Limitations of cross- and multigenerational plasticity for marine invertebrates faced with global climate change
Abstract
Although cross generation (CGP) and multigenerational (MGP) plasticity have been identified as mechanisms of acclimation to global change, the weight of evidence indicates that parental conditioning over generations is not a panacea to rescue stress sensitivity in offspring. For many species, there were no benefits of parental conditioning. Even when improved performance was observed, this waned over time within a generation or across generations and fitness declined. CGP and MGP studies identified resilient species with stress tolerant genotypes in wild populations and selected family lines. Several bivalves possess favourable stress tolerance and phenotypically plastic traits potentially associated with genetic adaptation to life in habitats where they routinely experience temperature and/or acidification stress. These traits will be important to help ‘climate proof’ shellfish ventures. Species that are naturally stress tolerant and those that naturally experience a broad range of environmental conditions are good candidates to provide insights into the physiological and molecular mechanisms involved in CGP and MGP. It is challenging to conduct ecologically relevant global change experiments over the long times commensurate with the pace of changing climate. As a result, many studies present stressors in a shock-type exposure at rates much faster than projected scenarios. With more gradual stressor introduction over longer experimental durations and in context with conditions species are currently acclimatized and/or adapted to, the outcomes for sensitive species might differ. We highlight the importance to understand primordial germ cell development and the timing of gametogenesis with respect to stressor exposure. Although multigenerational exposure to global change stressors currently appears limited as a universal tool to rescue species in the face of changing climate, natural proxies of future conditions (upwelling zones, CO2 vents, naturally warm habitats) show that phenotypic adjustment and/or beneficial genetic selection is possible for some species, indicating complex plasticity–adaptation interactions.
1 INTRODUCTION
Anthropogenic carbon dioxide (CO2)-induced climate change is altering marine ecosystems at a rate unprecedented on geological timescales (Zeebe, Ridgwell, & Zachos, 2016). Faced with this rapid change and associated stressors, it is not known if marine species will have the capacity to adjust in the short term through phenotypic plasticity or adapt in the longer term. Global warming is increasing seawater temperature (3°C warming projected for the 21st century) and the frequency of heat waves (Babcock et al., 2019; Gattuso et al., 2015; IPCC, 2014). In parallel, CO2-uptake by the ocean is reducing seawater pH (by 0.4 pH units in the 21st century), decreasing the availability of the calcium carbonate (CaCO3) minerals needed for calcification and increasing organism hypercapnia (Gattuso et al., 2015; IPCC, 2014).
While we have a good understanding of the impact of individual climate change stressors on single life history stages, as detailed in many reviews (Byrne, 2010, 2011, 2012; Byrne & Fitzer, 2019; Byrne, Lamare, Winter, Dworjanyn, & Uthicke, 2013; Byrne, Ross, Dworjanyn, & Parker, 2017; Foo & Byrne, 2017; Gazeau et al., 2013; Kroeker et al., 2013; Przeslawski, Byrne, & Mellin, 2015), most marine species have complex bentho-pelagic life cycles (Figure 1). Each life stage experiences different environmental conditions and has different physiological requirements, with the environment of the dispersive stage the least understood (Chan, Sewell, & Byrne, 2018). We need to understand the potential for environmental legacies across different stages of the life cycle and cross-generational impacts of global change stressors. Beneficial effects from parents to offspring may provide a temporal (phenotypic) buffer to facilitate evolutionary (genetic) adaptation to changing climate in the longer term (Foo & Byrne, 2016; Kelly, Padilla Gamiño, & Hofmann, 2013; Munday, Warner, Monro, Pandolfi, & Marshall, 2013; Ross, Parker, & Byrne, 2016; Sunday et al., 2014). Several studies show the benefits of parental conditioning to global change stressors for subsequent generations (e.g. Parker, O'Connor, Raftos, Pörtner, & Ross, 2015), but negative effects can accumulate (Borges, Figueiredo, Sampaio, Rosa, & Grilo, 2018; Gibbin, Chakravarti, et al., 2017; Lopes et al., 2019).
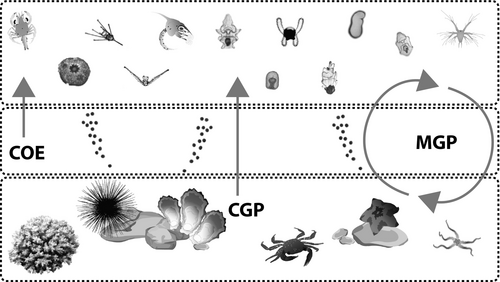
A legacy of environmentally induced effects between parent and offspring (Figure 1) is common in nature. For instance, it is well known that the temperature at which gametes develop influences the optimal temperature for embryonic and larval development, changing offspring phenotype (Andronikov, 1975; Byrne, 2010, 2011). Parental thermal history often determines the thermal tolerance of progeny, thereby enhancing their performance under thermal stress (Andronikov, 1975; Byrne, Selvakumaraswamy, Ho, Woolsey, & Nguyen, 2011; Chirgwin, Marshall, Sgrò, & Monro, 2018; Fujisawa, 1995). When linked to maternal effects, this type of phenotypic plasticity can be associated with loading of protective factors (e.g. metallothionines, chaperone proteins) in the eggs (Hamdoun & Epel, 2007; Lister, Lamare, & Burritt, 2015; Meistertzheim, Lejart, Le Goïc, & Thébault, 2009) or epigenetic effects (Eirin-Lopez & Putnam, 2019). The impacts of projected global change conditions across life stages and generations have been a focus of many recent studies of marine species (Table 1).
| Species |
Conditions tested pH/pCO2 µatm |
Method | Results | References |
|---|---|---|---|---|
| Cnidaria | ||||
| Pocillopora acuta | pHNBS 7.7/940, 7.96/482 | CG | +ve, ↑ survival, ↑ settlement, ↑ growth settled spat | Putnam et al. (2019) |
| Pocillopora damicornis |
pHNBS 7.8/805, 26.5°C 8.0/417, 9°C |
CG |
−ve, smaller larvae +ve, higher size-normalized metabolic rate |
Putnam and Gates (2015) |
| Stylophora pistillata | pHT 7.7, 8.15; CT, +5°C | CG | Neutral, weak to no carry-over effects | Bellworthy, Spangenberg, et al. (2019) |
| Annelida | ||||
| Hydroides elegans | pHNBS 7.8, 8.0 | CG | Growth ↑ if OA father, ↓ if OA mother ND if both parents from OA | Lane et al. (2015) |
| Ophryotrocha labronica | pHT 7.6, 8.0; Mean summer T, +3°C | CG |
W, −ve, ↓ hatching success; ↓ fecundity, ↓ egg volume A, −ve ↓ juvenile dev, ↓ egg volume OWA, no effect |
Chakravarti et al. (2018) |
| Ophryotrocha labronica | pHT 7.6, 8.0; Mean summer T, +3°C | MG, F0–F6 |
Warming +ve F3–F6 ↑, juvenile dev. rate; −ve, F3–F6 ↓ fecundity; F5–F6 ↓ size; F5 ↑, ROS; F2, F6 ↑ mito density, flux in mito capacity: F3,F4 ↓, F6 ↑ Acidification −ve F2 ↓ egg volume; F3 ↓ juvenile development, +ve F2 ↑ juvenile development; F5 ↑ juvenile development, warming + acidification: Flux in mito capacity: F3, F4 ↓, F6 ↑ |
F0–F2: Chakravarti et al. (2018); F3-F6: Gibbin, N'Siala, et al. (2017) |
| Ophryotrocha labronica |
pHNBS (variable history ~30 generations at 7.4–8.4) Experimental (F0–F6) 7.7/1,137; 8.0/460 |
MG, F0–F7 |
pH 7.7, +ve F1, F2 ↑ fecundity; F3–F7 no difference in fecundity; No difference in juvenile growth, devp; and survival or adult size |
Rodríguez-Romero, Jarrold, Massamba-N'Siala, Spicer, and Calosi (2016) |
| Ophryotrocha labronica | Mean summer T, +3°C |
WG (egg mass) MG, F0–F2 |
WG −ve ↓ egg volume, ↓ fecundity, ↓ hatching success, ↓ life span, +ve, ↑ juvenile growth; +ve F2 fecundity and lifespan restored, ↓ egg volume ↑ juvenile growth |
Jarrold et al. (2019) |
| Spirobranchus triqueter | pHT 7.6/1,490, 7.8/790, 8.1/380 | CG |
−ve pH 7.6 ↓ larval size, ↓ larval survival, −ve pH 7.6. 7.8, ↓ tube size No effect on settlement |
Díaz-Castañeda et al. (2019) |
| Mollusca | ||||
| Bivalvia | ||||
| Argopecten irradians | pHT 7.4/2,500, 7.9/600 | CG |
−ve ↓ larval size, ↓ larval survival, +ve ↑ metamorphosis No effect on egg size |
Griffith and Gobler (2008) |
| Mercenaria mercenaria | pHT 7.4/2,500, 7.9/600 | CG |
