Dynamic optical coherence tomography unveils subclinical, vascular differences across actinic keratosis grades I–III
Abstract
Actinic keratosis (AK) classification relies on clinical characteristics limited to the skin's surface. Incorporating sub-surface evaluation may improve the link between clinical classification and the underlying pathology. We aimed to apply dynamic optical coherence tomography (D-OCT) to characterize microvessels in AK I-III and photodamaged (PD) skin, thereby exploring its utility in enhancing clinical and dermatoscopic AK evaluation. This explorative study assessed AK I-III and PD on face or scalp. AK were graded according to the Olsen scheme before assessment with dermatoscopy and D-OCT. On D-OCT, vessel shapes, −pattern and -direction were qualitatively evaluated at predefined depths, while density and diameter were quantified. D-OCT's ability to differentiate between AK grades was compared with dermatoscopy. Forty-seven patients with AK I-III (n = 207) and PD (n = 87) were included. Qualitative D-OCT evaluation revealed vascular differences between AK grades and PD, particularly at a depth of 300 μm. The arrangement of vessel shapes around follicles differentiated AK II from PD (OR = 4.75, p < 0.001). Vessel patterns varied among AK grades and PD, showing structured patterns in AK I and PD, non-specific in AK II (OR = 2.16,p = 0.03) and mottled in AK III (OR = 29.94, p < 0.001). Vessel direction changed in AK II-III, with central vessel accentuation and radiating vessels appearing most frequently in AK III. Quantified vessel density was higher in AK I-II than PD (p ≤ 0.025), whereas diameter remained constant. D-OCT combined with dermatoscopy enabled precise differentiation of AK III versus AK I (AUC = 0.908) and II (AUC = 0.833). The qualitative and quantitative evaluation of vessels on D-OCT consistently showed increased vascularization and vessel disorganization in AK lesions of higher grades.
1 INTRODUCTION
Actinic keratoses (AK) predominantly occur as multiple lesions in field-cancerized skin, characterized histologically by dysplasia of epidermal keratinocytes.1-3 Caused primarily by repeated, long-term exposure to ultraviolet radiation (UV), AK is usually located in sun-exposed areas.1, 3 Without treatment, the disease often follows a chronic course, but individual lesions can undergo spontaneous regression or progress to cutaneous squamous cell carcinoma (SCC).1 Prior studies have demonstrated a low risk of progression to SCC, with estimates below 0.53% per AK per year in immunocompetent patients.4-6 However, accurately predicting which AKs will undergo this change is difficult, since existing clinical and histological classification systems carry no predictive information on the disease course.1, 7, 8
Although several clinical grading scales for AK have been proposed, there is no gold-standard in clinical practice to guide treatment strategy.1, 2, 9-11 Currently, the most widely used grading scale is the three-step Olsen classification scheme, which categorizes individual AK lesions according to their clinical thickness.12 A main limitation of classifying AK using this scheme is its poor correlation with histological grading.9, 13 Moreover, the scheme's subjective assessment demonstrates low reproducibility, impacting accuracy and reliability in clinical trials as well as in treatment decision-making.9, 13
Dermatoscopic examination, although beneficial for diagnosing AK, is constrained to superficial skin layers and relies on surface features.3, 14-16 The limitations in visualizing deeper parts of the skin, in particular assessing features obscured by thick hyperkeratosis, impedes comprehensive assessment across clinical AK grades.3, 14-16 Addressing these limitations, incorporating non-invasive imaging techniques to study additional features not sufficiently appreciated by Olsen grading or dermatoscopy of AK holds potential to improve the link between clinical grading and disease severity.
