Irisin promotes hair growth and hair cycle transition by activating the GSK-3β/β-catenin pathway
Yujin Kim and Jung Min Lee contributed equally to this work.
Abstract
Hair loss affects men and women of all ages. Myokines, which are mainly secreted by skeletal muscles during exercise, have numerous health benefits. VEGF, IGF-1, FGF and irisin are reprehensive myokines. Although VEGF, IGF-1 and FGF are positively associated with hair growth, few studies have researched the effects of irisin on hair growth. Here, we investigated whether irisin promotes hair growth using in vitro, ex vivo and in vivo patch assays, as well as mouse models. We show that irisin increases proliferation, alkaline phosphatase (ALP) activity and mitochondrial membrane potential in human dermal papilla cells (hDPCs). Irisin activated the Wnt/β-catenin signalling pathway, thereby upregulating Wnt5a, Wnt10b and LEF-1, which play an important role in hair growth. Moreover, irisin enhanced human hair shaft elongation. In vivo, patch assays revealed that irisin promotes the generation of new hair follicles, accelerates entry into the anagen phase, and significantly increases hair growth in C57BL/6 mice. However, XAV939, a Wnt/β-catenin signalling inhibitor, suppressed the irisin-mediated increase in hair shaft and hair growth. These results indicate that irisin increases hair growth via the Wnt/β-catenin pathway and highlight its therapeutic potential in hair loss treatment.
1 INTRODUCTION
Hair loss refers to the phenomenon of reduced hair density or thinning of hair strands and can be induced by various factors, including stress, aging and imbalances in hormones and nutrients.1 Although hair loss is not life-threatening, it affects self-esteem and the quality of life. The drugs minoxidil (MNX) and Finasteride are approved by the United States Food and Drug Administration (FDA) for hair loss treatment. However, their use is limited and transient because of unpredictable efficacy and side effects.2, 3 Thus, there is an unmet need for novel, effective hair growth agents.
The hair follicle (HF) is made up of the epidermal and dermal compartments. The mesenchymal compartment is made of specialized fibroblasts, which are divided into the dermal papilla (DP) and the dermal sheath. The DP is a cluster of specialized fibroblasts named dermal papilla cells (DPCs), which are surrounded by hair matrix keratinocytes. Thus, reciprocal interactions between the DP and surrounding epithelial cells are essential for the formation of new HFs and the regulation of hair growth.4 In adults, HFs undergo three cyclic phases, anagen, catagen and telogen. Specifically, DPCs play major roles in hair formation, growth and the physiological cycles of the HFs.5
Wnt/β-catenin signalling pathway plays an essential role in the hair-inductive properties of human DPCs (hDPCs), HF development, the hair cycle and regeneration.6, 7 Specifically, β-catenin regulates stem-cell niche activation and transition to the anagen phase.8
Although exercise benefits all body systems, research on its impact on hair health is limited. Exercise induces the secretion of myokines in the muscle, which enables the interaction between muscles and other organs in an autocrine/paracrine manner.9, 10 Previous findings indicate that myokines, such as vascular endothelial growth factor (VEGF), insulin-like growth factor 1 (IGF-1) and fibroblast growth factor (FGF), promote hair growth. VEGF increases HF size and hair thickness,11 whereas IGF-1 increases HF numbers and prolongs the anagen phase by downregulating TGF-α1 expression.12 FGF-10, FGF-1 and FGF-2 promote hair growth through the β-catenin signalling pathway.13 Moreover, FGF-21 knockout mice exhibit significantly slower hair regrowth, indicating that FGF-21 has a key role in increasing hair growth.14
Irisin is an exercise-derived myokine with anti-inflammatory, antioxidative and anti-apoptotic properties.15-17 It also has therapeutic effects against type 2 diabetes, myocardial infarction,18 osteoporosis19 and breast cancer.20 Interestingly, irisin increases the proliferation of the rat insulinoma cell line, INS-1, via the ERK signalling pathway, which is associated with HF morphogenesis, regeneration and growth.21, 22 However, there are no reports on the effects of irisin on hair growth.
