Redefine photoprotection: Sun protection beyond sunburn
Abstract
Excessive exposure to ultraviolet (UV) light leads to acute and chronic UV damage and is the main risk factor for the development of skin cancer. In most countries with western lifestyle, the topical application of sunscreens on UV-exposed skin areas is by far the most frequently used preventive measure against sunburn. Further than preventing sunburns, increasing numbers of consumers are appreciating sunscreens with a medium- to high-level sun protective factor (SPF) as basis for sustainable-skin ageing or skin cancer prevention programs. However, recent investigations indicate that clinically significant DNA damages as well as a lasting impairment of cutaneous immunosurveillance already occur far below the standard of one minimal erythema dose (MED) sunburn level, which contributes to the current discussion of the clinical value of high-protective SPF values. Ex vivo investigations on human skin showed that the application of SPF30 reduces DNA damage for a day long sun exposure (24 MED) drastically by about 53% but is significantly surpassed by SPF100 reducing DNA damage by approx. 73%. Further analysis on different SPF protection levels in UV-exposed cell culture assays focusing on IL-18, cell vitality and cis/trans-urocanic acid support these findings. Whereas SPF30 and SPF50+ sunscreens already offer a solid UVB cover for most indications, our results indicate that SPF100 provides significant additional protection against mutagenic (non-apoptotic-) DNA damage and functional impairment of the cutaneous immunosurveillance and therefore qualifies as an optimized sunscreen for specifically vulnerable patient groups such as immunosuppressed patients, or skin cancer patients.
1 INTRODUCTION
Solar ultraviolet (UV) radiation is linked to a specific spectrum of well known, immediate and chronic skin diseases. However, the main reason for dermatologists like Paul Gerson Unna, pharmacists like Eugene Schueller and chemists like Franz Greiter to develop photoprotective agents during the first decades of the last century was to create sufficient protection against cosmetically disturbing and painful sunburns.1, 2 Establishing the skin's mean erythematous reaction as the clinically visible correlate for UV-related damage in 1946, Greiter invented the sun protection factor (SPF) which, following Australia's acceptance of SPF as ‘the ratio of UV energy needed to produce a minimal erythemal dose’, became the global standard in sunscreen formulations until today.3, 4 Following ever increasing de-novo incidence rates of malignant melanoma and non-melanoma skin cancer, however, the dermatological core claim emphasizing the use of sunscreen nowadays is to prevent the induction and promotion of skin malignancies.5, 6 Despite established concepts emphasizing UV-induced erythema as important clinical marker for skin cancer risk, and consequently identifying people with a history of repeated erythematous exposures to sunlight as being prone to an increased risk to developing both melanoma and non-melanoma skin cancers.7 The incidence of skin cancer in Caucasian populations worldwide continues to increase despite increased use of sunscreens. Recent studies have lent strong evidence to the fact that the two pillars of skin carcinogenesis, significant DNA damage and UV-induced immune suppression, also occur with cumulative suberythemal UV exposure and that whilst commercially available sunscreen products may protect keratinocytes from lethal damage and apoptosis, they provide only insufficient protection against UV-induced impairment of the cutaneous8, 9 immune-surveillance.10-12 Contact hypersensitivity has been discussed by Yoshikawa et al.,13 and the side effects of UV-based phototherapy have been pointed out by Vieyra-Garcia et al.14
Consequently, data linking the SPF of a sunscreen with a specific protection against sub-apoptotic DNA-damage and photo-induced immune suppression are missing15 and efforts to establish a broadly accepted method of immune protection factor determination allowing direct comparison with the SPF so far failed.16 Recent advances in sunscreen galenic and filter technology allow the design of a new generation of ultra-high protective sunscreen formulations which are aiming to provide sufficient protection against suberythemal skin damage and impairment of the cutaneous immunosurveillance following ‘the higher, the better’ logic. Interestingly, recent clinical trials showed a comparably greater efficacy of an SPF100 versus an SPF50+ sunscreen in its classical domain of sunburn prevention, indicating that high-SPF sunscreens may provide more adequate protection even when applied by consumers at inadequate amounts as this has been shown by our group before.17, 18 Furthermore, broad spectrum sunscreens with high-SPF and corresponding UVA-PF also showed proportionately high protection against multiple cellular and immunological damage markers, such as thymidine dimers, p53, matric metalloproteinases and Langerhans cell depletion and migration.19
The following study aims to evaluate the impact of a modern, broad spectrum UVA and SPF100 sunscreen versus an established SPF50+ and SPF30 product in day long artificial MED exposition to show that the use of high protection for prolonged exposure to the sun is reasonable, particularly for vulnerable groups, to exclude longer-term damage.
