] ,or [DIC] in the ECM, and Ωarag, showed that all parameters were elevated with respect to the surrounding seawater (Schoepf, Jury, Toonen, & McCulloch, 2017; Sevilgen et al., 2019). pH elevation is achieved through PMCA activity, exchanging H+ and Ca2+ as described above. Most of the inorganic carbon for biomineralization comes from an internal pool (Erez, 1978; Furla, Galgani, Durand, & Allemand, 2000) and is delivered to the site of calcification either by CO2 diffusion or by 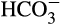 transporters (Tambutté et al., 1996). It has also been suggested that elevation of ECM DIC occurs via a carbon concentration mechanism in the calicoblastic cells to favor calcification (Allison, Cohen, Finch, Erez, & Tudhope, 2014; Comeau et al., 2017; McCulloch et al., 2012). Metabolic CO2 can then diffuse from the coral tissue to the ECM (Erez et al., 2011; Furla et al., 2000; Hohn & Merico, 2012). Alternatively, a bicarbonate transporter in the SLC4γ family, which has been immunolocalized to the calicoblastic layer (Barott et al., 2015; Zoccola et al., 2015), introduces bicarbonate anions from the cytosol of the calicoblastic cells to the ECM and so may both provide ECM DIC and raise ECM pH (Bhattacharya et al., 2016; Zoccola et al., 2015). Furthermore, a sensory mechanism has been identified that enables the coral to sense the internal pH via the enzyme soluble adenylyl cyclase (sAC) that is activated by bicarbonate ions to produce the secondary messenger molecule cyclic AMP (cAMP; Barott, Barron, & Tresguerres, 2017; Tresguerres, Barron, Barott, Ho, & Roa, 2013).
transporters (Tambutté et al., 1996). It has also been suggested that elevation of ECM DIC occurs via a carbon concentration mechanism in the calicoblastic cells to favor calcification (Allison, Cohen, Finch, Erez, & Tudhope, 2014; Comeau et al., 2017; McCulloch et al., 2012). Metabolic CO2 can then diffuse from the coral tissue to the ECM (Erez et al., 2011; Furla et al., 2000; Hohn & Merico, 2012). Alternatively, a bicarbonate transporter in the SLC4γ family, which has been immunolocalized to the calicoblastic layer (Barott et al., 2015; Zoccola et al., 2015), introduces bicarbonate anions from the cytosol of the calicoblastic cells to the ECM and so may both provide ECM DIC and raise ECM pH (Bhattacharya et al., 2016; Zoccola et al., 2015). Furthermore, a sensory mechanism has been identified that enables the coral to sense the internal pH via the enzyme soluble adenylyl cyclase (sAC) that is activated by bicarbonate ions to produce the secondary messenger molecule cyclic AMP (cAMP; Barott, Barron, & Tresguerres, 2017; Tresguerres, Barron, Barott, Ho, & Roa, 2013).
Although the macroscopic coral skeleton is clearly an extracellular feature, there are increasing suggestions that some part of the process of its formation may commence intracellularly. Early coral research of the mechanism of ion transport to the site of calcification suggested that the initial site of mineral formation is intracellular based on the observation of vesicles and calcareous elements in the calicoblastic cells (Bourne, 1899; Fowler, 1885; Hayes & Goreau, 1977; Kawaguti & Sato, 1968; Ogilvie, 1896; Von Heider, 1881). While this model was later replaced by the concept of transcellular and paracellular pathways, recent studies have revisited this component of biomineralization, indicating a strong biological control of skeletal formation via an intracellular pathway. There is evidence that high concentrations of calcium can be transported via vesicles to the site of calcification in S. pistillata (Barott et al., 2015; Mass, Drake, et al., 2017). Indeed, calicoblastic cells in rapidly calcifying portions of the calicodermis contain a higher concentration of such types of vesicles (Isa & Yamazato, 1981). Some intracellular vesicles, particularly those in calicoblastic cells, also contain a Na+/Ca2+ exchanger (Barron et al., 2018). Furthermore, Nano-SIMS reveals that intracellular sites of concentrated calcium colocate with aspartic acid-rich regions of the cell, such as those contained in highly acidic proteins later retained in coral skeleton (Mass, Drake, et al., 2017).