In this study, we investigated how irisin affects hair growth using in vitro, ex vivo and in vivo analyses. We found that irisin increases the proliferation of hDPCs the activity of alkaline phosphatase (ALP), mitochondrial membrane potential and the production of growth factors, such as epidermal growth factor (EGF), insulin-like growth factor-binding protein (IGFBP)-1 and − 2 (IGFBP-1 and IGFBP-2), and platelet-derived growth factor receptor α and β (PDGF-Rα and PDGF-Rβ), which are essential for hair growth in DPCs. Furthermore, irisin enhanced transition into the anagen phase and increased follicle numbers when compared with the control group. However, these effects were abolished by treatment with XAV939, a Wnt-β-catenin signalling inhibitor.
Collectively, our findings indicate that irisin promotes hair growth via Wnt/β-catenin signalling and highlight its therapeutic potential in hair loss prevention and/or treatment.
2 MATERIALS AND METHODS
2.1 Reagents
Recombinant mouse fibronectin type III domain-containing protein 5 was purchased from CSBio (TX, USA). Minoxidil (MNX), lithium chloride (LiCl) and XAV939 were purchased from Sigma–Aldrich (Steinheim, Germany). Antibodies against PGC-1α, Bcl-2, Bax, Wnt-10b, VEGF, IgG-FITC and β-actin were purchased from Santa Cruz Biotechnology (CA, USA). Antibodies against ALP and Ki67 were purchased from Thermo Fisher Scientific (MA, USA). Antibodies against Cyclin D1, Cyclin E, p-ERK-1/2 (Thr-202/Tyr-204), ERK-1/2, p-GSK-3β (Ser-9), GSK-3β, p-β-catenin (Ser-675), p-β-catenin (Ser-33/37/Thr-41), β-catenin, LEF-1 and IGF-I were purchased from Cell Signaling Technology Inc. (Beverly, MA).
2.2 Culture of hDPCs
hDPCs were purchased from Promocell (Heidelberg, Germany) and cultured in follicle dermal papilla cell growth medium (Heidelberg, Promocell) supplemented with growth medium Supplement Pack (Heidelberg, Promocell) at 37°C in a humidified incubator, with 5% CO2.
2.3 Cell proliferation assay
The hDPCs (3 × 103 cells/well) were cultured in 96-well plates (Corning Inc., NY, USA) for 24 h and then treated with irisin (0, 10, 30, 100 and 300 ng/mL) for 24 h. Cell viability was quantified using the WST-8 assay by measuring absorbance at 450 nm on a microplate reader (SpectraMax 340; Molecular Devices, Inc., CA, USA). Three independent experiments were performed to obtain mean cell proliferation values, which are presented as percentages of those obtained in the control group.
2.4 ALP activity assay
hDPCs were seeded at a density of 1 × 105 cells/mL and treated with irisin for 48 h. ALP activity was then assessed using enzyme-linked immunosorbent assay, using the SensoLyte™ pNPP ALP assay kit (Ana Spec, CA, USA) according to the manufacturer's instructions. Briefly, the cells were dissolved in 1× assay buffer containing 0.2% (v/v) Triton X-100 for 10 min at 4°C, with agitation. The cell lysate was then centrifuged at 2500g for 10 min at 4°C. The supernatant was then diluted in 1× assay buffer containing p-nitro phenyl phosphate substrate and then incubated for 1 h at room temperature (RT). Finally, a stop solution was added to each well, and absorbance was measured at 405 nm on a SpectraMax 340 microplate reader (Molecular Devices).
2.5 Mitochondrial membrane potential assay
Mitochondrial membrane potential was measured using a JC-1 mitochondrial membrane potential assay kit following the manufacturer's instructions. Briefly, hDPCs were seeded at a density of 3 × 103 cell/well and cultured for 24 h. They were then treated with various concentrations of irisin and cultured for 24 h. Next, they were stained with 1 μM of the JC-1 solution and incubated at 37°C for 30 min, followed by the measurement of the JC-1 aggregate/monomer ratio using a microplate reader (SpectraMax 340; Molecular Devices, Inc., CA, USA) at 535 nm (emission wavelength: 590 nm). The JC-1 monomer excitation wavelength was 475 nm (emission wavelength: 530 nm).
2.6 Western blot analysis
Protein extraction was done by lysing the cells with RIPA buffer. Equal amounts of protein samples were separated on 10% sodium dodecyl sulfate–polyacrylamide gels and then transferred onto nitrocellulose membranes (Cytiva, Amersham, USA). Membranes were then blocked using 5% skimmed milk in Tris-buffered saline (TBS)–0.1% Tween-20 and incubated with primary antibodies at 4°C, overnight. They were then washed and incubated with HRP-conjugated anti-mouse or anti-rabbit (Vector Laboratories Inc.) secondary antibodies. Immunodetection was done using an Amersham ECL kit (GE Healthcare, IL, USA) according to the manufacturer's instructions.