−ve ↓ larval size, ↓ larval survival, ↓ metamorphosis No effect on egg size |
Griffith and Gobler (2008) |
| Musculista senhousia | pH* 7.7, 8.1 | CG |
+ve ↑ egg size, ↑ dev rate, ↑ larval survival, ↑ metamorphosis; ↑ juvenile survival, shell growth effects ameliorated, juveniles metabolic plasticity—enhanced energy −ve ↑ spawning failure |
Zhao et al. (2019) |
| Mytilus chilensis |
500, 1,200 CT, −4°C |
CG |
+ve, larger larvae when progeny of acidification conditioned parents reared at ambient and warmer conditions No effect on egg size |
Diaz et al. (2018) |
| Mytilus edulus | 380–1,000 µatm | CG | +ve, biomineral switch from aragonite to calcite in juvenile shell | Fitzer et al. (2018) |
| Mytilus edulus | pHT 7.46/2,400, 7.75/1,120, 8.2/390 |
CG MG F1–F2 |
WG, −ve, ↓ larval size, −ve, ↑ survival at pH 7.46 F2 +ve, larger initial larval size, F2 −ve, ↓ larval growth rates at pH 7.46 Survival not improved No effect on egg size |
Thomsen et al. (2017) |
| Mytilus edulus | pHNBS 7.3/2,400, 7.7/1,100, 8.1/350 | CG | −ve pH 7.6, 7.3 ↑ embryo abnormality, larval size | Kong et al. (2019) |
| Ostrea lurida | pH* 7.3/3,045, 7.8/841 | CG (F0–F1) | +ve ↑ survival of outplanted juveniles (12 months at ambient), but magnitude differed between source and field site | Spencer et al. (2019) |
| Saccostrea glomerata | pHNBS pH 7.9/856, 8.2/380 | CG (F0–F1) |
Wild type: −ve ↓ survival, longer development time, +ve ↑ larval size Selected line: +ve, ↑ larval size, shorter dev time, similar survival |
Parker et al. (2012) |
| Saccostrea glomerata | pHNBS 8.2/380, pH 7.9/856 |
MG F1–F2 fieldb |
F1 from Parker et al., (2012) maturation in field (14 months at ambient) F2, +ve, ↓ abnormality, ↑ larval size, shorter dev time, ↑ juvenile, ↑ heart rate, similar survival F1: +ve, better regulation of acid–base homoeostasis as adults |
Parker, O'Connor, Raftos, et al. (2015) |
| Ruditapes philippinarum | pH* 7.7/1,000; 8.0/400 | CG | +ve, ↑ juvenile growth, ↑ adult shell growth; Juvenile—less costly ion regulation, adult, less energetically source of carbon, mitigation of effects on condition and metabolic rate | Zhao et al., (2017); Zhao, Zhang, et al. (2018) |
| Arthropoda | ||||
| Copepoda | ||||
| Acartia bifilosa a |
350–1,230 CT, +3°C |
CG |
A, −ve, ↓ hatching success & development rate W, +ve ↑ nauplii production W – increased egg production |
Vehmaa, Brutemark, and Engström-Öst (2012), Vehmaa et al. (2016) |
| Acartia erythracea | pHNBS 6.84/10,000, 7.4/2,000, 8.14 | CG |
−ve, ↑ mortality ↓ egg production at pH 6.84 |
Kurihara, Shimode, and Shirayama (2004) |
| Acartia tonsa | pHNBS 7.15/6,000, 7.4/3,000, 7.6/2,000, 7.8/1,000, 8.2/385 | CG | −ve, pH 7.1–7.6 ↓ hatching success ↑ larval mortality | Cripps et al. (2015) |
| Acartia tonsa | 200, 800 | MG, ~F0–F80, selected lines |
−ve, ↓ development rate, early hatching; fecundity and hatching success not affected Copepods in low pH fed algae from the same condition were smaller, but had a bigger body mass Beneficial effects not detected |
Langer et al. (2019) |
| Acartia tsuensis | pHNBS 7.3/2,830 | MG, F0–F1 | No effect of pH on fecundity and hatching success | Kurihara and Ishimatsu (2008) |
| Calanus finmarchicus | pH T 7.15/3,500,7.3/2,080, 7.6/1,080,8.0/430 | MG, F0–F2 |
+ve F1, pH7.3, amelioration of negative effect on development rate, −ve, F1, pH7.15, ↓ development rate, ↓weight, ↑ respiration F1−F2, no effect on fecundity and hatching |
Pedersen et al. (2014) |
| Calanus finmarchicus | pHNBS 6.95/8,000, 8.23 | CG |
−ve, ↓hatching No effect on egg production |
Mayor et al. (2007) |
| Calanus glacialis | pHNBS 6.9, 7.6, 8.2 | CG |
−ve, ↓ hatching at pH 6.9 No effect on egg production |
Weydmann, Søreide, Kwasniewski, and Widdicombe (2012) |
| Centropages typicus | pHNBS 6.7/9,830, 7.78/750, 7.85/620, 7.97/480, 8.04/380 | CG | No effect except at pH 6.7 ↓ egg production, ↓hatching | McConville et al. (2013) |
| Pseudocalalanus acuspes | pHT 7.54/1,550, 7.75/900.8.05/400 | MG, F0–F2 |
−ve, F2. ↓ fecundity (pH 7.54 & 7.75), ↓ hatching; ↑ respiration, ↓ gene expression +ve F2, negative effects of pH 7.54 on fecundity partially (50%) alleviated, metabolic stress alleviated |
De Wit et al. (2016), Thor and Dupont (2015) |
| Temora longicornis | pHNBS 6.7/9,830, 7.78/750, 7.85/620, 7.97/480, 8.04/380 | CG | No effect of pH on hatching | McConville et al. (2013) |
| Tisbe battagliai | pH* 7.67/550−650, 7.8/400−477, 7.95/280−310, 8.06/200−250 | MG, F0–F3 | −ve F3↓ copepodite size, ↓ nauplii production at pH 7.67, change to exoskeleton—↓ growth—reallocation of resources to maintain reproduction | Fitzer et al. (2019) |
| Tigriopus californicus | CT + 9°C | MG, F0–F10 | −ve little capacity to adapt to increased temperature | Kelly, Sanford, and Grosberg (2012) |
| Tigriopus japonicus | pH* 5.85/110,000, 6.3/3,700, 7.1/5,800, 8.16/430 | CG | −ve pH 6.3 ↓ hatching; pH 5.85↑ mortality | Kita et al. (2013) |
| Tigriopus japonicus | pH* 7.6/1,000, 8.0/400 | MG, F0–F3 | No effect of pH on embryo survival, development rate, number of larvae or fecundity | Li, Wang, and Wang (2017) |
| Amphipoda | ||||
| Gammarus locusta | pHT 7.7/820 8.0/400 | MG, F0–F1 | −ve ↓ survival, mate guarding duration, increase in egg number at pH 7.7 | Borges and Rando (2018) |
| Gammarus locusta |
A pH* 7.6/1,600, 8.1/400 CT, +4°C |
CG |
A −ve ↓ released embryos W −ve ↓ released embryos, +ve, ↑ development |
Cardoso et al. (2016) |
| Cirripedia | ||||
| Amphibalanus amphitrite | CT, +2–8°C | MG, F0–F2 | −ve +6–8°C ↓ larval survival | Morley, Nguyen, Peck, Lai, and Tan (2017) |
| Decapoda | ||||
| Chionoecetes bairdi | pHT 7.5/1,600, 7.8/800, 8.16/326 | CG |
−ve, pHT 7.5 ↓ larval size; ↓calcium content, ↑ mortality +ve, ↑ survival time |
Long et al. (2016) |
| Echinodermata | ||||
| Echinometra mathaei | pHT 7.65, 8.1, | CG |
−ve decreased male spawning No effect of adult conditioning, negative effects of acidification on larval development not ameliorated No effect on egg size |
Uthicke et al. (2013) |
| Heliocidaris erythrogramma |
A pHT 7.6, 80, W CT, +2–6°C, |
CG |
−ve ↑ juvenile metabolism +6°C, no effect of pH on metabolism No effect of pH or temperature on juvenile size |
Harianto (2019) |
| Psammechinus miliaris |
Adult—pHNBS 7.7/1,000, 8.0/560 Control parent offspring reared at pH 7.7 and 8.0 Acidification parent offspring only reared at pH 7.7 |
CG |
28 days −ve, ↓ larval survival, ↑ settlement 42 days, −ve, ↓ larval survival, +ve, ↑ larval size, ↑ settlement 70 days −ve, ↓ egg diameter, ↓ larval survival, +ve, ↑ larval size, ↑ settlement |
Suckling et al. (2014) |
| Sterechinus neumayeri |
Adult—pHNBS C—8.0/392, −0.3°C W—8.0/437, +1.5°C AW—7.7/925, +1.8°C AW—7.5/1,405, +2.1°C Offspring—pHNBS 8.0/560 |
CG |
−ve 6 months ↓ egg size, ↓ hatching, ↓ larval survival in AW, no difference larval size between control and 7.7/+1.8°C +ve 17 largest eggs in 7.5/+2.1°C, highest larval survival in W, ↑ abnormal development in 7.5, +2.1°C No difference hatching or larval size |
Suckling et al. (2015) |
| Strongylocentrotus droebachiensis | pHNBS 7.7/1,217, 8.1/400 |
CG 4, 16 months |
−ve 4 months, ↓ fecundity, ↓ larval, ↑ larval mortality, ↓ settlement, ↓ juvenile survival. 16 months, no differences in fecundity, larval survival, no effect on egg size (4 or 16 months) |
Dupont et al. (2019) |
| Strongylocentrotus intermedius | Control +3°C | CG | −ve ↓ hatching and larval size | Zhao, Zhang, et al. (2018) |
| Strongylocentrotus purpuratus |
pHT C—7.87/651, 17°C C—8.0/446, 15°C A—7.66/1,050, 15°C UW—7.57/1,330, 13°C |
CG | No effect on egg size, no effect on pluteus larval size, eggs had slightly more phospholipids from 7.87/17°C + ve ↑ prism size from 7.57/13°C | Wong et al. (2019) |
| Tripneustes gratilla |
pHT 7.7, 8.0 CT, +2.0°C |
CG |
−ve ↓ larval size A, W and AW, but −ve effects were decreased. −ve ↓ egg size in W |
Karelitz et al. (2019) |
Note
- If more than one parental conditioning period was used, the data are presented per time. F, generation, pH levels are listed as published on the total (pHT) or NBS (pHNBS) scales or no scale (pH*) if not indicated, pCO2 levels (µatm) indicated next to pH. Comments are added for clarification.