In recent years, development of dynamic optical coherence tomography angiography (D-OCT) has added information on cutaneous blood vessels to the structural features seen in tomographic OCT images.17, 18 D-OCT allows qualitative evaluation of cutaneous blood vessels and quantification of vessel density and diameter. Compared to dermatoscopy, D-OCT offers a deeper view of the skin microvasculature and provides both cross-sectional and en-face images.18 Previous studies using either dermatoscopy or D-OCT have identified vascular characteristics that correlate with disease progression in AK transforming into in-situ and invasive SCC.19-21 Additional studies have successfully employed D-OCT to differentiate nevi from melanoma, identify vascular differences between melanoma stages, distinguish melanoma of varying Breslow thicknesses and differentiate between basal cell carcinoma subtypes among other applications in dermatology.22-25 Building upon previous findings, it is plausible that assessing vascular features within AK grades could advance our understanding of the disease and varying disease course. Until now, no studies have correlated blood vessel characteristics on D-OCT with different AK grades. In efforts to improve assessment of AK, this study aimed to apply D-OCT for characterization of microvessels in AK grades I–III and PD skin, thereby exploring its utility in enhancing clinical and dermatoscopic AK evaluation.
2 MATERIALS AND METHODS
2.1 Design and ethical considerations
The study was a two-center explorative clinical trial evaluating D-OCT scans of AKs and surrounding non-lesional PD skin at the Department of Dermatology, Bispebjerg University Hospital in Copenhagen and Skin Center Mølholm, Mølholm Private Hospital in Vejle, Denmark. The study was conducted from October 2021–September 2022 as part of a larger investigation of AK patients and was approved by the Ethics Committee of Region Hovedstaden (case no. 78842) and registered in EudraCT (2021–0015860-21). The study was monitored by the unit for Good Clinical Practice and conducted in accordance with the Declaration of Helsinki. All patients signed an informed consent prior to study initiation.
2.2 Study set-up
Inclusion criteria were patients aged ≥18 years with clinically accessible AKs on the face or scalp. The study's exclusion criteria were any skin conditions other than AK within the test areas or any AK treatment up to 3 months before inclusion.
Upon inclusion, individual AK lesions were marked and numbered on a transparent film before assessment. In the clinical evaluation, AKs were graded by the same board-certified dermatologist (S.W.) according to the Olsen Classification Scheme from I (slightly palpable, better felt than seen), II (moderately thick, easily felt and seen) and III (thick, hyperkeratotic).10, 12 At least one AK of each grade I-III as well as adjacent PD skin in the same treatment site were selected for evaluation with dermatoscopy and D-OCT. Digital dermatoscopic images were obtained using a handheld dermatoscope (FotoFinder Handyscope®, Fotofinder Systems GmbH, Bad Birnbach, Germany) attached to a smartphone (Samsung® Galaxy S21, 5G, Samsung Electronics GmbH, Schwalbach, Hesse, Germany). Dermatoscopic images were retrospectively assessed (G.F.) for the presence of previously established features of facial AKs: scales, erythema, targetoid hair follicles, linear/wavy vessels, dotted or coiled vessels and the presence of the strawberry pattern.26, 27
2.3 Dynamic OCT—image acquisition
The D-OCT scans were acquired with a commercially available D-OCT scanner (Vivosight Dx, Michelson Diagnostics, Kent, UK) with a center wavelength of 1305 nm, lateral resolution of <7.5 μm, axial resolution of <5 μm and a field-of-view of 6 × 6 mm.17, 18 The Vivosight D-OCT scanner captures structural OCT images of the skin up to a depth of 1.5 mm. D-OCT images are generated automatically using the in-built Vivosight software. Individual en-face images of the vasculature at each depth are displayed overlayed on the structural en-face images. Vessel visualization is limited to ≤500 μm depth due to the interference of speckle and low signal to noise ratio in deeper parts of the skin.18, 28, 29 In our study, volume scans were captured with 250 B scans (in the x-z plane) and presented in the en-face view. The “fitted-en-face” tool in the in-built software detects and follows the curved skin surface, enabling presentation of en-face images at exact depths below the skin surface regardless of skin texture.
2.4 Dynamic OCT—qualitative microvascular analysis
D-OCT-based assessment of AK vasculature consisted of both a qualitative and quantitative analysis.
As previously reported, three predefined skin depths were initially selected for analysis: 150, 300 and 500 μm.19, 25, 28, 30 At 150- μm depth, D-OCT visualizes the capillaries in the dermal papilla, which are oriented perpendicular to the skin surface and appear as sharply delimited round vascular structures.28 The 300- and 500- μm depths provide a horizontal view of the superficial and deep dermal plexuses, respectively. In our study, projection artefacts and background interference of the D-OCT signal made identification of vessel structures challenging at 500- μm skin depth. Therefore, assessment of this skin layer was excluded.