2.7 Immunocytochemistry (ICC)
Cells were fixed with 4% paraformaldehyde (PFA) for 30 min, washed with phosphate-buffered saline (PBS) and blocked with a blocking solution containing 3% bovine serum albumin (BSA) and 0.2% Triton X-100 in PBS at RT for 1 h. They were then incubated overnight at 4°C with an anti-β-catenin antibody, washed with PBS and then incubated with an anti-rabbit IgG-FITC secondary antibody for 1 h at RT, in the dark. Cell nuclei were counterstained with DAPI (Immuno Bioscience Corp., Washington, USA). The cells were then examined and imaged on a confocal microscope (LSM 800, Carl Zeiss, Jena, Germany).
2.8 Dot blot array of growth factors
A human growth factor antibody array membrane kit (Abcam, Cambridge, UK) was used to measure irisin-induced growth factor changes. hDPCs (1 × 105 cells/well) were seeded and cultured to 80% confluence. They were then treated with 100 ng/mL of irisin for 48 h, after which the culture supernatants were harvested. Next, the assay was performed according to the kit manufacturer's instructions. The membrane was allowed to react with the culture supernatants and then imaged using a CCD camera (EZ-capture, ATTO, New York, USA). The resulting blots were analysed on ImageJ (Bethesda, MD, USA).
2.9 Spheroid formation and hanging drop culture
A total of 1.5 × 104 hDPCs were mixed with various concentrations of irisin and seeded into clear, 96-well, round-bottom ultra-low attachment microplates (Nexcelom Bioscience, Lawrence, USA). The sizes of the spheroids were assessed six and 24 h later under a microscope and then imaged (Leica, Wetzlar, Germany).
2.10 Reverse transcription quantitative PCR (RT-qPCR)
Total RNA was extracted from hDPCs using the TRIzol reagent (Invitrogen CA, USA) and then reverse transcribed into cDNA using a Prime Script TM RT Master Mix (Takara, Tokyo, Japan). Next, qPCR was performed using qPCR 2X PreMIX SYBR (Enzynomics Seoul, Korea) on a CFX96 Touch Real-Time PCR System (Bio-Rad, CA, USA) using the following program: initial denaturation at 95°C for 10 min, followed by 30 cycles at 95°C for 10 s, 60°C for 15 s and 72°C for 20 s. The results were calculated and reported as cycle threshold (Ct) values using the ΔCt quantification method. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the reference gene. The RT-qPCR primers are shown in Table 1.
| Gene | Primer sequence (5′–3′) | |
|---|---|---|
| EGF | F | CAGGGAAGATGACCACCACT |
| R | CAGTTCCCACCACTTCAGGT | |
| Human IGFBP-1 | F | ATCCTTTGGGACGCCATCAG |
| R | ATTCCAAGGGTAGACGCACC | |
| Human IGFBP-2 | F | GGTATGAAGGAGCTGGCCGTGTTC |
| R | CGCTGCCCGTTCAGAGACATCTTG | |
| Human PDGF-Rα | F | GTGCGAAGACTGAGCCAGATTG |
| R | CGATAAACAGAATGCTTGAGCTGTG | |
| Human PDGF-Rβ | F | GGACCTGCTATGAGGCTTTGGA |
| R | ACAAATGTGCAACCACCTGGAA | |
| Human ALP | F | ATTGACCACGGGCACCAT |
| R | CTCCACCGCCTCATGCA | |
| Human SHH | F | CTCGCTGCTGGTATGCTCG |
| R | ATCGCTCGGAGTTTCTGGAGA | |
| Human FGF-7 | F | ATCAGGACAGTGGCAGTTGGA |
| R | AACATTTCCCCTCCGTTGTGT | |
| Human BMP-2 | F | GAGGTCCTGAGCGAGTTCGA |
| R | TCTCTGTTTCAGGCCGAACA | |
| Human LEF1 | F | AGAACACCCCGATGACGGA |
| R | GGCATCATTATGTACCCGGAAT | |
| Human GAPDH | F | TGGGTGTGAACCATGAGAAG |
| R | GCTAAGCAGTTGGTGGTGC | |
2.11 Patch assay
Truncal skin was obtained from newborn C57BL/6 mice, washed with Ca2+- and Mg2+-free PBS, rinsed with povidone-iodine solution and then incubated with collagenase/dispase (2.5 mg/mL, Roche, Basel, Switzerland) at 4°C overnight. Inductive dermal and epidermal cells were then isolated and incubated with 0.25% trypsin–EDTA for 2 h at 37°C. DMEM–F12 medium (Gibco, New York, USA), supplemented with 5% FBS, was then added and the samples were filtered through a strainer. The cells were then centrifuged at 4000 revolutions per minute for 10 min at 4°C. Next, 2 × 106 dermal cells and 1 × 106 epidermal cells were resuspended in DMEM–F12 medium supplemented with 10% FBS and mixed with irisin (5 or 10 ng/mL) to a final volume of 100 μL. The cell suspension was then injected into the hypodermis of the BALB/c nude mice using a 26-gauge needle. The inhibitor was pretreated 30 minutes before irisin administration. The hHFs that were formed were stained with haematoxylin and eosin (H&E).