In addition to the potential for intracellular aggregations of Ca2+ and/or mineral precursors, it has been revealed that the ECM of an intact coral is not a prerequisite for aragonite formation. Isolated coral cells in culture on bare Petri dishes re-aggregate into protopolyps and precipitate aragonite both on the exterior of these protopolyps (Mass et al., 2012) and on the extracellular matrix surrounding individual cells (Mass, Drake, et al., 2017; Figure 7). In these cultures, cells killed with azide do not form aragonite (Mass et al., 2012) suggesting that such precipitation is actively controlled by the live cells. The three-dimensional structure of a coral should not be taken for granted, however, as it is precisely this which directs the aggregation of aragonite bundles into species-specific corallite patterns.

Further evidence that the precipitation of calcium carbonate is controlled biologically comes from the form of the crystal. It has been frequently suggested that major shifts in skeletal mineralogy of calcifying organisms, including corals, were driven by ocean Mg/Ca ratios (Morse, Wang, & Tsio, 1997; Porter, 2007; Stanley & Hardie, 1998). During periods with high Mg/Ca ratios, aragonite-forming organisms dominated, whereas low Mg/Ca seawater ratios promoted diversification of calcite-secreting organisms, in line with the control of abiotically produced polymorphs by Mg/Ca ratios (Berner, 1975). The Mg/Ca ratio of seawater is modulated by a number of processes, one of which is sea floor spreading rates with high spreading rates leading to the removal of Mg by hydrothermal processes and a release of Ca. Inflated mid-ocean ridges will also lead to higher sea levels and higher CO2 in the atmosphere (Tolstoy, 2015). Although, experimental and fossil record examples show that occasionally the Mg/Ca seawater ratio may indeed have direct influence on coral skeletal mineralogy (Higuchi et al., 2014; Stolarski, Meibom, Przeniosło, & Mazur, 2007; Webb & Sorauf, 2002), the main body of evidence suggests a remarkable evolutionary stability of coral skeletal mineralogy despite the major Mg/Ca fluctuations (Figure 3). For example, acroporid corals continued to form aragonitic skeletons from the Paleocene to the present, even as the seawater Mg/Ca increased from ca. 1.5 to 5.2 mol/mol (Stolarski et al., 2016) and aragonite is still detected among calcite in modern Acropora spp. when reared at Mg/Ca = 0.5 mol/mol (Higuchi et al., 2014, 2017; Yuyama & Higuchi, 2019). Furthermore, calcite-promoting conditions in the Cretaceous seas (the lowest Mg/Ca in the Phanerozoic) did not dictate the CaCO3 polymorph change in skeletons of corals that remained aragonitic through their entire fossil record (Janiszewska, Mazur, Escrig, Meibom, & Stolarski, 2017). These observations suggest that the skeletal formation process in corals is strongly physiologically controlled and these organisms are capable of biomineralization using the same mineral in geochemically altered sea conditions.
4.4 Effects of photosynthetic symbionts
Photosynthesis in shallow-water scleractinians is widespread but is neither a necessary nor sufficient process leading to biomineralization. Deep-water Scleractinia calcify, but are totally heterotrophic, while many “soft” corals have photosynthetic symbionts but do not produce an extracellular skeleton. In the shallow-water species that have photosynthetic symbionts, the alga is an intracellular dinoflagellate, formerly of genus Symbiodinium (Berkelmans & van Oppen, 2006; Blank & Trench, 1985; Schoenberg & Trench, 1980). There are many species within the family Symbiodiniaceae (LaJeunesse et al., 2018), commonly called zooxanthellae. The relationship between reef-building scleractinians and members of Symbiodiniaceae has been extensively described (e.g., Kirk & Weis, 2016; Roth, 2014).