To evaluate the impact of SPF100, the parameters IL-18 and cell vitality were determined in cell culture with and without sun protection to determine inflammation effects and direct cell death. Additionally, cis urocanic acid (UCA) and DNA damage were investigated for immunosuppression and as typical representatives of photodamage dependent on the applied SPF in excised human skin (Figure 1).
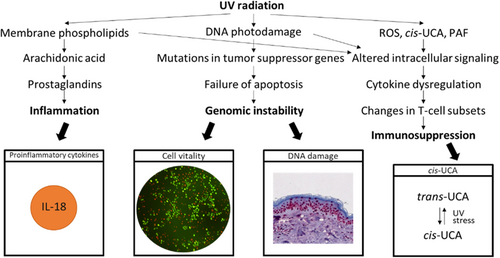
IL-18 was chosen because one of the earliest immune-functional responses made by viable keratinocytes in response to UV damage is the formation of an inflammasome within the cytoplasm. Through Caspase-1–mediated cleavage interleukin 1β (IL-1β) and interleukin 18 (IL-18) are processed into their secreted active forms. These secreted cytokines induce autocrine and juxtacrine signals of the skin damage status throughout the adjacent epidermal and dermal layers.20, 21
Unprotected exposure to UVA and UVB damages skin cells to various degrees. Cells induce protective mechanisms in response to UV exposure, but when the burden of exposure and damage becomes excessive, a programmed cell death is initiated to eliminate mutant cells from the epidermis. Sunburn cells are keratinocytes undergoing apoptosis after they have received a physiological UVB dose that irreversibly and severely damaged their DNA or other chromophores. If these cells would escape programmed cell death, a cancer prone phenotype could arise.22, 23 Thus, vitality/viability of skin cells in cell culture was first targeted, and for more sensitive evaluation, cyclobutane pyrimidine dimers (CPD+) and pyrimidine (6–4) pyrimidone photoproducts (6-4PP) were analysed in excised skin as typical representatives of photodamage.
Next to DNA damage trans-urocanic acid (UCA) has been recognized as a photoreceptor. Trans-UCA is normally found in the outermost layer of skin and isomerizes to the cis isomer upon exposure to UV radiation.24, 25 This isomerization to cis-UCA is one of the earliest events in UV-induced immunosuppression.26
2 MATERIALS AND METHODS
2.1 Test formulations
Three different sunscreen products from Eucerin® (Beiersdorf AG, Hamburg, Germany) were included in the experiments: Medical Device sunscreen Eucerin® Sun Actinic Control MD SPF100 (in vivo measured SPF120.4), Eucerin® Sun Face Cream SPF50+ (in vivo measured SPF71.1) and Eucerin® Sun Face Cream SPF 30 (in vivo measured SPF31.0).