2.12 hHFs culture
Ex vivo hHFs were obtained with written informed consent as per the study protocol approved by the Institutional Review Board of Severance Hospital at Dankook University College of Medicine (Seoul, Korea). Anagen phase hHFs were isolated using a stereomicroscope (Olympus, Tokyo, Japan) to avoid damage. Six to twelve hHFs were separated and used in the experiments. Isolated hHFs were cultured in Williams medium E (Gibco, New York, USA) containing 2 mM of L-glutamine (Gibco), 10 μg/mL of insulin (Sigma–Aldrich), 10 ng/mL of hydrocortisone (Sigma–Aldrich), 100 Units/mL of penicillin (Gibco) and 100 μg/mL of streptomycin (Gibco), at 37°C, with 5% CO2. After culturing for 1 day, the hHFs were imaged and their shapes and lengths were analysed. The hHFs were treated with irisin (100 ng/mL) for 7 days. If pretreated with XAV939, the hHFs were treated with irisin (100 ng/mL) and XAV939 (20 μM). They were then fixed in 10% formalin. The elongation of the hHFs was assessed using ImageJ (version 1.52a).
2.13 Histology and immunofluorescence (IF)
Mouse skins (back) were fixed overnight in 10% formalin, dehydrated in ethanol and embedded in paraffin blocks. They were then sectioned, stained with H&E and examined under an optical microscope (DM750, Leica) to determine HF number, skin thickness and the anagen/telogen ratio. For IF, the sections were incubated with antibodies against β-catenin or Ki-67 at 4°C, overnight. They were then washed and incubated with anti-rabbit-FITC secondary antibody, at RT for 1 h. The nuclei were counterstained with DAPI, followed by examination and imaging on a confocal microscope (LSM 800, Carl Zeiss, Jena, Germany).
2.14 Hair regeneration model
C57BL/6 mice were purchased from Saeron Bio Inc. (Gyeonggi-do, Korea) and acclimated for 7 days at 23 ± 2°C, 55% ± 10% humidity and a 12-h light/dark cycle. All animal experiment protocols adhered to the laboratory animal care guidelines of the National Institutes of Health and were approved by the Chung-Ang University Institutional Animal Care and Use Committee. Before the experiments, the mice were anaesthetised using Zoletil (40 mg/kg) and Rompun (5 mg/kg). To obtain telogen–anagen transition, C57BL/6 mice were shaved on the dorsal skin at the telogen phase of the hair cycle as described previously. The mice were randomly divided into normal control, the irisin 5 ng/kg and the irisin 25 ng/kg groups (N = 3 per group). Treatments were injected intravenously, three times a Week for 2 weeks. To compare the growth rate, the back skin of the mice was photographed using a digital camera on Days 10 and 14 after depilation. The growth area-to-total area ratio was calculated on ImageJ. The back skin of the mice was isolated on Day 14, fixed in 4% formalin, cut in the longitudinal and transverse directions, and then stained with H&E for histological analysis. HF numbers were counted on a cropped image and a fixed area (1 × 1 mm) using an optical microscope (DM750, Leica). To assess if the Wnt/β-catenin pathway mediates irisin-induced hair growth, four groups were intraperitoneally injected with the following treatments thrice a Week for 14 days: vehicle, irisin, irisin + XAV939 and XAV939 (n = 5 per group). Mice were intraperitoneally injected with XAV939 (10 mg/kg) or DMSO (saline at a 1:10 ratio) 1 h before irisin (50 ng/kg) administration.