- Abbreviations: +ve, positive; −ve, negative, A, acidification; AW, acidification + warming; C, control; dev, development; mito, mitochondria; ROS, reactive oxygen species; T, temperature; UW, upwelling conditions; W, warming.
- a Mesocosm study.
- b F1 juveniles translocated to field common garden for growth to adult.
2 CROSS GENERATION GLOBAL CHANGE STUDIES OF MARINE INVERTEBRATES
In this review, we focus on the impacts of exposure of adult marine invertebrates to warming and/or acidification with respect to outcomes for progeny, and where data are available, for subsequent generations. Our primary interest is to assess the capacity for phenotypic change in response to climate stressor history in consideration of the complex biphasic life cycles in marine invertebrates (Figure 1). We distinguish between adult conditioning involving one generation of germ cells (F0–F1) as cross generation plasticity (CGP), compared to multigenerational plasticity (MGP, F1–F2+), where more than one cycle of germ line development is involved. Given the connection between life stages, the term transgenerational plasticity (TGP) has been used in the literature for parental to offspring linkages. However, TGP has been used in a broad and confusing way in applications from carry-over effects (COE), to CGP and MGP (Donelson, Salinas, Munday, & Shama, 2018; Karelitz et al., 2019; Torda et al., 2017; Zhao, Zhang, et al., 2018). In light of this confusion and the need to clearly define exposure with regard to germ line development and gametogenesis (see Donelson et al., 2018), we define and use the term MGP here to make our context clear. While we advocate for MGP as being the clearer term, we consider that either MGP or TGP may be acceptable with the use of clear definitions with respect to germ line exposure (Box 1).
BOX 1. Critical considerations for mechanisms of cross- and multigenerational nongenetic inheritance
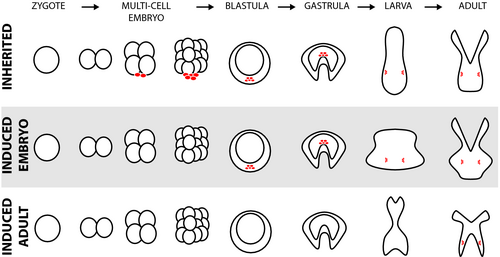
The processes of CGP and MGP require inheritance mechanisms beyond that of DNA. These mechanisms include direct parental provisioning (e.g. macromolecules and microbiome, Hamdoun & Epel, 2007), as well as epigenetic mechanisms (e.g. DNA methylation, miRNAs, chromatin modifications; Eirin-Lopez & Putnam, 2019), or some combination of these. Most CGP and MGP studies in marine invertebrates have focused on phenotype, while fewer have investigated mechanism. In particular, few studies have specifically designed experiments with respect to the development of the germ line (eggs and sperm cell lineages), with the exception of examples that exposed for the entirety of germ line development and gametogenesis (e.g. Gibbin, Chakravarti, et al., 2017; Karelitz et al., 2019). Thus, many studies cannot disentangle CGP from COE Donelson et al. (2018). Organisms differ in the timing and mode of primordial germ cell (PGC) generation (Box Figure 1; Extavour & Akam, 2003; Wessel et al., 2014). Thus, blanket statements about specific numbers of generations necessary for CGP and MGP, are not likely to apply to all taxa.
Primordial germ cells
Primordial germ cells are set aside to provide a protected germ line that becomes the next generation. PGC development can occur through early specification by maternally inherited determinants (i.e. preformation). Alternatively, continuous germ cell specification through an inductive mechanism is common in diverse taxa (Extavour & Akam, 2003). PGC specification through preformation occurs when germ cells are sequestered into cytoplasmic regions known as the germ plasm. Here, specific components (e.g. RNAs and proteins such as Vasa, Nanos and Tudor) of the germ plasm target the cells and initiate specification and often quiescence (Extavour & Akam, 2003). Conversely, inductive PGC development can occur in early life and even through the adult stage (Oulhen & Wessel, 2017) and is driven by cell–cell signalling (Extavour & Akam, 2003).
Once generated, PGCs can be protected by migration and sequestration. Typically, this sequestration occurs through physical location in a macromolecular PGC ‘niche’ (Cantú, Altshuler-Keylin, & Laird, 2016) and by arrival to the gonad. Physical sequestration is reinforced by molecular sequestration, through for example, quiescence obtained by targeting of transcription and translation, and reduction of metabolism and cell division (Oulhen & Wessel, 2017). For example, in sea urchins, small micromeres contain the germ line determinants and are transcriptionally silenced until they are physically sequestered to avoid being perturbed by somatic differentiation signals (Wessel et al., 2014). While these PGCs are quiescent, it is unlikely they are open to environmental influence. Furthermore, in the specification of PGC via induction, epigenetic reprogramming of somatic cells occurs, ‘wiping the slate’ through extensive modifications in histone and DNA methylation (Kota & Feil, 2010). Knowledge of the timing of PGC specification, type of sequestration and duration of quiescent state is essential for experimental design to test CGP and MGP.
Multigenerational epigenetic inheritance
Understanding of the mechanism(s) of nongenetic epigenetic inheritance is an ultimate endeavour of cross- and multigenerational research. In light of rapid climate change and threats for organism persistence, there is great interest in the potential of epimutations to contribute to nongenetic inheritance (Eirin-Lopez & Putnam, 2019) and provide a buffer against climate change through phenotypic plasticity (Torda et al., 2017). It is thought that for epigenetic marks to be transmitted to offspring that these modifications must be present in the germ line. In this line of thought, if a sequestered or quiescent germ line is not present, or if resetting occurs to clear marks, this would preclude the transfer of environmental ‘memory’ across generations. In a challenge to this thinking, nongenetic inheritance may also occur through parental provisioning providing the capacity to recapitulate epigenetic marks that have been erased. For example, de novo DNA methyltransferase, DNMT3, may be triggered or regulated by other macromolecule or molecular information to lay down marks associated with environmental history through a multiple information carrier means (e.g. maternal mRNA, small noncoding RNAs; Boskovic & Rando, 2018). Further work on the maternal RNA complement, the mRNA expression patterns of the maternal–zygotic transition, active epigenetic machinery and multiple epigenetic information carrier interactions are therefore critical.
Cross- and multigenerational plasticity in the climate change context have been investigated for species ranging from polychaetes to molluscs and echinoderms (Table 1). This research, spurred by interest in the potential for climate adaptation, is a burgeoning field. We provide a synthesis of the current understanding of the potential of marine species to produce a more favourable phenotype in response to climate change stressors by processes outside of selection, although multigeneration reciprocal transfer experiments have explored the potential that selection is involved (e.g. Gibbin, Chakravarti, et al., 2017; Gibbin, N'Siala, Chakravarti, Jarrold, & Calosi, 2017). Where enough data for phenotypic traits within groups were available, meta-analyses were conducted (See Supporting Information for methods) to test the hypothesis that the effects of parental conditioning are beneficial. Short generation life cycle (days, weeks) crustacean and polychaete species have been used as model groups to investigate the potential for evolutionary adaptation and acclimation over multiple generations (Table 1). For longer generation species (e.g. corals, Antarctic invertebrates), the adult conditioning, CGP approach has been used (Table 1).
While this growing body of literature showcases a role for CGP and MGP, it is also important to appreciate the habitat of study species, some of which are naturally stress tolerant as they experience acidification and temperature fluctuations (e.g. intertidal, upwelling zones) and others that occur over a broad range of conditions (e.g. large-scale latitudinal and depth gradients) and so may be tolerant generalist-type species (Byrne et al., 2011, 2017; Kelly, Padilla-Gamiño, & Hofmann, 2013; Pespeni, Chan, Menge, & Palumbi, 2013; Pespeni, Sanford, et al., 2013). For species that already experience acidification and warming at levels beyond projected for the near future, it is difficult to identify what level of these factors, and in what combination, are actually stressful. Species that live in highly heterogeneous and environmentally variable environments often have a greater capacity for phenotypic plasticity (Chevin & Hoffmann, 2017; Gaitán-Espita et al., 2017; Ghalambor, McKay, Carroll, & Reznick, 2007).
We focus on CGP and MGP in the marine invertebrate groups for which the most data are available (crustaceans, annelids, molluscs, echinoderms) although coral studies are discussed. Life history stage carry-over effects (COE, e.g. embryo to larvae, larva to juvenile; Figure 1) are also important (Pechenik, 2017; Ross et al., 2016) and are considered. We feel that defining the terms of COE, CGP and MGP within our study and in future work is particularly important with respect to understanding the mechanisms driving this plasticity. The variable levels of stressors used (see Table 1, note pH scales used, not stated in some studies) and traits analysed, often make direct comparisons difficult. Acidification studies dominate, with few studies incorporating temperature as a stressor and even fewer multistressor studies. This is a major gap in knowledge that is necessary to address, given the heterogeneity of marine environments and the current growing combination of global and local stressors.
2.1 Crustacea
Crustaceans are considered to be tolerant of acidification due to their ability to physiologically buffer the negative effects of hypercapnia and because their chitinous exoskeleton makes them less vulnerable to decreasing CaCO3 saturation than other calcifying taxa (Wang, Jeong, Lee, & Lee, 2018; Whiteley, 2011). Several multigeneration studies have availed of the short generation time of copepods and amphipods where F1 offspring are readily isolated from brooding females to establish additional generations. These crustaceans have been a focus because they are immensely important in marine ecosystems, often representing the highest biomass and play critical roles in trophic energy transfer.