To qualitatively evaluate vessels in D-OCT scans, our study used an adapted version of the guideline proposed by Ulrich et al. (Table 1).28 The terminology from the guideline was applied to describe different vessel shapes, patterns and directions in en-face images. In our study, the guideline was simplified by fusing subcategories of vessel shapes or patterns that had similar appearances.
| Terminology | Definition | Orientation (relative to skin surface) |
|---|---|---|
| Vessel Shapes | Each subcategory rated as preset if ≥3 vessels of same shape in a single en-face image. | |
| Dots | Small pin-points vessels to larger oval globules or spiral-like convoluted circles | Perpendicular |
| Linear | A vessel structure with length exceeding the width. Forms can be straight, curved or serpiginous | Horizontal |
| Branching | Linear vessels with branches that divides from a central main stem | Horizontal |
| Vessel Patterns | Determined by the predominant type of vascular network present in ≥4/5 of the en-face image | |
| Branched | Vessels connected in a reticular or branching pattern | Horizontal |
| Non-specific | A disorganized, polymorphous pattern with varying vessel shapes | Horizontal |
| Mottled | A pattern mainly consisting of perpendicular vessels | Perpendicular |
| Vessel Direction | Vessels orientation across the entire en-face image | |
| No direction | No specific orientation of vessels | % |
| Parallell | Vessels streaming parallell to each other | Horizontal |
| Radiating | Vessels with radial orientation towards the lesion center | Horizontal |
Using the adapted guideline, one unblinded investigator (G.F.) manually evaluated the complete set of en-face images. The analysis was performed in two steps. The first step was to evaluate the shape of individual vessels based on three predefined subcategories (dots, linear, branching; Table 1). Each subcategory was rated as present if ≥3 vessels of same shape appeared in the evaluated en-face image. All vessel shape subcategories could be present in a single image. Any particular arrangement of vessel shapes was also noted in the first step of the qualitative analysis.
The second step included a global analysis of vascular plexus architecture, based on the predominant vascular pattern and vessel direction across the entire en-face image. Vessel pattern was determined by the predominant pattern present in at least 4/5 of each en-face image, selected from three predefined subcategories (branched, non-specific, mottled; Table 1). Finally, analysis of vessel direction depended whether vessels displayed a specific direction, based on three predefined subcategories (no direction, parallel, radiating; Table 1). Each image could be classified as only one of the subcategories for vessel pattern and vessel direction.
2.5 Dynamic OCT—quantitative microvascular analysis
To quantitatively evaluate vessels in D-OCT scans, measurement of vessel density and diameter was performed using the in-built software at 150- and 300- μm depths. These measurements were performed in all AK grades I-III and compared to PD skin.
2.6 Statistics
For descriptive statistics, absolute and relative frequencies were calculated for categorical data, while mean and standard deviation (SD) were calculated for nominal data. To evaluate the association between the qualitatively evaluated vessel features with each of the different AK grades I-III and PD, univariate logistic regression was performed. Odds ratios (OR) with 95% confidence intervals (95% CI) and corresponding p-values were calculated, representing the effect of each vascular feature on the likelihood of a specific lesion type. Subsequently, multivariate regression with backward elimination was performed to identify the main distinguishing, qualitatively evaluated vessel parameters. The performance of D-OCT and dermatoscopy in differentiating between the different AK grades was expressed as area under the receiver operating characteristics curve (AUC). The PD skin represented the reference category in assessments of vascular differences between the different AK grades and PD skin. In the comparison across AK grades, the lowest grade on the Olsen Classification scheme was used as a reference.
Independent sample t-tests were used to compare vessel density and diameter values between each lesion type, as well as associations with qualitatively evaluated vessel features in each AK grade and PD skin. For all tests, p-values were two-sided, exact and considered statistically significant when <0.05. Statistical analyses were performed using SPSS Statistics software (Version 25; IBM Corp., Armonk, New York, USA).
