Vitamin C compounds mixture prevents skin barrier alterations and inflammatory responses upon real life multi pollutant exposure
Abstract
Cutaneous tissues is among the main target of outdoor stressors such as ozone (O3), particulate matter (PM), and ultraviolet radiation (UV) all involved in inducing extrinsic skin aging. Only a few reports have studied the multipollutant interaction and its effect on skin damage. In the present work, we intended to evaluate the ability of pollutants such as O3 and PM to further aggravate cutaneous UV damage. In addition, the preventive properties of a cosmeceutical formulation mixture (AOX mix) containing 15% vitamin C (L-ascorbic acid), 1% vitamin E (α-tocopherol) and 0.5% ferulic acid was also investigated. Skin explants obtained from three different subjects were exposed to 200 mJ UV light, 0.25 ppm O3 for 2 h, and 30 min of diesel engine exhaust (DEE), alone or in combination for 4 days (time point D1 and D4). The results showed a clear additive effect of O3 and DEE in combination with UV in terms of keratin 10, Desmocollin and Claudin loss. In addition, the multipollutant exposure significantly induced the inflammatory response measured as NLRP1/ASC co-localization suggesting the activation of the inflammasome machinery. Finally, the loss of Aquaporin3 was also affected by the combined outdoor stressors. Furthermore, daily topical pre-treatment with the AOX Mix significantly prevented the cutaneous changes induced by the multipollutants. In conclusion, this study is among the first to investigate the combined effects of three of the most harmful outdoor stressors on human skin and confirms that daily topical of an antioxidant application may prevent pollution-induced skin damage.
1 INTRODUCTION
Although humans arrived only recently in Earth's timeline, they have been driving major changes to the planet's ecosystems. Even now, the basic requirements for human life—air, water, shelter, food, nature, and culture—are rapidly transforming the planet. These changes have become so noticeable on a global scale that scientists believe we are living in a new chapter of the Earth's story: the Anthropocene, or Age of Humans.1 In this epoch, more than 50% of the population lives in the urban environment, typically associated with degraded and poor air quality.2
The “Lancet Commission on pollution and health” has recently concluded that chemical pollution is the leading environmental cause of chronic diseases and premature deaths in the world today.3 All forms of chemical pollution are thought to have caused 9 million premature deaths worldwide in 2015.3 A comparable number was published by the World Health Organization (WHO), postulating global excess mortality of 12.6 million in 2012 due to people living in unhealthy environments.4 Most likely, this number has been suggested to even increase in 2050 according to the projections of specialized agencies (World Health Organization, United Nations, European Commission, and World Bank) (Commission 2020).
Exposure to air pollution impacts the function of multiple organs, including heart, lungs, gut, and brain.5 Cutaneous tissue is for sure one of the main organs directly exposed to outdoor stressors and today the fact that pollution is able to induce or/and exacerbate a variety of skin conditions has been well proven by a multitude of scientific reports.6, 7 Although, UV light has been for a long time considered the main risk factor contributing to “extrinsic skin aging”, other pollutants are now considered to be involved in premature skin aging and affect skin conditions.8 Among them, ozone (O3) and particulate matter (PM) can play key roles in pollution-induced skin damage and eventually exacerbate the cutaneous noxious effect of UV exposure.9, 10 The specific mechanisms by which skin damage occurs can differ among the different environmental pollutants to which we are exposed. Indeed, while UV light is able to induce tissue damage by penetrating the skin, O3 cannot pass through the skin and its effect is limited to the interaction with the stratum corneum (SC). Continuous skin exposure to high levels of O3 result in the accumulation of peroxidation products and induction of stress responses in the active layers of the skin.11-13 In addition to its role in accelerating skin damage associated with aging, exposure to high levels of O3 is associated with urticaria, contact dermatitis, rash, and skin infections.7, 14 Exposure to ambient PM has been associated with several pathologic conditions and there is also a clear evidence that PM can accelerate skin aging, increase cutaneous spots, and induce skin inflammation.15, 16
Currently, it is not known whether PM can pass through the skin. It has been suggested that some PM may enter the lower layers of the skin through hair follicles although this can be negligible. Alternatively, rather than being permeated by the whole particle, the skin may absorb certain molecules (i.e. polycyclic aromatic hydrocarbons, PAHs) that are attached to the particle and induce an oxinflammatory response.17
Previous studies have shown the ability of UV to act synergistically with PM by exciting PAHs in the core structure of particulates.18 Another study has shown that the co-exposure of UV with O3 increases tissue peroxidation and decreases skin barrier proteins levels such as filaggrin and involucrin.10, 19
In general, outdoor stressors can affect the skin's physical properties by acting at both levels: “outside-in” and “inside-out”. The “outside-in” barrier consists of the SC, which protects against the penetration of pathogens, allergens, and other exogenous substances including PM.20, 21 Several distinct proteins in the epidermis are crucial to the terminal differentiation program and the barrier function of the skin, such as involucrin, loricrin, and keratins.22, 23 The “inside-out” barrier, mainly provided by tight junctions, prevents excessive transepidermal water loss (TEWL) and, therefore, protects the skin against dehydration.24
Several studies analysing single pollutants effects have shown that both O3 and PM are able to affect the skin by oxidizing SC components such as squalene and fatty acid in general. It is likely that PM and O3 disrupt the barrier function of the skin by modulating or even degrading the tight junctions via oxidative damage as shown already for lung tissues.25, 26
As of today, very few studies have analysed the eventual synergistic or additive effect of multiple pollutants on cutaneous tissues. Therefore, in the present study, we aimed to investigate whether exposure to O3 and PM could somehow implement the cutaneous damage induced by UV in human skin explants. Specifically, we have analysed protein related to skin tight junctions, differentiation and inflammatory responses. In addition, the possible topical protective effect of a commercially available cosmeceutical formulation mixture (AOX Mix) containing 15% vitamin C (L-ascorbic acid), 1% vitamin E (α-tocopherol), and 0.5% ferulic acid was also evaluated under the different pollutant exposure conditions.