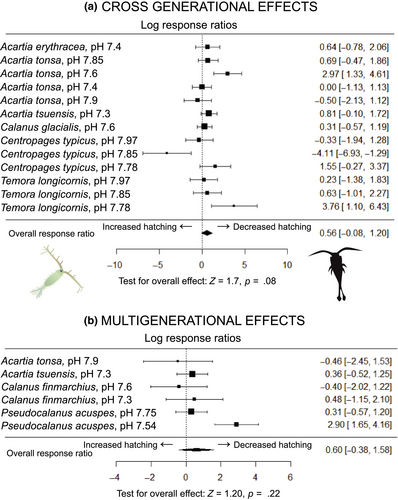
Data from cross- and multigeneration studies are available for 12 copepod species in response to acidification and/or warming (Table 1), allowing some comparisons to be made for the trait most reported on, hatching success (Figure 2). Studies that use extreme pCO2 levels to emulate carbon capture and storage scenarios show the high tolerance of several copepods (pH < 7.1, ~8,000+ µatm pCO2; see Table 1). For the meta-analysis, we only used data pH ≥ 7.3, to be closer to climate change scenarios (Figure 2).
Most studies involve calanoid and acartid copepods (Table 1). Cross generation studies of acidification effects on Acartia bifilosa and Acartia tonsa report decreased hatching success and offspring development at fairly moderate levels of acidification (Table 1). The nauplii were particularly sensitive with higher mortality (Cripps, Lindeque, & Flynn, 2014). However, very low pH (pHNBS 7.3) did not have negative effects on hatching, development rate and offspring size for Acartia tsuensis (Kurihara & Ishimatsu, 2008). The only study that also included exposure of the male parent prior to mating found strong negative cross generation effects on hatching, indicating the importance to consider paternal effects (Cripps, Lindeque, & Flynn, 2015).
In a study of A. tonsa over 80 generations (3.5 years), beneficial phenotypic change in offspring in response to acidification was not observed (Langer et al., 2019). The negative legacy of acidification was seen in the reduced developmental rate and early hatching, but not on hatching success. After 80 generations, copepods reared in 800 µatm pCO2 and fed algae cultured in the same conditions were smaller in length but had a bigger body mass, pointing to food as a stressor offset. Food offsetting of stress effects is seen in acidification and thermal stress studies of marine invertebrates (e.g. Bellworthy, Spangenberg, & Fine, 2019; Thomsen et al., 2010; Uthicke et al., 2016).
For Pseudocalanus acuspes, multigenerational exposure to acidification (at two levels pHT 7.54 and 7.75) resulted in decreased fecundity in the F2 generation and reduced gene expression, including downregulation of RNA transcription genes (De Wit, Dupont &Thor, 2016; Thor & Dupont, 2015). For the F2 generation maintained at pHT 7.54, there was some alleviation (~50%) of the decreased fecundity seen in progeny from ambient conditioned parents. This change was associated with a change in metabolism (Thor & Dupont, 2015).
For Calanus finmarchicus, the effect of acidification on development of the F1 generation was ameliorated at pHT 7.3, but not at pHT 7.15 (Pedersen et al., 2014). The latter pH level was deleterious across generations, elevating metabolism and reducing growth. There was no effect on hatching to the F2 at either pH level. With regard to hatching success, the threshold for deleterious effects of acidification in C. finmarchicus was estimated to be between 2,080 and 3,080 µatm pCO2 (Pedersen et al., 2014). High tolerance to acidification (pHNBS 6.95) in this species (Mayor, Matthews, Cook, Zuur, & Hay, 2007) is suggested to reflect its adaptation to low pH (pH 5.0) during diapause (see De Wit et al., 2016) and vertical migration through pH strata (see below). Hatching of C. glacialis embryos is also resilient to pHNBS 7.6 (Table 1).
There are several studies of acidification across multiple generations of benthic copepods. Tisbe battagliai reared to F3 in acidification conditions exhibited negative effects on larval size and production and changes to the exoskeleton (Fitzer et al., 2012). Reduced growth and skeletal changes were associated with a reallocation of resources to maintain reproduction. For Tigriopus japonicus, pH 7.1 had no effect on hatching in a cross generation study (Kita, Kikkawa, Asai, & Ishimatsu, 2013) or on embryo survival, development and larval production in a multigeneration study (Li, Wang, & Wang, 2017).
For the amphipod Gammarus locusta, exposure to pHT 7.7 enhanced female fecundity, but this was followed by negative effects in the F1 generation with reduced fecundity, reduced survival and altered behaviour (shorter mate guarding time; Borges et al., 2018). Reduced hatching success was observed in a cross generation study of G. locusta at pH 7.6 (Cardoso et al., 2018). A recent study where several stress markers were analysed (e.g. lipid peroxidation, DNA damage) showed strong negative effects in the F1 generation of G. locusta (Lopes et al., 2019). Warming (+4°C) also reduced the number of embryos, but those that did hatch developed faster. In a study of Sunamphitoe parmerong where brooding females were maintained in ambient and +4°C through progeny development and F1 rearing, the warm conditioned line had higher thermal tolerance (Campbell, 2018). A multigeneration study of this species showed that warming (+2–4°C) had no effect on F1 development, but increased F2 brood number with the magnitude of change depending on diet (Ledet, Byrne, & Poore, 2018). Finally, for the commercially important Tanner crab, Chionoecetes bairdi, cross generation exposure to pHT 7.5 had negative effects on the larvae (Long, Swiney, & Foy, 2016).
One multigenerational study that investigated temperature as a single stressor revealed that the high intertidal benthic copepod, Tigriopus californicus, has little capacity to adapt to warming even after 10 generations (Kelly, Sanford, & Grosberg, 2012). Although this appears surprising considering the thermally dynamic habitat of this species, other studies suggest that intertidal invertebrates living in extreme habitats have low acclimatory capacities (Somero, 2010; Stillman, 2003). T. californicus lives in high intertidal pools, a habitat associated with low genetic diversity and this is suggested to contribute to its poor adaptive capacity (Kelly et al., 2012). In a follow-up study where warm and cool adapted populations of T. californicus were cross-bred, heat tolerance was observed in selected lines (to F5), but with a cost of reduced fecundity (Kelly, DeBiasse, Villela, Roberts, & Cecola, 2016).
The importance to consider habitat and vertical migration in interpreting the sensitivities of copepods to acidification is shown in a study of Arctic under ice conditions (to 200 m depth; Lewis, Brown, Edwards, Cooper, & Findlay, 2013). Calanus species are exposed to a wide pH range during their daily migration and were tolerant of low pH (pHT 7.6). In contrast, surface dwelling (non-migratory) copepods were more sensitive. For Arctic copepods, sensitivity to acidification parallels the natural levels experienced. Similarly, along a latitudinal gradient of acidification due to upwelling along the coast of Chile, A. tonsa from lower and more variable pH areas were more tolerant of acidification than conspecifics living at higher pH (Vargas et al., 2017). These studies provide the link between environmental variability and tolerance of copepods to acidification (see also Thor et al., 2017), but the key selective forces associated with this variability (e.g. pH mean, range, minimum, maximum) are not known (see below, Section 5).
For copepods, the weight of evidence from eight cross and six multigeneration studies across 12 species indicates that acidification (≥pH 7.6) has little impact on hatching success (Table 1). Meta-analyses of hatching success revealed that female conditioning did not affect embryo hatching rates. The effect sizes overlapped zero (Figure 2a). In the meta-analyses of hatching data over multiple generations, acidification effects were more variable (Figure 2b). Negative effects were only seen for P. acuspes. Overall, the analyses show no significant effect of acidification indicating that copepods have considerable phenotypic plasticity with regard to pH level for the hatching trait (Figure 2b). For copepods, it will be important to not only quantify the response of the progeny from adults (males and females) conditioned in control treatments but also those from adults conditioned in acidification treatments to execute a fully crossed factorial experimental design to allow a better understanding of the impacts of stressors (see Supporting Information for experimental designs). This would allow a full estimation of whether conditioning reduces the impacts of low pH on copepods.
2.2 Annelida
Cross- and multigeneration studies of the Annelida with respect to climate change have largely involved one polychaete species, Ophryotrocha labronica using populations from the Mediterranean (Table 1). This cosmopolitan invasive species occurs in fouling communities (Simonini, Massamba-N'Siala, Grandi, & Prevedelli, 2009). With its short generation time (17 days), weekly production of egg masses and parental care, this species is a good model for multigeneration studies (Chakravarti et al., 2016).
A laboratory strain of O. labronica maintained for ~30 generations in fluctuating pH (pHNBS 7.4–8.4) and then exposed to unvarying pHNBS 7.7 for a further six generations showed initial (F1, F2) negative effects of acidification (Table 1). By F3–F7, however, fecundity levels were restored and offspring performance improved (Rodríguez-Romero, Jarrold, Massamba-N'Siala, Spicer, & Calosi, 2016). Thus, this strain restored its fitness in an acidification environment through phenotypic plasticity within two generations and maintained this fitness for the additional four generations investigated. There was also no difference in metabolic rate in the F7 generation (acidification vs. control), indicating physiological acclimation and perhaps some genetic selection over the 30 generations in fluctuating pH.