3 RESULTS
3.1 Study population
A total of forty-seven participants (40 men and 7 women) with Fitzpatrick skin type I–III were included in this study. For the analysis, 207 AKs located on the face (n = 166) and scalp (n = 41) with clinical grades I (n = 93), II (n = 65) or III (n = 49) as well as PD skin (n = 87) were assessed (Table 2).
| Patients (Male:Female) | 47 (40:7) | ||||
|---|---|---|---|---|---|
| Number of Lesions n | PD = 87 | AK I = 93 | AK II = 65 | AK III = 49 | Total (n) |
| Location n | |||||
| Face | 67 | 72 | 50 | 44 | 233 |
| Scalp | 20 | 21 | 15 | 5 | 61 |
| D-OCT scans n | |||||
| 150 μm | 87 | 93 | 65 | 49 | 294 |
| 300 μm | 87 | 93 | 65 | 49 | 294 |
| 500 μm | 27 | 15 | 5 | 2 | 49 |
| Dermatoscopic images n | |||||
| 72 | 82 | 58 | 38 | 250 | |
- Abbreviations: AK, actinic keratosis; PD, photodamaged skin.
3.2 Qualitative vessel evaluation in D-OCT scans
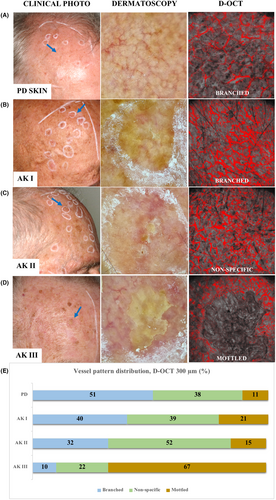
Qualitative vessel evaluation in D-OCT scans revealed subclinical vascular differences between the various AK grades and PD skin (Figure 1). At a depth of 150 μm, the en-face images mostly depicted the perpendicularly oriented vessels of the dermal papilla.28 However, the specific arrangement of these vessels allowed for differentiation between AK and PD skin. At 300-μm depth, D-OCT visualized distinct vessel patterns and directions among AK I-III as well as when compared to the adjacent PD skin (Table S1). A detailed description of our key-findings from the qualitative evaluation is provided below.
3.3 Vessel shapes
Vessels of dotted, linear and branching shapes appeared in both AK I-III and PD (Table 3). Dots were present in all D-OCT scans from 150- and 300- μm depths (Table S1). Linear and branching vessels also figured in AK I-II at 150 μm depths but were more common in the 300 μm scans. At this depth, linear and branching vessels appeared more frequently in AK I-II and PD than AK III, possibly due to thick hyperkeratosis of AK III which often obscured the visualization of these horizontally oriented shapes (Table S1).
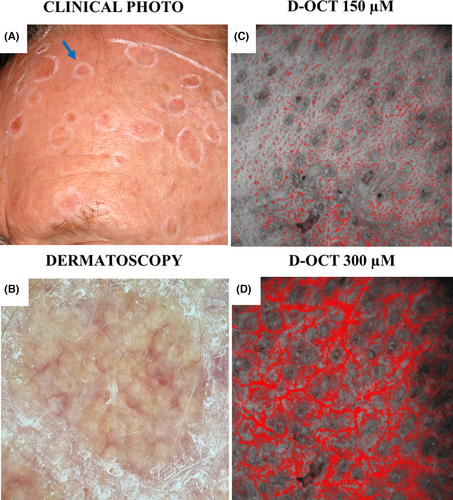
Of note, at both depths, a particular arrangement of either dotted (150 μm) or branching vessels (300 μm) around follicular openings appeared in a subset of AK I and II (Figure 2). The presence of these perifollicular vessels differentiated AK I (17%; 17/93) (OR 3.41, 95% CI 1.19–9.75, p = 0.022) from PD at 150- μm depth and AK II (150: 22%; 14/65, 300: 35%; 23/65) from PD at both depths (150 μm; OR 4.50, 95% CI 1.53–13.25, p = 0.006 and 300 μm; OR 4.75, 95% CI 2.01–11.18, p < 0.001) (Table S2).