2 MATERIALS AND METHODS
2.1 Culture and exposure of ex vivo human biopsies
As previously described,9 human Caucasian skin, obtained from three different healthy subjects (age 35–45) undergoing routine elective abdominoplasty, was purchased from Hunstad/Kortesis/Bharti Cosmetic Surgery clinic (Huntersville, NC, USA) (as the skin was purchased as “consumable material”, IRB and ethical approval was not request). For each tissue sample, collected following informed consent, 12 mm full thickness punch skin biopsies were taken. The skin biopsies were then rinsed with Phosphate Buffer Solution (PBS) and were cultured in DMEM containing 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in 5% CO2 with the upper part of the epidermis exposed to the outside environment. The next day, medium was changed and an antioxidant mixture containing 15% vitamin C (L-ascorbic acid), 1% vitamin E (α-tocopherol) and 0.5% ferulic acid (CE Ferulic, SkinCeuticals Inc., New York, NY) was topically applied. After 24 hrs of pre-treatment, biopsies were exposed to the following pollutants, as previously reported9: (1) 200 mJ UVA/UVB light alone; (2) 200 mJ UVA/UVB light and then 0.25 ppm of O3 for 2 h in an O3 chamber; (3) 200 mJ UVA/UVB light then 30 min of DEE; (4) 200 mJ UVA/UVB light, O3, and then DEE. Samples were collected 24 h after the first exposure or continued to be exposed for 4 days. DEE was generated by a Kubota RTV-X900 diesel engine (3-cylinder, 4-cycle diesel with overhead valves, 1123 cc that has 24.8 HP at 3000 rpm).
2.2 Immunofluorescence
Skin biopsies were collected at the indicated timepoints and fixed in 10% Neutral Buffer Formalin for 48 h at 4°C. Then, samples were dehydrated in different gradients of alcohol and xylene and embedded in paraffin. Paraffin blocks were then sectioned using a microtome to obtain 4 μm paraffin tissues sections. For immunofluorescence staining, sections were deparaffinized in xylene and rehydrated in decreasing alcohol gradients and eventually rinsed in double deionized water (DDI) water. Antigen retrieval was achieved using heat-based epitope retrieval with sodium citrate buffer (Thermo Fisher Scientific, USA) (pH 6.0) at a sub-boiling temperature in a 500 W microwave for 10 min. After cooling, sections were washed in PBS, blocked with 5% BSA in PBS, and incubated overnight at 4°C with primary antibodies diluted in PBS-BSA 0.25% as following: Keratin 10 (dil. 1:50) (sc-23 877, Santa Cruz Biotechnology Inc., Dallas, TX, USA); Desmocollin 1 (dil. 1:50) (sc-398 590, Santa Cruz Biotechnology Inc.); Claudin 1 (dil. 1:50) (sc-166 338, Santa Cruz Biotechnology Inc.); Aquaporin 3 (dil. 1:50) (sc-518 001, Santa Cruz Biotechnology Inc.); NLRP1 (dil. 1:50) (sc-166 368, Santa Cruz Biotechnology Inc.) and ASC 1:100 (NBP1-78977, Novus Biologicals, LLC, Centennial, CO, USA). Sections were then washed in PBS and incubated with fluorochrome-conjugated secondary antibodies (dil. 1:1000) (Alexa Fluor 568, A11004 or Alexa Fluor 488, A11055) in PBS- BSA 0.25% at RT, and then washed with PBS. Nuclei were stained with DAPI (D1306, Thermo Fisher Scientific, Waltham, MA, USA) in PBS, and sections were then washed with PBS. Sections were mounted using PermaFluor™ Aqueous Mounting Medium (Thermo Fisher Scientific) and imaged on a Zeiss Z1 AxioObserver LSM10 confocal microscope. Images were quantified using ImageJ software.