- SPF100 formula: Aqua, Alcohol Denat., Butylene Glycol Dicaprylate/Dicaprate, Butyl Methoxydibenzoylmethane, Ethylhexyl Salicylate, Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine, Diethylamino Hydroxybenzoyl Hexyl Benzoate, Dibutyl Adipate, Distarch Phosphate, Ethylhexyl Triazone, Homosalate, C12-15 Alkyl Benzoate, Phenylbenzimidazole Sulfonic Acid, Titanium Dioxide (nano), Silica Dimethyl Silylate, Glyceryl Stearate, Hydrogenated Rapeseed Oil, Stearyl Alcohol, Glycerin, Xanthan Gum, Sodium Stearoyl Glutamate, Silica, Chondrus Crispus Extract, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Dimethicone, Sodium Chloride, Phenoxyethanol, 1,2-Hexanediol, Pentylene Glycol, Sodium Hydroxide, EDTA.
- SPF50+ formula: Aqua, Homosalate, Polymethylsilsesquioxane, Butyl Methoxydibenzoylmethane, Ethylhexyl Salicylate, Octocrylene, Alcohol Denat., Tapioca Starch, Phenylbenzimidazole Sulfonic Acid, Cyclomethicone, Behenyl Alcohol, Cetearyl Alcohol, Methylpropanediol, Silica Dimethyl Silylate, Glycerin, Sodium Stearoyl Glutamate, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Carbomer, Xanthan Gum, Sodium Hydroxide, Sodium Chloride, Trisodium EDTA, Ethylhexylglycerin, Phenoxyethanol.
- SPF30 formula: Aqua, Glycerin, C12-15 Alkyl Benzoate, Alcohol Denat., Butyl Methoxydibenzoylmethane, Butylene Glycol Dicaprylate/Dicaprate, Ethylhexyl Salicylate, Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine, Cetearyl Alcohol, Dibutyl Adipate, Ethylhexyl Triazone, Phenylbenzimidazole Sulfonic Acid, Tapioca Starch, Dimethicone, Glyceryl Stearate, Phenoxyethanol, Sodium Hydroxide, Ethylhexylglycerin, Sodium Stearoyl Glutamate, Trisodium EDTA, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Carbomer, Xanthan Gum, Sodium Chloride.
2.2 Skin models: Cell culture, skin explants and excised human skin
Primary human keratinocytes were isolated from skin biopsies obtained from Alphenyx (Marseille, France) and isolated as described.27 Subsequently, these were cultured in EpiLife medium (ThermoFisher Scientific, Waltham, MA, USA) supplemented with human keratinocytes growth supplements (HKGS; ThermoFisher Scientific), 1% penicillin/streptomycin, and maintained in standard culture conditions at 37°C and 5% CO2. During UV irradiation, the cell medium was replaced by phenolred-free medium without supplements (keratinocyte basal medium, Sigma Aldrich/Merck KGaA, Darmstadt, Germany).
Ex vivo human skin explants (Genoskin, Toulouse, France) were cultured in 12-well plates with 1 mL of provided medium (Genoskin) for 24 h according to manufacturer's instructions.
Excised human Caucasian skin from plastic surgery was used for histological studies. The investigations were approved by the Ethics Committee of the Charité—Universitätsmedizin Berlin (EA1/324/19). Informed written consent was obtained from the participants and all procedures complied with the Declaration of Helsinki. The skin was used for histological examination within 24 h after plastic surgery.27
2.3 UV irradiation of keratinocytes and skin explants
Poly(methyl methacrylate) (PMMA) plates (WW5, Schönberg, Germany) coated with three different sunscreen products or glycerin as untreated control were prepared (1.3 mg/cm2) acc. to ISO 24443:2012: ‘Determination of sunscreen UVA photoprotection in vitro’. Keratinocytes or skin explants were covered with these PMMA plates and were exposed to either 24 MED (600 mJ/cm2) or 0.25 MED (6.25 mJ/cm2) solar simulated light (SSR) using an Oriel-1600 W (Newport Corporation, Stratford, CT, USA) (Figure S1). Irradiances were measured using an ILT 1400 Radiometer Photometer with an SEL240 sensor (International Light Technologies, Peabody, MA). Considering that 1 SED is equivalent to an effective erythema radiation exposure of 100 J/m2 in the 250 nm and 400 nm range, and after weighting the dose according to the erythema action spectrum, it was shown that 24 MED from the SSR is equivalent to 42.6 SED and 0.25 MED correspond to 0.4 SED. Unstressed cells/explants (covered with aluminium foil) served as experimental controls. After UV exposure, the medium of keratinocytes was changed to medium with supplements or skin explants were placed in 1 mL fresh medium, respectively, and were incubated for additional 24 h.