3.4 Vessel pattern
The D-OCT evaluation showed significant vessel pattern alterations in AK II-III compared to PD skin at 300-μm depth (Figure 1). A branched pattern was characteristic of PD skin (51%; 44/87) when compared with all AK grades combined (OR 0.23, 95% CI 0.11–0.49, p < 0.001). AK I lesions resembled PD skin, presenting most often with a branched pattern (40%; 37/93). In contrast, the predominant vessel pattern changed to a non-specific pattern in AK II (Figure 1). As such, this pattern appeared in most of AK II (52%; 34/65) and was significantly associated with this lesion grade compared with PD skin (OR 2.16, 95% CI 1.07–4.38, p = 0.033). A mottled pattern predominated in AK III (67%; 33/49), presenting significantly more often in these lesions than in AK I (OR 12.21, 95% CI 4.12–36.19, p < 0.001), AK II (OR 13.86, 95% CI 4.15–46.24, p < 0.001) and PD skin (OR 29.04, 95% CI 9.06–93.06, p < 0.001) (Table S2).
3.5 Vessel direction
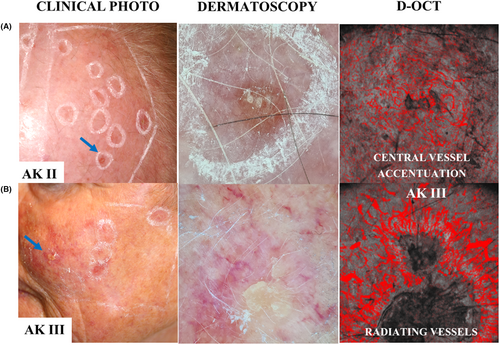
While vessels had no specific direction in PD skin, D-OCT visualized an alteration of the vessel's direction in AKs. In AKs, there was an apparent increase in number of vessels towards the hyperkeratotic center, termed a “central vessel accentuation” in our study (Figure 3). A central vessel accentuation frequently occurred in combination with a radiating vessel direction, with both features presenting more frequently in AKs of increasing grade. Thus, a central vessel accentuation was identifiable in some AK I (31%; 29/93) but occurred significantly more often in AK II (48%; 31/65) (OR 2.01, 95% CI 1.05–3.88, p = 0.036) (Table S2). In AK III, this feature appeared in most lesions (67%; 33/49), presenting with a significantly greater frequency than in AK II (OR 2.26, 95% CI 1.05–4.89, p = 0.038) and AK I (OR 4.55, 95% CI 2.17–9.55, p < 0.001). Correspondingly, radiating vessels were more often associated with AK III (35%; 17/49) compared with AK II (15%; 10/65) (OR 2.92, 95% CI 1.20–7.15, p = 0.019) and AK I (2%; 2/93) (AK III vs. I; OR 24.17, 95% CI 5.29–110.47, p < 0.001, AK II vs I; OR 8.27, 95% CI 1.75–39.16, p = 0.008).
3.6 D-OCT-based quantification of vessel density and diameter
Quantified vessel density was similar in AK I and II at both analysed skin depths (p ≥ 0.293), but higher than in PD skin (150 μm; p ≤ 0.025, 300 μm; p ≤ 0.020) (Table S1). In contrast, the quantification of vessel diameter showed no difference between groups at any skin depth (p ≥ 0.125).
Although qualitative evaluation revealed a trend of vessel disorganization and an apparent increase in vessel density towards the lesion center in AK III, thick hyperkeratosis commonly limited vessel quantification. Therefore, the quantified measurements from AK III could not be compared across AK grades. All quantified measurements are outlined in Table S1.
3.7 Vascular features on dermatoscopy
Assessment of dermatoscopic features in AK I-III and PD skin is presented in Figure 1 and Tables S1 and S3.
A predominant feature on dermatoscopy of PD was the presence of linear-wavy vessels, observed in the majority of dermatoscopic images (81%; 58/72). In contrast, erythema and a strawberry pattern were the most characteristic features of AK. Notably, the prevalence of erythema increased in AKs of increasing grade, appearing significantly more often in AK I (46%; 38/82), II (76%; 44/58), III (82%; 31/38) than in PD skin (24%; 17/72) (Table S2). The strawberry pattern figured across all AK grades and PD skin, though its prevalence varied significantly, being most common in AK I (60%; 49/82), followed by AK II (40%; 23/58), AK III (37%; 14/38) and PD (29%; 21/72) (Table S1 & Table S3).