Recent molecular clock recalibration suggests that members of family Symbiodiniaceae diversified during the Jurassic period (LaJeunesse et al., 2018), coincident with the radiation of shallow-water scleractinians (Park et al., 2012; Stanley, 2003). There are several lines of evidence for photosymbiosis of fossil corals, determined from modern symbiotic stony corals and observed in the fossil record. Skeletal growth band spacing is regular in symbiotic corals and irregular in asymbiotic corals (Frankowiak et al., 2016). Additionally, while δ13C of skeletal organic matter is not significantly influenced by symbiotic status, δ15N is significantly depleted (Muscatine et al., 2005; Tornabene, Martindale, Wang, & Schaller, 2017) and skeletal U/Ca exhibits a “clear and systematic decrease” (Inoue et al., 2018) in symbiotic versus nonsymbiotic corals. Differences in δ18O and δ13C have also been used to support the hypothesis of photosymbiosis in fossil corals (Frankowiak et al., 2016).
Photosymbiosis benefits stony corals, and specifically calcification in a variety of ways. The most obvious is the in-house provision of fixed carbon provided by the dinoflagellate to the animal host (Falkowski, Dubinsky, Muscatine, & Porter, 1984; Muscatine, McCloskey, & Marian, 1981). While there is recent evidence that the animals themselves may respond to light of specific wavelengths with increased calcification rates (Cohen, Dubinsky, & Erez, 2016), historically the increase in calcification rate by symbiotic corals during the day, termed “light-enhanced calcification” is attributed to photosynthesis and subsequent translocation of photosynthate to the coral host (Chalker & Taylor, 1975; Goreau, 1959; Goreau & Goreau, 1959; Kawaguti & Sakumoto, 1948), with higher rates of calcification observed in some coral species attributed to the assimilation of the fixed carbon by the animal (Gattuso, Allemand, & Frankignoulle, 1999; Moya et al., 2006) or by increased O2 production (Chalker & Taylor, 1975; Galli & Solidoro, 2018; Rinkevich & Loya, 1984). The process is further enhanced by the reflective and refractive properties of the coral skeleton itself, although it may also increase susceptibility to “bleaching,” a phenomenon that leads to the expulsion of alga from the host animal (Enríquez, Méndez, Hoegh-Guldberg, & Iglesias-Prieto, 2017; Swain et al., 2018).
4.5 Skeleton micromorphology
The relatively simple anatomy of scleractinian corals facilitates the study of early stages of coral biomineralization by analyzing the skeletal surface from intact coral branches and sectioning of various skeletal structures (typically perpendicularly to the growth direction; Barnes, 1970; Cuif & Dauphin, 1998; Cuif & Dauphin, 2005b; DeCarlo et al., 2019; Goldberg, 2001b; Hidaka, 1991). Traditionally, based on such observations, a two-step model was proposed that emphasized differences in the time of formation of “centers of calcification” (first step) and the fibers (second step; Cuif et al., 2003). However, studies of longitudinal sections of the skeleton (thus showing ontogenetic succession of skeletal deposition; Stolarski, 2003) and combined Nano-SIMS and SEM observations of isotopically labeled skeleton (86Sr) reveal that the skeleton is formed continuously although skeletal growth dynamics are different in various regions (Brahmi, Domart-Coulon, et al., 2012; Domart-Coulon et al., 2014; Houlbreque et al., 2009). The distal-most regions of septa, columella, and thecae form significantly faster than lateral skeletal layers (Brahmi, Kopp, Domart-Coulon, Stolarski, & Meibom, 2012). The fast-deposited regions are called rapid accretion deposits (RADs), whereas slower growing regions are termed thickening deposits (TDs).