2.4 Cell vitality
Twenty-four hours after UV stress, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (MTT; Sigma-Aldrich) was conducted according to manufacturer's instructions, except for leaving the plate for 5 min on a shaker instead of incubating overnight. The data were normalized per donor (n = 14) to untreated and unstressed medium control (=100%).
2.5 IL-18 immunoassay
Twenty-four hours after UV stress, keratinocytes (n = 13) and supernatants were harvested. Cells were lysed using Complete Lysis-M (Roche, Basel, Switzerland) and analysed using a BCA Protein Assay Kit (ThermoFisher Scientific), whilst supernatants were analysed using an IL-18 Proquantum High-Sensitivity Immunoassay Kit (ThermoFisher Scientific). Both kits were conducted according to the manufacturer's instructions, and samples were measured using a microplate reader (Tecan M1000 pro spectrometer, Tecan Group AG, Männedorf, Switzerland) or a 7900HT Fast Real-Time PCR System (Applied Biosystems/ThermoFisher Scientific). IL-18 levels were normalized to total protein content, and the 24 MED stressed positive control was set as 100%.
2.6 Urocanic acid (UCA) determination
Twenty-four hours after UV stress, skin explants (n = 13) were cut in four pieces and shock frozen. For analytical preparations, 1/4 of each skin explant was thawed, weighted and incubated for 4 h in 150 μL 0.1 M potassium hydroxide solution at 32°C (adapted from28). Skin residues were discarded, and the pH of the remaining solution was adjusted with phosphoric acid to a value of 5. Subsequently, the solution was filtered (0.2 μM pore size) and centrifuged (4°C, full speed, ~30 s), and 60 μL was stored at −20°C. For analysis, these samples were thawed and without further processing, cis-UCA and trans-UCA were measured by means of HPLC-MS/MS (Agilent HPLC 1200 series; Agilent Technologies GmbH, Waldbronn, Germany) coupled to a 3200 QTRAP mass spectrometer (AB Sciex, Darmstadt, Germany). Separation was performed on a Hamilton HPLC anion exchange column PRP 100125 × 2 mm, 10 μm (Sigma-Aldrich) with an injection volume of 2 μL and flow rate of 400 μL/min. Cis-UCA (μg/mL) level was normalized to total UCA level (sum of cis- and trans-UCA (μg/mL)). Values below the detection limit of the method were excluded from statistical analysis.
2.7 LIVE/DEAD staining (supplements)
Twenty-four hours after UV stress, keratinocytes (n = 3) were washed with PBS and stained with LIVE/DEAD® Viability/Cytotoxicity Kit (Thermofisher Scientific) containing calcein AM and ethidium homodimer as dyes. The concentrations were used according to the manufacturer's instructions, and the cells were incubated for 30 min in the incubator at 37°C. Fluorescent images of the stained cells were acquired using an EVOS FL microscope (Thermofisher Scientific). The images were overlaid with ImageJ software.