In a study of a different population of O. labronica, cross generation exposure to warming or acidification had negative effects on fecundity, egg volume, hatching success and juvenile development (Chakravarti et al., 2016). Some of these negative effects were ameliorated over subsequent generations in offspring reared in the same conditions (Gibbin, Chakravarti, et al., 2017; Gibbin, N'Siala, et al., 2017). Combined warming and acidification had a dampening effect on negative responses, indicating that they acted as opposing vectors of stress. Warming accelerated development through generations while acidification had a retarding effect. However, while the O. labronica developed faster at higher temperature, they reproduced at a smaller size, resulting in lower fecundity. This indicated a trade-off between reproducing earlier but with a lower output. In a recent study that investigated warming (+3°C) as a single stressor (Jarrold et al., 2019), negative effects observed in the cross-generation part of the study were reversed in the F2 generation. However, as trade-offs were evident in Gibbin, Chakravarti, et al. (2017) and Gibbin, N'Siala, et al. (2017), beyond F2 deleterious effects would be expected.
Over generations, the responses (positive or negative) of O. labronica to acidification in seven traits (adult, developmental, physiological) depended on number of generations. The initial benefits of parental conditioning to increased temperature were lost as warming became broadly deleterious. A consistent change or improvement over generations was not evident (Gibbin, Chakravarti, et al., 2017; Gibbin, N'Siala, et al., 2017). After five generations, reactive oxygen species production (ROS) was double that of controls indicating high stress levels, as the polychaetes coped with warming (+3°C). Most negative effects were attributed to increased temperature (Table 1). While the benefits of parental conditioning were evident in the early generations, this was lost over generations leading to a strong decline in fitness (Gibbin, Chakravarti, et al., 2017; Gibbin, N'Siala, et al., 2017).
We need more studies of polychaetes, especially groups with planktonic larvae such as serpulids that form calcareous tubes that are vulnerable to acidification (Lane, Mukherjee, Chan, & Thiyagarajan, 2013). In a cross-generation study of Spirobranchus triqueter, the larvae generated by adults conditioned at pHT 7.6 and reared in this treatment were smaller and had lower survival than those from ambient parents (Díaz-Castaneda et al., 2019). At pHT 7.6 and 7.8, the tubes of the F1 worms were smaller than those of controls. A study of Hydroides elegans, a locally abundant serpulid, produced an unexpected result showing that juvenile growth and calcification were faster if the acidified conditioned parent was a male, with the opposite result for maternal effects and no effects if both parents were conditioned acidification (Lane, Campanati, Dupont, & Thiyagarajan, 2015). For H. elegans, there were negative COE of acidification from larva to juvenile stages (Lane et al., 2013). Juveniles resulting from larvae reared at pHNBS 7.7 and 7.9 were unable to make their tube. Serpulids are major biofouling species across the globe and the results for H. elegans and S. triqueter indicate that their dominance in fouling communities may change due to ocean acidification (Díaz-Castaneda et al., 2019). Reduction in serpulid density is evident at CO2 vent sites (Kroeker, Micheli, Gambi, & Martz, 2011).
In a cross-generation study of the effects of temperature as a single stressor on the calcareous tube worm Galeolaria caespitosa where some adults were conditioned at cool (~−4°C), and others conditioned at warm (+2°C) temperature, offspring survival was higher when parent and offspring environmental temperatures matched (Chirgwin et al., 2018).
Multigeneration studies as undertaken for O. labronica are rare yet are needed to provide insights into the response of species to changing marine environments. These studies quantified many traits including cellular and physiological health markers (Table 1). The health markers (e.g. ROS) were particularly insightful with regard to how fitness tracked over generations, showing the importance to consider these traits, as well as morphological ones. Studies with O. labronica also incorporated reciprocal transfers between experimental and control environments and showed that some of the trait changes were reversible (indicating acclimation), while others were fixed (indicating adaptation; Gibbin, Chakravarti, et al., 2017; Gibbin, N'Siala, et al., 2017). Due to the mixed outcomes for progeny, these authors suggest that six generations are insufficient to be able to discern between plasticity and adaptive capacity.
2.3 Mollusca
All cross-generation climate change studies of molluscs have involved bivalves, including clams, scallops, mussels and oysters (Table 1). These bivalves have received emphasis due to their ecological and commercial importance. Most studies have involved adult conditioning.
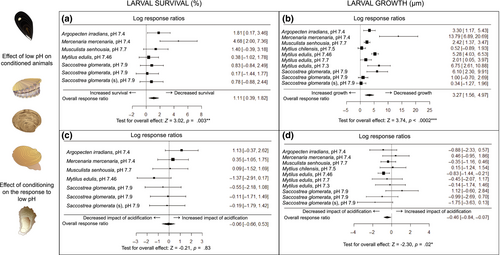
Conditioning of wild type and selected families of the Sydney rock oyster (Saccostrea glomerata) in pHNBS 7.9 during gametogenesis produced beneficial effects for offspring (Parker et al., 2012). The progeny of both oyster types were less sensitive to low pH than the larvae of ambient treatment adults, with the larvae of the selected families being more resilient (Figure 3). The larvae of acidification treatment oysters had a shorter development time and were larger. The F1 generation juveniles of the wild-type oysters were transferred to common garden field ambient conditions until the adult stage and were then similarly conditioned in pHNBS 7.9. In the resulting F2 larvae, the positive effects of the original parental exposure were maintained, showing beneficial MGP (Parker, O'Connor, Raftos, et al., 2015). Moreover, offspring generated from the acidification treatment adults also performed better at ambient pH.
A multistressor study showed that while S. glomerata larvae produced by acidification conditioned adults, were, as expected, resilient to acidification as a single stressor, they were more sensitive when thermal stress, reduced salinity and low food conditions were combined (Parker, O'Connor, Byrne, et al., 2017). Adult conditioning was maladaptive when parental and offspring environments did not match. The authors suggest that this maladaptive response might not have occurred if the parents were also conditioned to the same multistressor environment.
In a recent study of Ostrea lurida, where juveniles generated from adults that were maintained in acidification treatments (pH 7.3) through gametogenesis (60 days) were outplanted at several sites, survival was higher in the acidification treatment progeny (Spencer et al., 2019). The magnitude of this effect depended on the parental source and out planting site.
There are several cross and multigeneration studies of mytilids. For Mytilus edulis, a study comparing the progeny of adults from the naturally low pH environment of the Western Baltic Sea and nearby high pH areas showed that the larvae of Baltic mussels were more tolerant to acidification, an indication of CGP to acidification in nature (Thomsen et al., 2017). In a 2 year, multigenerational experiment, families (individual male–female crosses) from the Baltic Sea mussels were reared in three pH levels (Table 1). One-third of these families were tolerant to low pH (pHT 7.46) and were then reared to maturity in control and acidification conditions to generate the F1, and so on, to the F2. Beneficial effects were evident in the larger initial size of the larvae, their calcification performance and increased survival of the F1 and F2 generation (Figure 3c,d). However, over time, the beneficial effects of parental exposure to acidification with respect to progeny performance were not realized (Thomsen et al., 2017).
Interestingly, the larvae generated by M. edulis conditioned to 1,000 µatm pCO2 over 6 months, and reared in these conditions, metamorphosed into juveniles with an altered shell minerology (Fitzer, Phoenix, Cusack, & Kamenos, 2014). The juveniles produced calcitic rather than aragonitic shells, the latter being more soluble in acidification conditions. If such calcification plasticity is a genetic trait of M. edulis, this may be an important adaptation to resist habitat acidification. For M. chilensis, parental conditioning to acidification resulted in larger larvae when reared at ambient pH and in low pH-warm treatments (Table 1).
For the invasive fouling mussel Musculista senhousia, cross generation parental exposure to acidification (pH 7.7) was broadly beneficial for all larval traits investigated (development rate, survival and metamorphosis; Table 1). These positive effects were linked to the larger eggs spawned by acidification conditioned females (Zhao et al., 2019). The positive effects of parental conditioning for progeny continued to the juvenile stage which had higher survival. The enhanced performance of the juveniles appeared to be due to a change in metabolism. The broad ranging positive effects are suggested to be associated with the high stress tolerance and natural phenotypic plasticity of M. senhousia (Zhao et al., 2019).
For the scallop Argopecten irradians and the clam Mercenaria mercenaria conditioning of adults at low pH (pHT 7.4) did not benefit offspring (Griffith & Gobler, 2017). These adults produced larvae that were equally or more sensitive to low pH than larvae originating from ambient pH-treatment adults (Figure 3c,d). The larvae were smaller, had lower survival and were more vulnerable to other factors (low food, harmful algae).
For the Manila clam, Ruditapes philippinarum parental conditioning (pH 7.7) resulted in faster growth in the resulting juveniles and adults grown in the same conditions (Zhao, Schöne, Mertz-Kraus, & Yang, 2017; Zhao, Yang, et al., 2018). The juveniles switched to a less costly ion regulation mechanism and availed of a less costly source of carbon, facilitating calcification and growth. It was suggested that phenotypic plasticity in R. philippinarum was associated with its adaptation to fluctuating conditions in its intertidal habitat.
Carry-over effects of acidification have also been investigated in the embryo to larva and larva to juvenile transition in bivalves. For O. lurida, there were strong negative COE (Hettinger et al., 2012). Juveniles generated by larvae cultured in acidification and out planted into the natural environment did poorly (40% lower growth rate) compared with juveniles generated in ambient conditions. In contrast, for A. irradians and M. mercenaria, the COE from larva to juvenile were beneficial (Gobler & Talmage, 2013). For M. mercenaria, although 80% of the larvae died in acidification culture, the survivors had greater success as juveniles when transferred to natural conditions. These juveniles grew faster and had higher survival compared to those sourced from ambient cultures. A similar result was initially seen for A. irradians, but after 10 months, the juveniles were smaller than those generated by ambient treatment adults (Gobler & Talmage, 2013). For the Ostrea angasi, the low pH that surrounds brooded offspring during valve closure (to pHNBS 7.46) is suggested to convey larval resilience to acidification (Cole et al., 2016), but this was not the case for O. chilensis (Chaparro, Montory, Segura, & Pechenik, 2009).