2.15 Statistical analyses
Data are presented as means ± standard deviation (SD) of at least three independent experiments. Data were analysed using a one-way analysis of variance, followed by a Bonferroni post hoc test. All statistical analyses were done on GraphPad Prism, version 7.0 (GraphPad Software Inc., CA, USA). All experiments were repeated at least three times. p < 0.05 indicates statistically significant differences. *, **, *** and **** indicate p < 0.05, < 0.01, < 0.001 and <0.0001, respectively.
3 RESULTS
3.1 Irisin increases the proliferation of hDPCs
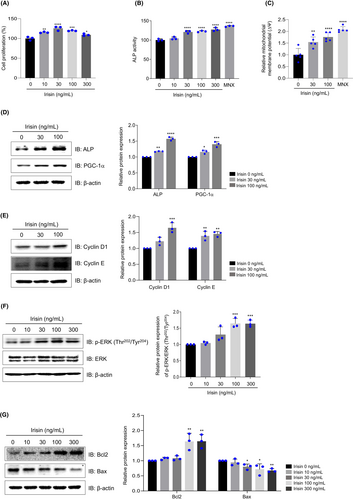
At 10, 30, 100 and 300 ng/mL, irisin increased cell viability by 20%–40% (Figure 1A). ALP activity has been identified as a critical marker for hair growth.23, 24 Our analysis revealed a significant increase in ALP activity following treatment with irisin or MNX (Figure 1B). The mitochondrial membrane potential of the hDPCs was assessed through JC-1 staining, where red and green colours indicate high and low activity, respectively. Treatment with irisin at 30 and 100 ng/mL dose-dependently increased mitochondrial membrane potential by 175.4% and 201.8%, respectively (Figure 1C). Moreover, 1 μM MNX increased mitochondrial potential by 224.2% (Figure 1C). Irisin increased PGC-1α and ALP expression levels dose-dependently (Figure 1D). Next, evaluation of the effects of irisin on the expression of cell cycle regulatory proteins revealed that Cyclin D1 and Cyclin E protein levels were upregulated in cells treated with irisin at 30–100 ng/mL (Figure 1E). Additionally, irisin increased ERK phosphorylation (Figure 1F) while reducing the expression of Bax, a mitochondrial apoptosis marker. However, the levels of Bcl2, an anti-apoptotic factor, were increased by 23% upon treatment with irisin at 100–300 ng/mL (Figure 1G). These findings indicate that irisin enhances hDPC proliferation.
3.2 Irisin activates Wnt/β-catenin signalling in hDPCs
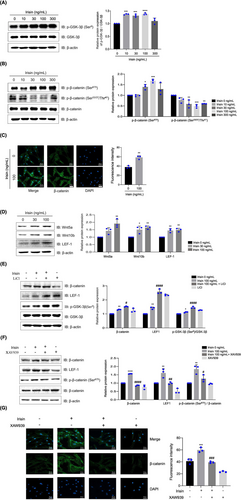
The phosphorylation of glycogen synthase kinase-3β (GSK-3β) on Ser-9 increased in a dose-dependent manner, indicating GSK-3β inactivation (Figure 2A). Consequently, β-catenin phosphorylation on Ser-33/Thr-41, a GSK-3β substrate, decreased, whereas its phosphorylation on Ser-675 increased (Figure 2B). β-catenin nuclear translocation was increased in irisin-treated hDPCs when compared with the controls, and irisin significantly increased Wnt5a, Wnt10b and LEF-1 protein levels (Figure 2C,D). Treatment with LiCl, a GSK-3β inhibitor, promoted irisin-induced GSK-3β phosphorylation on Ser-9 and enhanced β-catenin and LEF-1 expression (Figure 2E). Conversely, XAV939, a Wnt/β-catenin signalling inhibitor, suppressed irisin-mediated β-catenin phosphorylation on Ser-675 and the expression of β-catenin and LEF-1 (Figure 2F). Furthermore, XAV939 inhibited nuclear translocation of β-catenin by treatment with Irisin (Figure 2G). These results indicate that irisin increases the expression of β-catenin and LEF-1 via a GSK-3β-dependent mechanism.