2.8 Analysis of DNA damage after UV irradiation
To assess skin DNA damage, an UVB lamp containing also a small UVA fraction (280–400 nm, 0.041 mW/cm2;TH-1E; Cosmedico Medizintechnik, Stuttgart, Germany) (Figure S1) was applied to induce 0.25, 1 and 24 MED in excised human skin. Irradiances were measured with an ILT 1400 Radiometer Photometer equipped with a SEL240 sensor (International Light Technologies, Peabody, MA). Twenty-four MEDs were chosen because for skin type II (classification based on Fitzpatrick),29 one MED is reached within approximately 20 min and a daily exposure of 8 h would result in 24 MED. To prevent a sunburn, a sunscreen with SPF 30 at minimum was selected. For the UVB lamp, 24 MED corresponds to 39.5 SED, 1 MED to 1.7 SED and 0.25 MED to 0.4 SED.
In total, the following examinations were performed per excised piece of skin: non-irradiated, 0.25 MED (7.5 mJ/cm2), 1 MED (30 mJ/cm2) and 24 MED (720 mJ/cm2). Furthermore, 24 MED applied with various cream formulations (SPF30, SPF50+ and SPF100) was analysed. The cream was applied according to DIN ISO 24444: 2 mg/cm2.
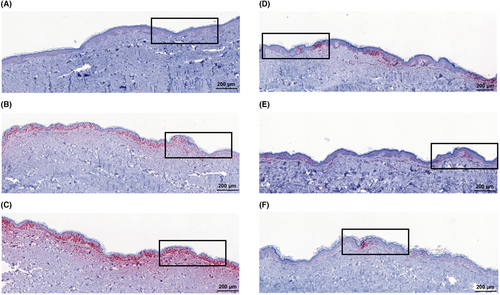
To evaluate the protective effect of the different cream formulations on skin, DNA damage was detected immunohistochemically via expression of cyclobutane pyrimidine dimers (CPD+) and pyrimidine (6–4) pyrimidone photoproducts (6-4PP) on 1–2 μm paraffin sections as previously described.30 Histological images of the stained skin sections were acquired using an AxioImager Z1 microscope (Carl Zeiss MicroImaging, Inc.). The percentage of positively stained cells in the epidermis was evaluated in a blinded manner. For this purpose, three randomly selected areas of 100 cells, each, were counted with respect to positive and negative cells (classical evaluation).
2.9 DNA damage classification algorithm
Because the DNA damage is not homogenous, the analysis of the whole section would be preferable but cannot be done by eye. Thus, a semantic model for the prediction of DNA damage in the epidermis of histological slides stained for CPD+ and 6–4 PP DNA damage was used as previously described elsewhere.31 For data augmentation, the epidermis was additionally masked with a separate segmentation model based on the U-Net/64 convolutional network typically used in biomedical image segmentation. This ensures that only information from the epidermis was considered. For all models, a fourfold cross validation was executed32
The nuclei score (Snuclei) was estimated with a regression model based on a standard VGG16 model followed by a concatenation of global max and mean pooling and one additional ReLU layer followed by one output Sigmoid neuron.33, 34 All images are down sampled by a factor of four. The model was optimized using Adamax (with learning rate 0.001) minimizing binary crossentropy per pixel. The nuclei score model can only be applied on the same areas as used for training the model.
The area score Sarea is calculated as the ratio of the area of the epidermis and the area of semantic segmentation maps of damaged cells in the epidermis. For the segmentation, U-Net was used, where the filter size was not changed and a sliding window approach was chosen. All images are down sampled by a factor of two. U-Net/64 with a base feature dimension of 64 yielded the best results for the application. U-Net was trained for 50 epochs with 100 steps per epoch. The area model can be used for other sizes than trained. This allows the analysis of whole thin sections which is important when the damage occurs non-homogeneously.
2.10 Statistical analysis
Data analysis was performed using the statistical programs: IBM SPSS® Statistics Version 27.0 (IBM; Armonk, New York, USA) or SAS 9.4 for Windows (SAS; Cary, NC, USA). The analysis was based on the Wilcoxon test for dependent samples. A significance level of p ≤ 0.05 was used. A significant trend was described at p ≤ 0.1.