The results of eight cross- and two multigeneration acidification studies across seven bivalve species are mixed with respect to outcomes for offspring (Table 1). The weight of evidence indicates that depending on the pH level used, parental conditioning is beneficial for few species. Meta-analyses of the pH effect (See Supporting Information for experimental design) revealed that parents conditioned in acidification treatments produced larvae that were still significantly negatively affected by acidification with respect to survival and growth (Figure 3a,b). Overall, conditioning of parents did not remove the negative impact of low pH on offspring reared in the same conditions.
The effect of conditioning was assessed by comparing the response to low pH of offspring produced by conditioned and non-conditioned parents (Figure 3c,d; see Supporting Information for experimental design). These data from studies that used a fully orthogonal design are insightful with regard to the influence of conditioning on offspring performance. Parental exposure to low pH reduced the magnitude of the impact of acidification on offspring survival for M. edulis, but not for the other species (Figure 3c,d). Larval growth was rescued for M. edulis from the Western Baltic Sea at low pH (pHT 7.46) and to some extent for S. glomerata (especially from the selected family line) at moderate acidification (pHNBS 7.9; Figure 3d). Overall, conditioning of parents significantly reduced the negative impacts of acidification on larval growth, likely reflecting the subset of resilient survivors.
2.4 Echinodermata
For echinoderms, cross generation studies have involved eight echinoid species. While most of these studies have involved conditioning of mature adults, one study of Tripneustes gratilla reared animals from the juvenile stage in warming (+2°C) and acidification (pHT 7.8) treatments in a fully crossed experimental design for the entirety of gonad/germ line development and gametogenesis to the mature adult (F0 parents) (Karelitz et al., 2019). These adults were used to generate the F1 generation. Progeny of the acidification and the acidification + warming parents were resilient to low pH, while those from the warming and the acidification + warming parents were resilient to increased temperature. The larvae of experimental treatment parents were better able to grow their skeleton in acidification/warming conditions than those of control treatment parents. However, negative effects were evident as the larvae from experimental treatment parents were overall smaller than control larvae (Karelitz et al., 2019). The eggs of the warm treatment urchins were smaller and this may have influenced this result.
For Strongylocentrotus droebachiensis, there was a difference in the outcome for progeny depending on the time that parents were exposed to acidification (pHNBS 7.7). The progeny generated by parents after 4 months exhibited increased mortality, decreased settlement and low juvenile survival (Dupont, Dorey, Stumpp, Melzner, & Thorndyke, 2013). After 16 months, many of these negative effects were ameliorated. Parental fecundity was lower in the acidification treatment at 4 months, but not at 16 months, indicating that gametogenic timing influenced these results. After 16 months, the urchins would have gone through their annual gametogenic cycle and the gonads would have fully matured under experimental conditions, providing an opportunity for the gametes to be ‘imprinted’ to the progeny environment thereby promoting beneficial ‘anticipatory’ plasticity (positive CGP) for offspring (see Box 1).
Exposure of the Antarctic sea urchin Sterechinus neumayeri to a range of acidification and warming conditions also had different outcomes for offspring depending on parental exposure time (Suckling et al., 2015). The S. neumayeri were conditioned in control, warm (+1.5°C) and two acidification-warming treatments (pHNBS 7.7/+1.8°C; 7.5/+2.1°C). With this design, it is not possible to tease out the impacts of warming and acidification as individual stressors. After 6 months, the effect of adult conditioning was negative, with reduced egg size and embryo hatching (Table 1). Decreased larval survival was seen in the warm-control pH treatment. After 17 months of adult conditioning, the outcomes were beneficial with the largest eggs produced by urchins maintained at pHNBS 7.5/+2.1°C, although with high larval abnormality. There was no difference in larval size indicating that adult conditioning in warm-acidification conditions ameliorated the negative effects of acidification on growth and calcification. This result is likely due to the effect of +2.0°C countering the stunting effect of acidification on larval calcification in S. neumayeri (Byrne, Ho, et al., 2013). As expected, increased temperature resulted in faster development. With the longer conditioning time, the gonads of S. neumayeri would have fully matured in treatments and imprinted on future offspring environment, promoting beneficial CGP (Box 1).
The study by Suckling et al. (2014) where Psammechinus miliaris was conditioned in acidification (pHNBS 7.7) for different durations (28–70 days) also showed that outcomes differed between incubation times (Table 1). There were positive effects for progeny after 42 and 70 days conditioning but not after 28 days. The marked reduction in larval survival across all adult conditioning times, however, indicated deleterious effects. Following the longer conditioning period, the surviving larvae were larger, indicating they were better able to calcify in low pH conditions, but it is not known how they would have performed at ambient pH as this treatment was not included (Suckling et al., 2014). The incubation times used would not have allowed the gonads of P. miliaris to mature fully in conditions with potential to generate negative effects on gamete quality (Box 1).
Following a 7-week conditioning of Echinometra mathaei in pHNBS 7.7, the larvae produced did not differ from those of control parents grown in the same conditions (Uthicke, Soars, Foo, & Byrne, 2013). Adult conditioning did not ameliorate the negative effects of acidification on larval skeletogenesis and abnormality. As gametogenesis in E. mathaei takes months, the conditioning period used would have been too short to provide beneficial outcomes for progeny and may have reduced gamete quality (Box 1), as seen in poor spawning of males.
For Strongylocentrotus purpuratus from the California Current Upwelling System (CCUS), there is evidence of adaptation to local temperature and pH conditions (Kelly et al., 2013; Pespeni, Chan, et al., 2013). In a reciprocal breeding experiment using parents from high and low CO2 sites, the impact of acidification on larvae was lower if the maternal parent originated from the high CO2 site, indicating the presence of phenotypic plasticity in oogenesis (Kelly et al., 2013). In a recent study where adult S. purpuratus were conditioned to a range of acidification and temperature treatments to emulate the upwelling and non-upwelling conditions (Table 1), there were minimal to no effects of any treatment for progeny morphology (Wong, Kozal, Leach, Hoshijima, & Hofmann, 2019). This outcome reflects the resilience of the S. purpuratus from populations that routinely experience upwelling and appear to be genetically adapted low pH conditions (Pespeni, Chan, et al., 2013; Pespeni, Sanford, et al., 2013). The larvae of this species exhibit only a slight decrease in arm growth at pH 7.7 (Kelly et al., 2013; Yu, Matson, Martz, & Hofmann, 2011). In a global meta-analysis, S. purpuratus from the CCUS showed the highest tolerance to acidification, whereas conspecific larvae from other areas were more sensitive (Byrne, Lamare, et al., 2013). Similarly, for Loxechinus albus along the coast of Chile, larval growth in populations exposed to variable pH due to upwelling was less sensitive to acidification (Gaitán-Espitia et al., 2017). More variable pH is correlated with exposure to lower extreme pH, but we do not know which aspect of this variability makes S. purpuratus and L. albus larvae less sensitive to acidification (see below, Section 5).
Although the morphological responses of the progeny of S. purpuratus were muted, gene expression in gastrulae differed and when grouped according to parental treatment, the highest expression levels were found in embryos derived from upwelling treatment adults (Wong, Johnson, Kelly, & Hofmann, 2018). There were also differences in DNA methylation (Strader, Wong, Kozal, Leach, & Hofmann, 2019; see Section 3). These findings highlight the importance to consider mechanistic (cell and molecular) underpinnings in cross generation climate change studies.
For the juvenile offspring of Heliocidaris erythrogramma that had been conditioned to acidification and warming treatments in a fully crossed experimental design over the entire gametogenic cycle, only the highest temperature used (+6°C) influenced juvenile performance, causing increased respiration (Harianto, 2019). A study on the effects of warming as a single stressor on Strongylocentrotus intermedius across one generation found decreased hatching and larval size (Zhao, Zhang, et al., 2018).
Carry-over effects of acidification have been investigated in the larva to juvenile transition in sea urchins. For S. droebachiensis, larval exposure to acidification reduced juvenile survival (by 95%; Dupont et al., 2013). For Arbacia lixula, the larvae were robust to acidification surviving to metamorphosis, but the resulting juveniles were smaller than those generated in control conditions (Wangensteen, Dupont, Casties, Turon, & Palacín, 2013). Similarly, warming and acidification experienced during the larval stage of H. erythrogramma had negative COE for the juvenile stage with an increased abnormality and decreased calcification, although the negative effects of acidification were ameliorated by modest warming (+2°C, but not +4°C; Byrne et al., 2010, 2011). The impacts of these stressors differed with larvae being more sensitive to pH and temperature and juveniles more resilient to both stressors, although this also reflects the subset of larvae that survived and were able to metamorphose under these conditions (Foo, Dworjanyn, Poore, Harianto, & Byrne, 2016). In the single study of the juvenile to mature adult transition, T. gratilla was reared from the very small juvenile to adulthood in acidification and warming treatments. Those reared at pHNBS 7.6 and +3°C gave rise to smaller adults with poor gonads, while those in pHNBS 7.8 and +3°C were similar to controls and had well-developed gonads (Dworjanyn & Byrne, 2018).