3.3 Irisin elevates hair growth markers
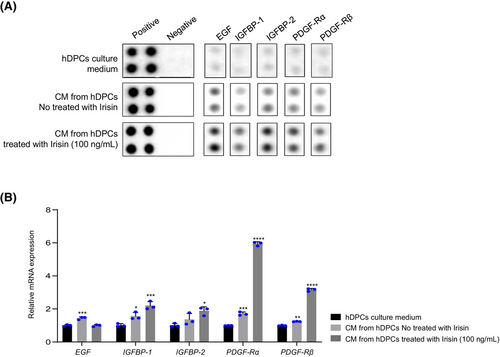
To investigate whether irisin influences the secretion of growth factor proteins by hDPCs, we performed a dot blot analysis of a human growth factor antibody array. This analysis revealed that irisin enhanced the secretion of EGF, IGFBP-1, IGFBP-2, PDGF-Rα and PDGF-Rβ (Figure 3A). This observation was validated at the RNA level in irisin-treated hDPCs, with these genes exhibiting dose-dependent upregulation in response to irisin treatment (Figure 3B). These findings indicate that irisin enhances hair growth by promoting growth factor secretion.
3.4 Irisin increases hDPCs inductivity and hair growth marker expression
The size of hDPC spheres is closely associated with hair shaft diameter25 and reduced hDPC sphere sizes have been observed during HF miniaturization in patients with alopecia.26 Therefore, we investigated whether irisin regulates hDPC sphere size and observed that spheroids from irisin-treated hDPCs were bigger than those from vehicle-treated hDPCs (Figure 4A). Moreover, RT-qPCR analysis revealed that irisin upregulated the expression of the hair growth-related genes, ALP, SHH, FGF-7, BMP-2 and LEF-127 (Figure 4B). Irisin also elevated the protein levels of VEGF and IGF-1 in hDPCs (Figure 4C). The inductivity of hair-like structures was validated using an in vivo patch assay. Mixed cells treated with irisin at 5 ng/mL and 25 ng/mL were injected into the hypodermis of BALB/c nude mice. After 3 weeks, full-thickness skin was harvested and significant clusters of hair-like structures were observed in the hypodermis (Figure 4D, patch image). The pigmentation and the number of HF-like structures were significantly higher in the irisin-treated group when compared with the vehicle-treated group (Figure 4D). Irisin led to, approximately, a 2.1-fold increase in the number of HFs when compared with vehicle-treated mixed cells (Figure 4D,E H&E image). Additionally, treatment with XAV939 inhibited irisin-induced new hair formation (Figure 4F). These data indicate that the irisin-induced increase in hair inductivity is regulated by Wnt/β-catenin signalling.
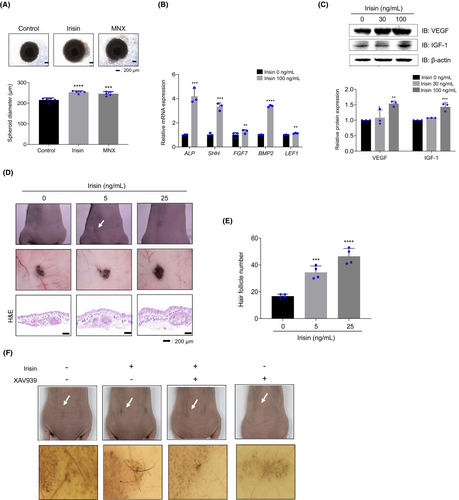
3.5 Irisin improves human hair growth and HF morphogenesis
To examine the effect of irisin at the hair organ level, cultured human scalp HFs were treated with irisin (200 ng/mL) for 7 days. This analysis revealed that irisin-treated HFs exhibited greater hair shaft elongation than the control hHFs (Figure 5A). IF analysis of β-catenin and the proliferation marker, Ki-67, revealed that when compared with the vehicle, irisin increased β-catenin and Ki-67 expression in the HFs (Figure 5B,C). Furthermore, treatment with XAV939 prevented irisin-mediated HF growth (Figure 5D). These results suggest that irisin increases HF growth via the Wnt/β-catenin signalling pathway.