For the parameter cis-UCA, the Wilcoxon's signed rank test, if necessary, with p-value adjustment according to the Bonferroni–Holm procedure was used, and for cell vitality and IL-18, the Shapiro–Wilk test for normal distribution of the data was performed. If the normal distribution hypothesis was rejected, the Blom-transformed ranks of the original data (IL-18) were analysed, otherwise original data (cell vitality). The analysis of variance for repeated measures was applied, if applicable with simulation-based p-value adjustment.
3 RESULTS
Different formulations containing sun protection factors (SPF) of SPF30, SPF50+ and SPF100, respectively, were analysed in cell-based assays with human primary keratinocytes as well as ex vivo skin explants for their ability to protect from UV-induced cell death, inflammation, immunosuppression and DNA damage.
Before simulated solar irradiation, the cultured skin cells and ex vivo skin explants, respectively, were covered with a sunscreen product-coated plate and compared to a glycerin-coated control plate (Figure 2) and a non-irradiated control.
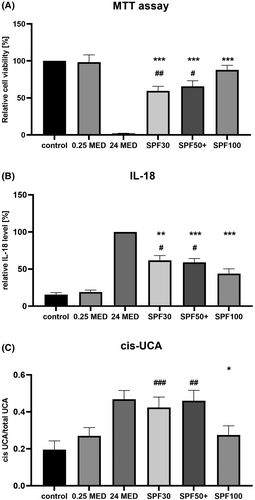
The SPF100 sun product is superior to the SPF50+ and SPF30 sun products in preventing cellular death (Figure 2A, readout cell vitality), (Figure 2B, readout IL-18), and immunosuppression (Figure 2C, readout cis-UCA). Regarding the parameters cell vitality as well as IL-18 also the two sunscreen products with lower SPF show significant protection from the UV-induced changes but to a lesser extent compared to the SPF100 product. This can be visualized by the live/dead cell staining (Figure S2). Significant changes between SPF30 and SPF50+ have not been found. For the parameter cis-UCA, only the SPF100 product significantly prevents formation of cis-UCA after UV stress (p = 0.0351). The SPF50+ product shows a similar trend but didn't reach the significance level of 0.05 (p = 0.0626), whereas the SPF30 product does not prevent cis-UCA formation (p = 0.1484).
Photochemical damage to the genetic material is considered to be an important factor in skin carcinogenesis. A dose-dependent damage of the DNA can be illustrated in Figures 3 and 4, Figures S3 and in S6 for CPD+, and in Figures S4, S5, and S7 and Table S1 for 6–4 PP. For CPD+ and for 6–4 PP, this positive correlation is highly significant compared to non-irradiated skin (Table 1).
| CPD+'s | Methods | 0.25 MED | 1 MED | 24 MED + SPF30 | 24 MED + SPF50+ | 24 MED + SPF100 |
|---|---|---|---|---|---|---|
| Non-irradiated | Classical | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| AI: nuclei | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| AI:area | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 0.25 MED | Classical | <0.001 | <0.001 | <0.001 | <0.001 | |
| AI: nuclei | <0.001 | <0.001 | 0.02 | <0.001 | ||
| AI:area | 0.005 | 0.001 | 0.001 | <0.001 | ||
| 1 MED | Classical | <0.001 | <0.001 | <0.001 | ||
| AI: nuclei | <0.001 | <0.001 | <0.001 | |||
| AI:area | <0.001 | <0.001 | <0.001 | |||
| 24 MED + SPF30 | Classical | 0.394 | 0.1 | |||
| AI: nuclei | 0.334 | 0.016 | ||||
| AI:area | 0.277 | 0.02 | ||||
| 24 MED+ SPF50+ | Classical | 0.171 | ||||
| AI: nuclei | 0.022 | |||||
| AI:area | 0.003 |
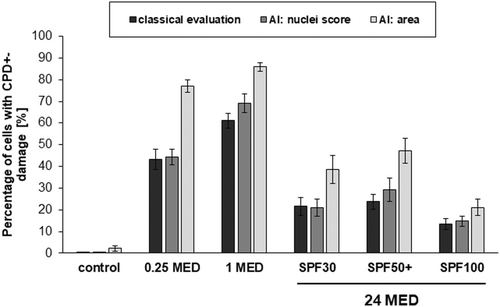
The histological images illustrate that the DNA damage for 0.25 and one MED is homogeneously distributed over the whole epidermal layer. The DNA damage increases non-linearly with increasing dose. The application of a sunscreen decreases the DNA damage of 24 MED below the DNA damage of one MED for all SPFs significantly. Interestingly, the DNA damage is not homogenously distributed in the epidermis when sunscreen is applied. Nevertheless, for all SPF a comparable reduction in CPD+ was observed—no matter which method of readout was applied. The analysis using AI nuclei score resulted in comparable percentages whereby the area score applied on the whole thin section showed higher values. The relative changes with regard to the sum of damage remain comparable (Figure S6). For one MED, the area model presents significantly less CPD+-damage compared to the other methods.