Initially, the mineral deposits at the growing edges of skeletal structures were characterized as “randomly oriented fusiform crystals” (Gladfelter, 1982; Hidaka, 1991) “patches of microcrystals” (Stolarski, 2003), “tiny isodiametric crystals” (Cuif & Dauphin, 2005a) or “granular nanocrystals” (Clode & Marshall, 2003b; Figure 8). The use of scanning helium ion microscopy (SHIM), which bridges the gap between SEM and transmission electron microscopy in terms of spatial resolution, provides ultrahigh-resolution three-dimensional images of RADs (Figure 8o; Von Euw et al., 2017) These are in the form of randomly arranged, spherical, nano-sized CaCO3 particles ca. 100 nm in diameter, which are characteristics typical of ACC nanoparticles as observed in in vitro experiments (Gal et al., 2014). Recently, a more complete picture of the earliest phase of biomineralization has been revealed using X-ray photoemission electron spectromicroscopy (X-PEEM). ACC has been detected in very new skeletal growth—minutes to hours old—of recently terminated S. pistillata; the newest material is hydrated ACC that dehydrates and then crystalizes as aragonite (Mass, Giuffre, et al., 2017), similar to noncrystalline units identified in sea urchin embryonic spicules (Politi et al., 2008). Similar ACC phases have also been detected within “centers of calcification” (COCs, sometimes alternatively called RADs) in the skeleton of Madracis sp. (DeVol et al., 2015) using X-PEEM. Furthermore, Mg-ACC (Akiva et al., 2018) and Mg-calcite (Neder et al., 2019) were detected in the initial mineral deposits of primary polyps of pocilloporid corals, which may explain the rapid production of skeleton during initial development and settlement.
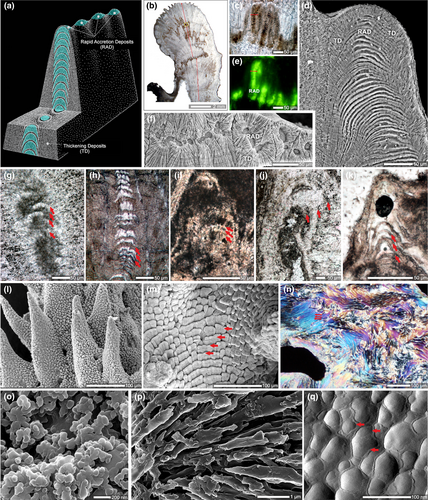
Together, SHIM (Figure 4o) and X-PEEM (Mass, Giuffre, et al., 2017) observations provide strong evidence that ACC nanoparticles are initially deposited and then transform into aragonite crystals. Such a pathway to crystallization during which an amorphous solid phase is initially deposited seems to be a widespread strategy in the formation of biogenic crystals, including phosphates (Beniash, Metzler, Lam, & Gilbert, 2009; Mahamid, Sharir, Addadi, & Weiner, 2008), oxalates (Evan et al., 2007; Ihli et al., 2015; Nakata, 2003), and carbonates (Beniash, Aizenberg, Addadi, & Weiner, 1997; DeVol et al., 2015; Griesshaber et al., 2009; Politi, Arad, Klein, Weiner, & Addadi, 2004; Politi et al., 2008; Weiss, Tuross, Addadi, & Weiner, 2002), and is likely achieved through Mg/Ca ratio modifications as well as inclusion of highly acidic proteins in the calcifying milieu (Evans, Webb, Penkman, Kröger, & Allison, 2019).