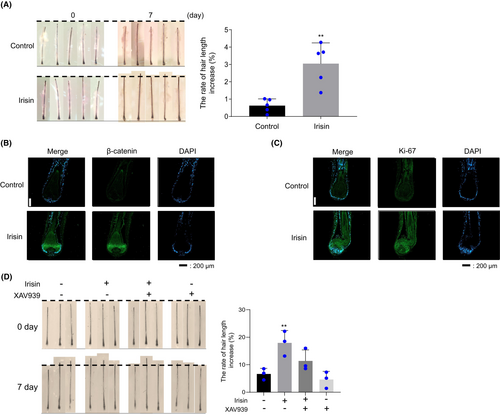
3.6 Irisin stimulates hair cycle telogen–anagen transition in mice
In C57BL/6 mice it is possible to visually evaluate hair growth by observing changes in skin colour during the hair cycle.28 As the hair cycle progresses from the telogen to the anagen phase, the skin colour transitions from pink to black. By Day 10 following depilation, the skin colour of mice treated with irisin (5 and 25 ng/kg) became noticeably darker when compared with the control group (Figure 6A). As a result, the irisin-treated group had a higher dorsal skin colour score than the vehicle-treated group (Figure 6D). Furthermore, the irisin-treated group achieved 90% hair growth by day 14, compared with 70.4% in the vehicle-treated group (Figure 6E). The irisin-treated group had a higher number of total HFs in the longitudinal sections and anagen HFs in the transverse sections when compared with the vehicle-treated group (Figure 6B,C,F,G). Irisin-treated mice exhibited stronger β-catenin expression when compared with the vehicle-treated group (Figure 6H). These results indicate that irisin accelerates hair growth by stimulating anagen transition in mice.
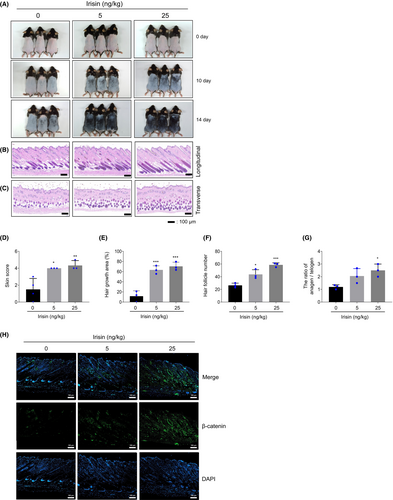
3.7 XAV939 attenuates irisin-mediated hair growth in mice
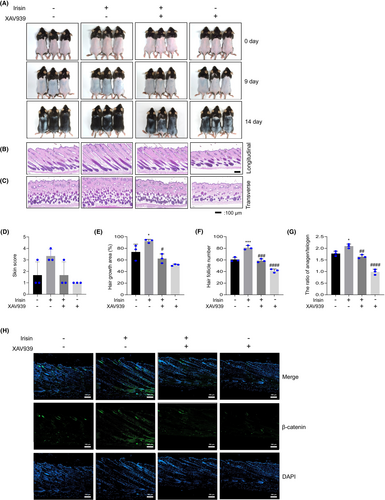
To investigate whether irisin's impact on mouse hair growth is associated with β-catenin signalling, mice were intraperitoneally injected with XAV939 (10 mg/kg) or a DMSO solution (saline 1:10) 1 h before irisin administration (25 ng/kg). The irisin-treated group exhibited increased hair growth when compared with the vehicle group. However, pretreatment with XAV939 suppressed irisin-induced skin score and hair growth improvement (Figure 7A,D,E). Additionally, to determine the hair growth phase, we evaluated number and morphological changes of HFs using H&E. This revealed that in representative longitudinal and transverse sections obtained on Day 14 (Figure 7B,C), The pretreatment with XAV939 caused a decrease in the number of hair follicles and a reduced ratio of the anagen/telogen phase (Figure 7F,G). IF analysis revealed increased β-catenin levels during hair growth in the irisin-treated group, and this was reversed by XAV939 administration (Figure 7H). These results indicate that in mice, irisin promotes hair growth through Wnt/β-catenin signalling.
4 DISCUSSION
Although physical exercise effectively improves human health, its impact on alopecia is unclear. Previous studies suggest that exercise may enhance blood flow to the scalp, thereby facilitating the supply of nutrients and oxygen to HFs,29 which nourishes hair roots, potentially stimulating the anagen phase of the hair growth cycle. However, exercise might also contribute to alopecia by elevating oxidative stress, testosterone levels and 5α-reductase activity.30, 31
During exercise, skeletal muscle contraction triggers the release of myokines, which have autocrine, paracrine or endocrine effects.32 Irisin, a well-known exercise-induced cytokine, mediates beneficial effects of exercise, including adipocyte browning, thermogenesis and the maintenance of metabolic homeostasis.33, 34 In this study, we evaluated the impact of irisin on hair growth using in vitro, ex vivo and in vivo models. Irisin was associated with enhanced hair shaft elongation in an ex vivo model (Figure 5) and hair growth via the stimulation of anagen transition in a mouse model (Figure 6). Additionally, we show that irisin regulates hair growth through the Wnt/β-catenin signalling pathway (Figure 7).