3.8 Utility of D-OCT in enhancing clinical and dermatoscopic AK evaluation
Overall, D-OCT proved helpful in differentiating between AK I-II and AK III. While the use of dermatoscopy alone showed low performance in distinguishing between AK grades, D-OCT evaluation was beneficial, either when applied alone or in combination with dermatoscopy. The main distinguishing features are presented in Table S4.
The combination of D-OCT and dermatoscopy enabled a precise differentiation between AK I versus AK III (AUC = 0.939) (Figure 4). Applying D-OCT alone was also effective in the differentiation between these two grades (AUC = 0.908). In comparison, relying solely on dermatoscopic examination showed low accuracy (AUC = 0.733).
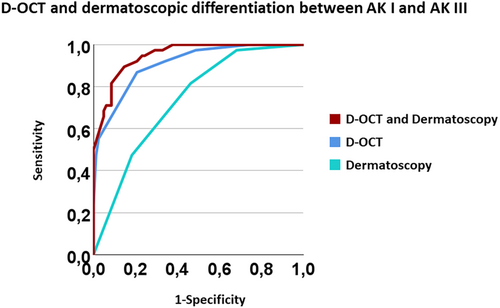
While the combination of D-OCT with dermatoscopy demonstrated low discriminative ability between AK II and III(AUC = 0.598), the use of D-OCT alone was valuable in the differentiation between these two AK grades (AUC 0.833). Dermatoscopic examination alone did not allow differentiation between AK II and III.
Combining D-OCT with dermatoscopic evaluation provided little benefit in the differentiation between any AK grade and PD, or between AK I-II.
4 DISCUSSION
By applying D-OCT to characterize microvessels in AK I-III and adjacent PD skin on face or scalp, our study identified subclinical differences across AK grades I-III and PD skin. Both the qualitative and quantitative analyses consistently showed increased vascularization and vessel disorganization in AK lesions of higher grades. This vascular progression seen across AK grades underscores the substantial role of vessels in the growth of lesions.21, 31, 32 In addition, our results demonstrate the potential benefit of incorporating D-OCT in clinical classification and dermatoscopic evaluation.
Although the three-step Olsen classification scheme appears to imply a stepwise, fixed progression of AK, this scheme primarily relies on clinically assessed thickness with no correlation to underlying histopathology or the risk of progression.13 By incorporating sub-surface evaluation of AK with D-OCT, we were able to characterize the different clinical AK grades in more detail. Most notably, we observed a global change in the microvasculature across AK I-III. The AK I resembled PD skin, mainly presenting a structured, branched pattern (Figure 1). However, despite this similarity, our quantitative measurements revealed higher vessel density in both AK I and II than in PD skin, consistent with findings from prior D-OCT studies on AK microvasculature.18, 19 Moreover, specific features were distinctive for certain AK grades, such as the perifollicular vessels in AK I-II, varying vascular patterns across all AK grades and an altered vessel direction in AK II-III. In comparison to dermatoscopy, D-OCT provided a more detailed differentiation between AK grades. In particular, D-OCT enhanced the differentiation between AK I-II from AK III. These findings indicate that the integration of D-OCT into AK management may offer a more nuanced diagnostic workup.
The identification of specific vessel characteristics within a subset of AK suggests pathological changes not captured by clinical AK grading alone. Notably, perifollicular vessel growth seen in a subset of AK I-II on D-OCT resembles the dotted vessels surrounding hair follicles seen on dermatoscopy of AK with increased atypia21, 33 (Figure 2). Previous evidence suggests that follicular involvement of atypical keratinocytes may be associated with treatment failure or recurrence following topical treatment.34, 35 The potential link between follicular atypia on histology and perifollicular vessels on D-OCT needs further investigations, as this may have implications for the therapeutic approach.