The application of SPF100 compared to SPF30 decreases the CPD+-damage significantly: The reduction when using the conventional analysis method was by ca. 40%, a 54% reduction was shown for machine learning (AI: area). Remarkably, the SPF50+ cream exhibits higher CPD+ values than the SPF30 one, although not significantly. The reduction from SPF50+ to SPF100 is significant for analysis with the machine learning models but not for the analysis by conventional method (Table 1).
The 6–4 PP values are much lower compared to the CPD+-values, but they follow the CPD+ changes. The photoproduct increases for one MED and decreases using sun protection, but no significant differences could be found between the different SPF applications (Figure S4). For 6–4 PP, the relative changes due to the different applications normalized to the sum of the damage (Figure S4) are very comparable regarding the different analysis methods. Only one significant difference at SPF50+ application between the classical readout and the area mode appears.
4 DISCUSSION
The results show that the application of a sunscreen with SPF30 increases the cell vitality compared to unprotected cells but not to a level as measured for a 0.25 MED that is just considered reasonable for vitamin D production.35 Higher doses of sun exposure do not provide any benefit to the skin and body. Thus, a sunscreen should at least provide a protection where the values of interest lie beyond those obtained for 0.25 MED.
For cell vitality, this is reached only for SPF100. Regarding IL-18, SPF100 does not reach this value but reduces the values significantly better than SPF30 and 50+ (Figure 2A,B).This is of high relevance because in melanoma, inflammasome-activated IL-18 has an inflammatory and/or immunosuppressive effect in tumorigenesis depending on tumour grade and the tissue in which the inflammasome is activated.36 In non-melanoma skin malignancy, which is typically presented as a spectrum of progressive dysplasia manifesting from benign actinic keratosis (AK) through to intra-epidermal carcinoma (IEC), and eventually, aggressive cutaneous squamous cell carcinoma (SCC), IL-18 was shown to be non-significantly upregulated in AK, but became significantly upregulated in the skin cancer type IEC, and even more pronounced upregulated in SCC. Also, its levels correlated with skin lesion phenotype severity.37 The inhibition of inflammasomes or neutralization of their products, mainly IL-1β and IL-18, has profound effects on carcinogenesis and tumour progression. Thus, inflammasomes are promising therapeutic targets in cancer-related clinical conditions.38
For cis-UCA, the reduction due to SPF30 and SPF50+ is not significant but a trend can be observed for SPF50+, whereas SPF100 clearly prevents cis-UCA formation, reaching similar values induced by 0.25 MED (Figure 2C) indicating an optimal protection against immunosuppression. It may be speculated that a certain threshold of UV protection is needed to effectively reduce cis-UCA formation. Skin exposure to UV radiation induces immunosuppression which can manifest in actinic keratosis and subsequently skin cancer.25 By binding to a serotonin receptor (5-HT2A),25 cis-UCA leads for instance to a modification of antigen-presenting cell function, suppression of both contact hypersensitivity and delayed type hypersensitivity responses, and delay of allograft rejection.39 This cis-UCA induced reduction of cell-mediated immunity can subsequently result in carcinogenesis.40, 41 Our results agree with findings in the literature where sunscreen has been shown to be an efficient strategy to reduce UV exposure and its consequences like the formation of cis-UCA28 and when used regularly, sunscreens are able to prevent the formation of actinic keratosis and eventually carcinogenesis.42, 43