To promote hair shaft elongation, an adequate energy supply is required. Previous studies on HF metabolism indicate that glycolysis and mitochondrial metabolism are key sources of the energy required for hair growth.35-37 Fan et al. reported that increased mitochondrial oxidative phosphorylation promotes the proliferation of hDPCs.38 Our analysis revealed increased mitochondrial membrane potential after irisin treatment (Figure 1C). Additionally, treatment with irisin dose-dependently increased PGC-1α expression (Figure 1D). PGC-1α is thought to be a master regulator of mitochondrial biogenesis and function and to play a crucial role in various processes, including oxidative phosphorylation and reactive oxygen species detoxification.39 Furthermore, PGC-1α acts as a transcription cofactor to stimulate irisin synthesis and secretion.40 Taken together, these results suggest that irisin promotes the proliferation of hDPCs by increasing mitochondrial activity and PGC-1α expression, thereby providing energy for HF cell proliferation.
hDPCs are a major component of a hair DP, which is an inductive structure that sends and receives signals.25 The generation of new HFs and the maintenance of hair growth rely on continuous, close interaction with the hair matrix epithelium through the native extracellular matrix.41 To determine whether irisin promotes hair growth factor secretion by hDPCs, we conducted antibody dot blot array analyses using conditioned media from hDPCs. Several cytokines including EGF, IGFBP-1, IGFBP-2, PDGF-Rα and PDGF-Rβ were strongly secreted (Figure 3A). VEGF, which is secreted in dermal papilla cells, is involved in hair growth.42 Moreover, VEGF can reduce hair loss by promoting blood circulation and the formation of new blood vessels around HFs. Our analysis showed that irisin increases VEGF expression (Figure 4C). Therefore, it is plausible that the induction of these growth factors by irisin promotes hair growth by regulating the secretion of various cytokines, which in turn, transmit signals through autocrine and paracrine mechanisms.
A limitation of this study is that our findings do not directly support the possibility that exercise positively affects hair health, although they suggest that the exercise-derived myokine, irisin, can positively affect hair growth. Since exercise induces the secretion of several myokines, complex interactions occur. Thus, additional research is needed to confirm whether hair growth is improved through exercise in the mouse model of hair loss and to validate the effects of irisin in mice subjected to exercise.
Cell communication is an essential feature of multicellular organisms. Ligand–receptor binding, which activates specific cell signalling pathways, is a fundamental form of cellular communication and is closely associated with HF growth. By critically regulating reciprocal interactions between hDPCs and epithelial cells, these cell signalling pathways play a crucial role in HF growth regulation.43 Previous studies have reported that αVβ5 integrin acts as an irisin receptor in osteocytes, adipocytes and enterocytes.44, 45 Determining whether αVβ5 integrin is the irisin receptor in HFs would provide important insight into the mechanism through which irisin promotes hair growth.
5 CONCLUSION
The results of this study provides first evidence that irisin robustly stimulates hair growth, as demonstrated in hDPCs, human hair shafts, patch assay and in vivo mouse models. Its effects are attributable to the modulation of mitochondrial membrane potential, new hair induction and Wnt/β-catenin signalling activation. The findings from this study offer insight into the positive impact of myokines on hair growth. However, further research, using exercise models, is required to determine the overall effects of myokines on hair growth.
AUTHOR CONTRIBUTIONS
Yu-jin Kim: methodology, data curation, investigation, formal analysis and writing. Jung Min Lee: investigation and data curation. You Na Jang: investigation and data curation. A Yeon Park: investigation and data curation. Su-Young Kim: investigation and data curation. Beom Joon Kim: conceptualization and project administration. Jung Ok Lee: writing, reviewing, editing and supervision.
ACKNOWLEDGEMENTS
This research was supported by the Chung-Ang University Research Scholarship Grants in 2023.
FUNDING INFORMATION
This work was supported by the National Research Foundation of Korea through a grant funded by the Korean government (MSIT) (Grant No: NRF-2019R1A2C1005916).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing financial interests or personal relationships that could appear to influence the work reported in this paper.
Open Research
DATA AVAILABILITY STATEMENT
All data generated and analysed in this study are included in this article. Further inquiries can be directed to the corresponding authors.