For sea urchins, the weight of evidence (Table 1) indicates that the effects for progeny depend on acidification/warming level and the duration of adult conditioning. A global analysis indicated that (pCO2 1,000 µatm) approximates the tipping point for significant effects of acidification on reduced larval calcification (Byrne, Lamare, et al., 2013). The outcomes for sea urchins (Table 1) show the importance of gamete imprinting over the complete gametogenic cycle so that the gonads fully develop and mature under experimental conditions (Box 1).
2.5 Cnidaria
Given their economic, cultural and scientific importance, the response of reef building corals to climate change has been a prominent area of study, with a focus on mechanisms of rapid adaptation (Torda et al., 2017). Three studies have examined CGP in response to adult conditioning in pocilloporids (Table 1) that brood larvae of both sexual and asexual origin (Combosch & Vollmer, 2013), making the identification of CGP or COE difficult. Two studies of Pocillopora acuta/damicornis documented positive responses to climate change stressors in offspring when their parents were conditioned. Adult conditioning during brooding to combined low pH and increased temperature resulted in metabolic enhancement of larvae (Putnam & Gates, 2015). In Putnam, Ritson-Williams, Cruz, Davidson, and Gates (2019), adult conditioning to low pH had positive impacts on larval survival, settlement and growth, but this enhanced performance was no longer detectable by 6 months post settlement. Conditioning of adult brooding Stylophora pistillata to warming (+3–5°C) and reduced pH (pHT 7.6) for 26 and 33 days resulted in reduction in symbiont cell density, chla/c2 and photosynthetic performance in the F0 (Bellworthy, Menoud, Krueger, Meiboom, & Fine, 2019). This reduced photosynthetic performance was also present in the F1 generation, but no significant CGP/COE were observed in host physiology, recruitment and mortality due to parental treatment, which the authors interpreted as stress resistance.
The dearth of studies for reef building corals reflects their long generation time and challenging nature of these experiments. It is essential, however, to understand the eco-evolutionary responses in such an important and threatened group, especially as modelling efforts that include the potential for acclimatization and adaptation differ vastly from those that do not (Logan, Dunne, Eakin, & Donner, 2014).
3 INSIGHTS FROM AQUACULTURE PRACTICE VIA EPIGENETIC SELECTION
The dire trajectories of marine systems (Lotze et al., 2019) have increased the urgency for solution-based science and human interventions to secure their functions, goods and services. This has prompted research into approaches such as assisted evolution or accelerating naturally occurring evolutionary processes (van Oppen, Oliver, Putnam, & Gates, 2015). Among these approaches, COE and CGP are used to condition, or environmentally harden marine organisms. This hardening includes sublethal exposure to one or more stressors, with the goal of eliciting adaptive gains. One mechanism underlying hardening is acclimatization through epigenetics, or the change in gene expression and function, without any change in DNA bases (Eirin-Lopez & Putnam, 2019). These markings on the DNA (DNA methylation), or packaging and formatting of the genetic material (chromatin and histone modifications) can provide cross- and multigenerational signals (Feil & Fragga, 2012) that could generate differences in offspring physiological and ecological performance following adult conditioning.
Insights that adaptive acclimatization and genetic evolution may be possible are shown by bivalve aquaculture where improved/hardy phenotypes are selected for over decades (Nell & Perkins, 2005) and CGP conditioning practices have also shown gains (Parker et al., 2012). Cross generation conditioning of brood stock to environmental factors, including increased temperature, is routine in bivalve aquaculture to generate more resilient and productive offspring (Gavery & Roberts, 2017). The long-time practice of conditioning aquaculture brood stock, with respect to the gametogenic cycle, reflects the presence of anticipatory-type plasticity in gamete development (Box 1). This intentional induction of ‘epigenetic memory’ within or between generations is used to produce desired phenotypes (Gavery & Roberts, 2017).
The Sydney rock oyster presents an insightful case study for assisted evolution in a global change context. For S. glomerata, environmental hardening and potential for epigenetic selection (sensu Gavery & Roberts, 2017) to facilitate persistence of species in a changing ocean has been undertaken. For example, adult conditioning in tandem with selective breeding for fast growth and disease resistance traits over decades (Nell & Perkins, 2005), has in parallel, created families that are more resilient to acidification in the larval (Figure 3) and adult (Fitzer et al., 2018, 2019; Parker, O'Connor, Raftos, et al., 2015; Parker, Ross, & O'Connor, 2011) stages, which may have occurred through genetic or epigenetic selection. Transplanting tolerant individuals back into the natural environment demonstrated acidification tolerance in the F2 generation, although there were limits in response to multiple stressors (Parker, O'Connor, Raftos, et al., 2015; Parker, O'Connor, Byrne, et al., 2017).
The selected families of S. glomerata are not only more tolerant of acidification, but actually outperform wild-type oysters in these conditions (Fitzer et al., 2018, 2019), although are less tolerant of warming in the field (McAffe, O'Connor, & Bishop, 2017; Parker et al., 2011). Due to their ability to maintain shell structure, change metabolic rate and with plasticity in carbon source for shell building, they are also better able to calcify in estuarine regions naturally low in pH and CaCO3 saturation (Fitzer et al., 2018, 2019; Parker et al., 2012). Larvae produced through selection of Crassostrea gigas also had better growth and settlement under pHT 7.5 than wild-type larvae (Durland, Waldbusser, & Langdon, 2019).
In eastern Australia, S. glomerata has evolved in estuarine habitats which often experience sulphate soil acidification that lowers habitat pH (e.g. pH < 7.0, in Amaral, Cabral, & Bishop, 2012), including near aquaculture leases (pHNBS 7.3; Fitzer et al., 2018). Due to this environmental evolutionary history, and the fact that oysters possess the genetic tools to cope with stress (Powell et al., 2018; Zhang et al., 2012), S. glomerata is likely to be naturally adapted for acidification resilience. The moderate acidification levels (pHNBS 7.9) used for adult conditioning in Parker et al. (2012) routinely occur in the coastal estuaries where this species lives (Fitzer et al., 2018), and so is likely to be well within the tolerance limits of S. glomerata. In contrast, negative COEs were evident for A. irradians and M. mercenaria, but at a much lower pH (pHT 7.4; Griffith & Gobler, 2017). This level of acidification may have been beyond the ‘adaptive range’ of these species. These results highlight the importance to understand the stressor conditions experienced in nature when interpreting the outcomes of cross generation studies and to place experimental treatments in an ecological context. There is likely to be a limit to beneficial COE with regard to stressor level, in evolutionary rescue and assisted evolution efforts. In addition, near shore coastal and estuarine environments present multiple co-occurring abiotic and biotic stressors and the ability to condition juveniles for the stressor mix that they experience in nature remains a challenge.
As shown for S. glomerata, production of commercial bivalves that are more resilient to global change stressors, in tandem with advances from selective breeding efforts, hold promise in designing strategies for evolutionary rescue of key aquaculture resources to help climate proof shellfish industries. A better understanding of calcification physiology and mechanisms is needed, as recent studies show that this process is phenotypically plastic and can be adjusted with respect to environmental conditions (Fitzer et al., 2014, 2018, 2019; Zhao, Zhang, et al., 2018).
4 INSIGHTS FROM CONTEMPORARY ENVIRONMENTAL ANALOGUES OF GLOBAL CHANGE
Exposure to environmental stressors in nature and adaptation to local conditions are important to consider (Sanford & Kelly, 2011). Many species exhibit the potential to adapt to acidification, as seen in populations that inhabit low pH environments, including examples presented above (e.g. S. purpuratus from the CCUS; S. glomerata in estuarine acidification). Animals that experience natural acidification (e.g. CO2 vents: Ischia, Papua New Guinea), high temperature (e.g. Red Sea) and both acidification and warming (e.g. New Caledonia Lagoon) all their lives provide examples of acclimatization/adaptation to global change conditions (Camp et al., 2017, 2018, 2019; Fine, Gildor, & Genin, 2013; Foo, Byrne, Ricevuto, & Gambi, 2018; Hall-Spencer et al., 2008; González-Delgado & Hernández, 2018). These systems also incorporate environmental factors, such as nutrients, currents and species interactions, not easily replicated in the laboratory. Natural acidification/warming environments, while not perfect analogues of future marine conditions (see Camp et al., 2018), support a diversity of species providing the opportunity to investigate acclimatization and local adaptation in animals that have lived in these conditions for a long time. A few examples are presented here.
Due to the natural pH gradients generated—including those that match IPCC projections—CO2 vents have been used as analogues for ocean acidification (Foo, Byrne, Ricevuto, et al., 2018; González-Delgado & Hernández, 2018; Hall-Spencer et al., 2008), although it is essential to confirm vent systems as ocean acidification (e.g. CO2 only) analogues, because gas release can be associated with metal ions and sulphides as reflected in bioaccumulation of trace elements in some resident species (Bray, Pancucci-papadopoulou, & Hall-spencer, 2014; Tarasov, 2006; Vizzini et al., 2013).
Sea urchins living at CO2 vents provide an insightful case study of tolerance for acidification. A. lixula, Paracentrotus lividus and Echinometra sp. can tolerate low pH conditions at vents and can even outperform conspecifics from ambient pH sites (Foo, Byrne, & Gambi, 2018; Uthicke et al., 2016). It appears that a mean environmental pHT 7.8 approximates the lower level of acidification for sea urchins to succeed at CO2 vents (Calosi et al., 2013; Foo, Byrne, Ricevuto, et al., 2018), although pH can drop to pHNBS 6.9 (Foo, Byrne, & Gambi, 2018). These sea urchin studies highlight the potential for traits across physiological systems (e.g. reproduction, immune, growth) to exhibit plastic adaptive responses to acidification.