The different analysis methods for DNA damage including machine learning yielded in comparable results but especially the algorithm area, which takes the whole thin section into account, could provide higher significances compared to the methods using only excerpts from the full section (Figure 3). Such an analysis could overcome the problem of inhomogeneous damage due to the limitation of uniform distribution of the sunscreen, which is known to occur both macroscopically and microscopically.44, 45
For the DNA damage in excised human skin, SPF30 reduces the damage already below the one obtained by 0.25 MED (Figures 3A and 4). Nevertheless, SPF100 reduces DNA damage further significantly by 30 to 50% compared to SPF30 and SPF50+. This is relevant for vulnerable persons who are immunosuppressed due to transplantation or other drug-related immunosuppression.46 Skin cancer is the most common malignancy in solid organ transplant recipients (SOTRs), occurring at an incidence of 10–45% at 10 years posttransplantation, and appears to be more aggressive in this population.47, 48 Further individuals with special risk are xeroderma pigmentosum (enzymatic defect of DNA repair), and patients treated with high doses of PUVA. X-rays, tar and arsenic are also known skin carcinogens.49 For lupus erythematosus patients, sun protection is also highly relevant.50
5 CONCLUSION
The application of a sunscreen with SPF100 protects the skin significantly better against skin damage by preventing the alteration of the inflammation marker IL-18, cell vitality, cis-UCA levels and DNA damage compared to SPF30 and SPF50+ by up to 40%. No difference between SPF30 and SPF50+ was found. This illustrates the superior efficacy of sunscreens with SPF100 which significantly protect the skin against inflammation, immunosuppression, and thus, the risk of cancer development.
AUTHOR CONTRIBUTIONS
L.K., M.H., C.U., M.v.B. and M.C.M. conceived and designed the concept of the study and methodology, M.K., D.F.Z.D, S.B.L., H.K., M.v.B., N.M., C.K., A.V. and Z.M. performed the experimental investigations. Data analysis and interpretation of the data was done by R.K.C.M., M.H., M.v.B., L.K., J.G., C.G., C.U., J.M.W., S.B.L. and M.C.M. M.K., S.B.L., J.M.W., C.U. and M.C.M prepare the original draft. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGEMENTS
The authors wish to express their thanks to Gabi Sperling for her assistance during the cell culture experiments, Juliane Lüttke for her valuable support in the statistical analysis and Dr. Jens-Peter Vietzke and Thorsten Burkhart for measuring the UCA level. Open Access funding enabled and organized by Projekt DEAL.
We thank Sabine Schanzer and Heike Richter for assistance with the irradiation experiments ex vivo. Furthermore, the authors thank IPATH.Berlin (iPATH.Berlin-Immunopathology for Experimental Models, Core Facility of the Charité-Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Charitéplatz 1, 10117, Berlin, Germany.) for performing the histological staining.
Furthermore, we thank Maximilian Springenberg, Patrick Wagner and Jackie Ma from the Department of Artificial Intelligence, Fraunhofer Heinrich Hertz Institute, 10587 Berlin, Germany for the development of the machine learning algorithms.
The research was funded by Beiersdorf AG.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to declare. M.L.v.B., N.M., C.K., A.V., Z.M., M.H., J.M.W., L.K., J.G. and C.G. are employees of Beiersdorf AG.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