Although stable ACC solid phases have been observed in vivo (Akiva-Tal et al., 2011; Raz, Testeniere, Hecker, Weiner, & Luquet, 2002), more frequently occurring metastable ACC solid phases, or “transient amorphous precursor phases,” are replaced by more stable phases (i.e., commonly, their crystalline counterparts such as aragonite and calcite) at a later stage of the biomineralization process (Addadi, Raz, & Weiner, 2003; Radha, Forbes, Killian, Gilbert, & Navrotsky, 2010). In coral, a close look at the RAD (or COC) in direct comparison to TD (sometimes referred to as fibers) regions in skeletal cross sections sheds light on the crystal growth process (Benzerara et al., 2011; Cuif & Dauphin, 2005b; Stolarski, 2003; van de Locht et al., 2013; Von Euw et al., 2017). In RAD skeletal regions, fast skeletal deposition incorporates a relatively high amount of organic matrix (Cuif & Dauphin, 2005b; Mass et al., 2014; Stolarski, 2003); therefore, the nano-sized spheres are easily distinguished well after initial deposition (even in well-preserved fossil corals; Stolarski & Mazur, 2005), and are not randomly arranged but are instead crystallographically aligned (Benzerara et al., 2011). In contrast, the TD regions consist of elongated fibers and have a much more compact structure which is an outcome of a significantly smaller amount of organics involved in their formation. Consequently, transformation of ACC to a crystalline phase occurs in a much more discreet way. Nonetheless, because the formation of RAD and TDs occurs via an amorphous precursor phase, even fully crystalline aragonite TDs, the principal “building blocks” of the coral skeleton, show a highly textured surface (Figure 8). They are clearly distinct from the classical image of an inorganic crystal exhibiting a smooth, faceted surface derived from different crystallographic planes (Figure 8). Such a nanoparticulate texture has been observed across biomineralizing taxa (Gal et al., 2014; Gal, Weiner, & Addadi, 2015) and is associated with crystallization by particle attachment (De Yoreo et al., 2015), particularly in invertebrates such as at the surface of mollusk nacre aragonite crystals (Dauphin, 2001; DeVol et al., 2015; Macías-Sánchez, Willinger, Pina, & Checa, 2017).
Combining all lines of evidence (Brahmi, Domart-Coulon, et al., 2012; Brahmi, Kopp, et al., 2012; Domart-Coulon et al., 2014; Mass, Giuffre, et al., 2017; Neder et al., 2019; Stolarski, 2003; Stolarski et al., 2016; Von Euw et al., 2017), the coral skeleton deposition can be described as follows: (a) a transient amorphous precursor phase in the form of ACC nanoparticles is initially deposited in actively formed skeletal regions (in RADs, the individual nanograins are embedded in significantly higher amounts of organic matrix than in slower growing TD regions); (b) the ACC nanoparticles subsequently attach to one another so that (c) nascent aragonite crystals start to grow by the accretion of amorphous nanoparticles; and this process progressively results in the formation of (d) acicular aragonite crystals following the crystallization of their ACC nanoparticulate building blocks. The full process by which ions are brought to the ECM by the paracellular pathway remains to be settled; this is an exciting resolution that we look forward to in the coming years.
5 CONCLUSIONS
This overview of the evolutionary history of stony corals and their biomineralization process clearly shows that, although these organisms have experienced several major mass extinction events over geological time, reef-forming corals have continued to survive these events, as well as periods, such as the PETM, when atmospheric CO2 increased rapidly and markedly and was accompanied by a decrease in ocean pH. The biomineralization process in corals is highly regulated by the host animal cells at a molecular level. While we do not understand the entire process in detail, much has been elucidated over the past century or more. It is clear, for example, that coral biomineralization is not simply a function of the aragonite saturation state. Over geological time, natural selection facilitated the adaptation to changing ocean conditions, such that, although coral species inevitably change, the group as a whole has survived. While coral cover is likely to decline further in the coming centuries due directly or indirectly to human activities (Hoegh-Guldberg et al., 2019), that corals, in some form, have survived multiple traumas over the past 540 million years, strongly suggests that they will almost certainly survive our disruption of this planet.
ACKNOWLEDGEMENTS
JLD was supported by a National Science Foundation (United States) (Award #1611943). PGF acknowledges the National Science Foundation (United States) (Award #EF-1416785). TM acknowledges the Israel Science Foundation (Grant 312/15) and the European Research Council (ERC; Grant #755876). JS acknowledges the National Science Centre (Poland) (Grant #2017/25/B/ST10/002221). SVE was supported by the European Commission under the Marie Sklodowska-Curie grant (Agreement #793861).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.