Additional vessel characteristics observed in AK of various grades include a central vessel accentuation and radiating vessels (Figure 3). Dermatoscopic studies have previously linked the presence of these features with progression and growth of AK.21 Interestingly, D-OCT revealed increased vascularization and radiating vessels in a subset of AK II-III. This observation suggests a potential association between the pronounced vascular changes in these thicker AK grades and their growth. Such an association could offer insights into the difficulties encountered in treating AK II-III compared to the thinner AK I lesions.36-38 Identification of these subclinical, vascular differences underscores the benefit of D-OCT's deeper visualization. The ability to visualize sub-surface features is crucial, as subtle nuances may not be readily apparent on the skin's surface.
Imaging the skin is associated with various potential confounders, with skin anatomy being a notable contributing factor. Differences in vascular patterns seen on dermatoscopy and D-OCT can, in part, be explained by variations in skin's anatomy. Additionally, the thickness of a skin lesion influences the vascular visualization, as vessels come into view at different depths in the en-face images.17 On dermatoscopy, vessels located immediately underneath the epidermis appear bright red and well-defined, whereas those in the superficial dermis are pink and indistinct.39 In comparison, D-OCT enables visualization of deeper vessels and smaller capillaries (≥20 μm) and provides associated depth measurements. In our study, qualitative D-OCT evaluation revealed a subclinical distinction between AK grades, particularly useful for differentiating AK I-II from the thick AK III. In contrast, D-OCT provided little benefit in the differentiation between AK and PD skin as well as between thin AK I-II lesions. According to these findings, a combined approach, using D-OCT and conventional dermatoscopy, should be integrated to achieve optimal AK assessment.
In comparison to qualitative D-OCT evaluations, our quantified measurements revealed a significantly higher vessel density in both AK I and II compared to PD, highlighting the importance of quantitative assessments in distinguishing AK from the surrounding field-cancerized skin. For future research, overcoming the challenge of imaging through hyperkeratosis remains crucial, as this limitation prevented us from acquiring reliable quantified measurements in AK III.
Strengths of this study include the large sample size, differentiation between AK grades in the in-vivo D-OCT vessel assessment and comparison to PD skin. The study employed a systematic qualitative image analysis following a terminology consensus, but the unblinded single-assessor evaluation of vessels remains a main limitation. We deem the limitation acceptable considering the study's explorative aim of determining D-OCT's utility in detecting distinct vascular features in AKs I-III and PD skin. Another limitation was the use of the observer-dependent clinical AK grading. Application of this grading scheme may partly explain why some AK grades demonstrate overlapping features. A more reproducible grading system that also accurately assesses underlying histology, would provide a more powerful comparison with D-OCT. Vessel evaluation at D-OCT's maximum penetration depth of 500 μm was excluded from our study due to projection artefacts and background interference of the D-OCT signal. Although projection artefacts arise at all skin depths, they are most prominent in deeper parts of the skin.24 Overcoming the limit of projection artefacts is therefore an important subject for future studies.
4.1 Conclusion
Through qualitative vessel evaluation and quantification of vessel density and diameter, D-OCT enabled in-vivo characterization of microvascular differences between AK I-III and PD skin. Both qualitative and quantitative assessments consistently revealed increased vascularization and vessel disorganization in AK lesions of higher grades. The incorporation of D-OCT into the clinical and dermatoscopic evaluation of AK may assist in diagnosis and holds potential to optimize management strategies.
AUTHOR CONTRIBUTIONS
Study conception and design: MH, SW. Acquisition of data: GF, SW, FA. Analysis and interpretation of data: GF, CSF, EW, GU, PP. Drafting of manuscript: GF. Critical revision: CSF, EW, GU, PP, SW, FA, PB, MH.
ACKNOWLEDGEMENTS
The work was completed within the framework of the Skin Cancer Innovation clinical academic group (SCIN-CAG)/Greater Copenhagen Health Science Partners (GCHSP) and the Danish Research Center for Skin Cancer, a public-private research partnership between the Private Hospital Mølholm, Aalborg University Hospital and Copenhagen University Hospital, Bispebjerg and Frederiksberg. The authors would like to thank Jon Holmes of Michelson Diagnostics for expert discussions.
CONFLICT OF INTEREST STATEMENT
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.