A physiological system analysis of P. lividus resident at CO2 vent and control sites revealed no differences in coelomic acid–base balance, immune cell composition, metabolic rate, nitrogen excretion and skeletal mineralogy, indicating acclimatization of these traits to acidification (Migliaccio et al., 2019). In contrast, the immune cells, the sentinels of environmental stress responses in sea urchins, had an altered proteomic profile in the vent P. lividus. These cells showed a modified metabolism in a shift towards higher levels of protective antioxidant expression. The biochemistry of the immune cells showed an upregulation of phagosome and microsomal proteins, ammonium metabolism enzymes and modulation of carbon metabolism proteins. Thus, the vent P. lividus exhibited phenotypic plasticity in their immune system and a rearrangement of defensive abilities that are likely to facilitate success under acidification.
The eggs of A. lixula from a vent site, but not those of individuals from nearby ambient pH conditions, produce a jelly coat that is resilient to acidification, indicating plasticity in oogenesis (Foo, Byrne, & Gambi, 2018). As the jelly coat is a key structure in enhancing fertilization success, this was suggested to be a strategy to maintain gamete function in low pH.
Echinometra sp. from a vent site were larger than conspecifics from ambient sites (Uthicke et al., 2016). Due to higher CO2 at the vent, algal food levels were higher, and this promoted sea urchin growth despite low pH, an indirect and ecologically important effect of CO2 enrichment. The importance of abundant food in naturally acidified areas to offset deleterious effects on energetically costly processes such as calcification is reported for many species (Connell, Kroeker, Fabricius, Kline, & Russell, 2013; Thomsen et al., 2010). Success of the Echinometra sp. adults to vent conditions, however, did not convey OA resilience to progeny (Lamare, Liddy, & Uthicke et al., 2016).
Corals living in warm/acidified environments are also insightful examples. In the warm conditions of the Red Sea, corals live well beyond the temperature levels that cause bleaching elsewhere due to selection for heat-resistant genotypes (Fine et al., 2013), as also found for corals in warm back reef areas (Barshis et al., 2013). In the warm (+2°C) and low pH (to pH 7.3) sites in New Caledonia Lagoon resident corals show physiological plasticity to facilitate survival in this habitat (Camp et al., 2017).
As these analogues are open, small spatial scale systems, resident populations receive gene flow from larvae generated in surrounding ambient conditions and so it is unlikely that local genetic adaptation has occurred. Aside from species with short distance larvae (e.g. brooding corals), it is unlikely that larvae (Figure 1) recruit back to the parental habitat. This reduces the opportunity for investigation of MGP. These environments are likely to exert strong selection on recruiting larvae. Selection post-settlement may promote genetic differences as seen in the selective survival of sea urchin larvae reared under acidification, although with reduced genetic diversity (Lloyd, Makukhov, & Pespeni, 2016; Uthicke et al., 2019). Resident species that lack a dispersal phase (e.g. brooders, egg layers; Lucey et al., 2015) provide the opportunity to assess MGP in situ and the potential for genetic selection to life in these extreme habitats.
5 CONCLUSION
Although parental conditioning within (CGP) and across generations (MGP) has been identified as a mechanism of acclimatization to global change, potentially at timescales shorter than the rate of changing climate (Munday et al., 2013; Ross et al., 2016), the weight of evidence from experimental studies (Table 1) indicates that this is not broadly true for marine invertebrates. For many species reviewed here, there were no benefits of parental conditioning for the traits examined. Even when improved performance was observed, this waned within and over generations and fitness declined. With the experimental approaches taken to date and the species studied, it appears that MGP is unlikely to be a universally useful tool for species rescue. That said, many CGP and MGP studies have identified key ecologically and economically important species that are stress tolerant or broad generalists that have tolerant genotypes in wild populations (Fine et al., 2013; Kelly et al., 2013; Thomsen et al., 2017) or in selectively bred lines (Parker et al., 2011, 2012; Parker, O'Connor, Raftos, et al., 2015). Here, the ‘ghost of selection past’ (sensu Ghalambor et al., 2007) in maintaining plastic responses may contribute to resilience. It is important to identify stress tolerant and generalist-type species as they are likely to be the winners in the climate change stakes. These species are also good candidates to provide insights into the mechanisms involved in CGP plasticity to climate change stressors.
The results of several studies (Table 1) support the idea that highly variable environments and exposure to extreme conditions contribute to plastic or adaptive tolerance (Barshis et al., 2013; Rivest, Comeau, & Cornwall, 2017; Safaie et al., 2018). For example, the larvae of sea urchins that live in pH flux-upwelling regions were resilient to acidification. Environmental variability was suggested to be most important in this outcome (Gaitán-Espitia et al., 2017). The plasticity of the immune system of urchins living in variable CO2 vent conditions is another example (Migliaccio et al., 2019). Many bivalves live in heterogeneous near shore and estuarine environments where they routinely experience fluctuations in temperature and pH and so are also likely to have some adaptive tolerance to these stressors (Rivest et al., 2017). While heterogeneous environments may select for greater MGP, we do not know which aspects of environmental variability facilitates this plasticity (e.g. mean, range, extremes). This is an important knowledge gap to address.
For bivalves, interesting trends are emerging in that some species are phenotypically plastic with regard to the form of calcium carbonate that they can use to make their shells (Fitzer et al., 2014), in the metabolic pathways/enzyme systems employed (Zhao et al., 2017) and in the source of carbon for calcification (Fitzer et al., 2019; Zhao, Zhang, et al., 2018), depending on level of acidification. Such plasticity provides a platform to manipulate outcomes for future generations and is likely to contribute to the success of parental conditioning to enhance resistance to acidification. This highlights the need to understand the mechanism(s) of plasticity, an exciting avenue of research.
Although the outcomes of comparatively short-term laboratory studies provide equivocal evidence for the capacity for ‘climate adaptation’, natural proxies of future conditions such as upwelling zones, estuarine gradients, CO2 vents and warm habitats show the potential for phenotypic adjustment and/or beneficial genetic selection.
An important caveat not considered here, are the effects of genetic drift and loss of genetic diversity in experimental evolution studies with small laboratory populations that have low standing genetic variation (Lloyd et al., 2016; Reusch & Boyd, 2013; but see Langer et al., 2019). Loss of the initial benefits of CGP and MGP over generations may be influenced by selection and the loss of genetic variation. The potential for genetic selection has generally not been tested for, although would be expected for instance across 80 generations of A. tonsa (Langer et al., 2019). In one of the few studies that tested for selection, this was not evident across seven generations for a short generation polychaete (Gibbin, Chakravarti, et al., 2017). This prompts the question—how many generations are required to see evidence of evolutionary rescue, a particularly daunting endeavour for long generation species. For these species, CGP is likely to be influential and so parental effects remain a key area of investigation.
We also did not expand on the interactions between plasticity and genetic adaptation, but these are important to consider. Adaptation to climate change will involve phenotypic plasticity as well as evolution through selection, but these are not independent (Fox, Donelson, Schunter, Ravasi, & Gaitán-Espitia, 2019). Importantly, plasticity may be maladaptive in the face of new conditions (Ghalambor et al., 2015; Parker, O'Connor, Byrne, et al., 2017). By increasing our knowledge of both adaptive and acclimatory processes and their potentially antagonistic or synergistic evolutionary interactions, we will be better equipped to forecast the trajectory of marine organisms under rapid climate change.
Due to logistical constraints to conduct experiments over the long times (~decades) commensurate with the pace of changing climate, many studies use shock-type stressor exposure at rates much faster than projected change. While these experiments are useful to determine stress tolerances and have been important to identify resistant species, they are less informative with respect to potential outcomes for more sensitive species. With more gradual introduction of projected future conditions over much longer experimental durations and in context with the fluctuating conditions that species are currently acclimatized and/or adapted to, the outcomes might be different for sensitive species. It is a challenge to design conditioning strategies to better match multistressor environments. We also have to be cognizant that extreme events such as heat waves are becoming more prevalent (Babcock et al., 2019). Such events, compared to gradual change (e.g. IPCC scenarios) provide little opportunity for acclimatization or adaptation.
Finally, CGP and MGP studies of marine invertebrates in context with global climate change are a rapidly expanding field, but the diversity of species investigated so far is narrow. Our comparisons were limited by the small number of traits assessed across studies. We need a better understanding of the effects of parental conditioning and over multiple generations (where possible) for more species, with incorporation of (a) natural fluctuations; (b) multiple interactive stressors; (c) a greater breadth of traits assessed from the molecular to whole organism physiology as we see traits can respond differently to CGP and MGP; and (d) a clear understanding of the reproductive biology and development of PGCs and gametes. It will also be important to test for selection and the role of plasticity in adaptation (Fox et al., 2019). Stressor levels and rates of change commensurate with what species currently experience in nature should be considered to assess adaptive capacity with respect to environmental and evolutionary history. Thus, it is essential to have a habitat level understanding of current environmental conditions and how these are changing to assess impacts on marine species.
ACKNOWLEDGEMENTS
We thank Dr. Elliot Scanes and Dr. Kennedy Wolfe for assistance with figures and Hamish Campbell for assistance with the manuscript and grant support from the ARC (DP150102771; MB), NSW Environmental Trust 2016RD0159 (MB, PR), US National Science Foundation, Grant/Award Number 1921465, Foundation for Food and Agriculture Research (Development of Environmental Conditioning Practices to Decrease Impacts of Climate Change on Shellfish Aquaculture: HMP) and Pembroke Foundation International Ltd. We are grateful to the reviewers for providing insightful comments that improved the manuscript. This is contribution number 252 of the Sydney Institute of Marine Science.