2.3 Statistics
Statistical analyses were performed by using GraphPad Prism 6 software (GraphPad Software Inc., La Jolla CA). For comparisons between groups, analysis of variance (ANOVA) followed by Bonferroni's post-hoc test was conducted. All data were expressed as means ± SD. p ≤ 0.05 was considered as significant in all cases.
3 RESULTS
3.1 Combined pollutants exposure enhances UV-induced skin barrier structure impairment and topically applied antioxidant mix prevents skin damage
Prolonged exposure to pollutants can affect skin health and barrier function as also displayed in several skin conditions related to air pollution.27, 28 Therefore, we investigated the levels of Keratin 10 (K10), a member of the keratinocyte-derived keratin family involved in the formation of the epidermis cytoskeleton, which confers structural resistance against mechanical trauma. Keratin 10 is the main keratin involved in the early keratinocytes differentiation within the spinous/suprabasal layer of the skin and it is therefore used as marker of early cutaneous differentiation.29 As shown in Figure 1, we observed a decrease in Keratin 10 expression levels after the different combined pollutants exposure at DAY 4 (Figure 1) while no difference was noticed at an earlier time point (DAY1, data not shown). Furthermore, pre-treatment with the antioxidant mix (AOX) was able to significantly restore the loss of Keratin 10 levels induced by pollutants exposure after 4 days. No significant difference in Keratin 10 levels were observed at DAY 1 (data not shown).
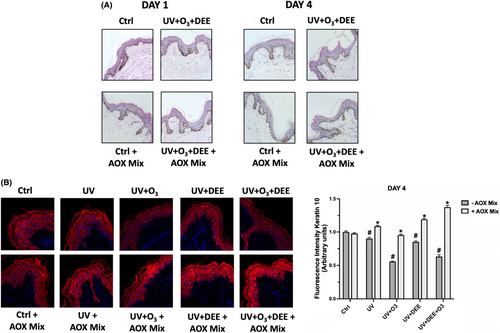
3.2 Combined pollutants exposure affects proteins related to cutaneous integrity and water channels. Protective effect of AOX mix topical application
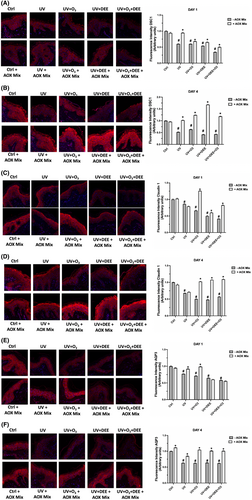
The tight junctions (TJs) together with the stratum corneum contribute to the establishment of the cutaneous barrier against the environment, preventing the penetration of external antigens or leakage of internal constituents such as water and nutrients.24 Since both UV radiations and pollutants have been shown to affect TJs distribution within the skin, leading to an impairment of skin barrier function,30 we investigated whether O3 and DEE were able to enhance UV-induced skin damage by evaluating the protein levels of Desmocollin 1 (DSC 1), one of the main components of the skin cell–cell desmosomes junctions, and the TJ Claudin-1. As shown in Figure 2, skin biopsies exposed to the different pollutant's combination displayed decreased levels of Desmocollin 1 compared to unexposed tissues on DAY 1 (Figure 2A) and this effect was even more noticeable on DAY 4 (Figure 2B). Interestingly, O3 and DEE seemed to exacerbate the UV-induced skin damage by enhancing the loss of Desmocollin 1 at both time points. Moreover, the treatment with AOX mix was able to prevent Desmocollin 1 decrease starting from DAY 1 (Figure 2A), displaying an even more evident effect on DAY 4, as it was able to completely restore the protein loss (Figure 2B). A similar trend was observed for Claudin 1, whose expression levels were impaired at both time points, on DAY 1 (Figure 2C) and on DAY 4 (Figure 2D); and in particular after the combination of the 3 pollutants. The treatment with AOX mix was able to prevent this loss at both time points respectively, especially on DAY 4. Skin integrity and barrier function are essential characteristics to prevent water loss and retain water, allowing the skin to be hydrated and maintain the exchange of water and micronutrients between cells.31 Since pollutant exposure has been shown to compromise the skin barrier structure, we wondered if this impairment could also affect the ability of the skin to retain water. Therefore, we evaluated the protein levels of Aquaporin 3 (AQP3), a protein channel involved in the cutaneous bidirectional cellular water flow.32 As depicted in Figure 2E,F, pollutants exposure exacerbated the UV-induced decrease in Aquaporin 3 (AQP3) at both time points, while the prolonged treatment with AOX mix prevented this loss at DAY 4 (Figure 2F). Of note, the rescue effect of the pretreatment with the AOX mix was also noticed already on DAY 1, in particular against UV + O3 and UV + O3 + DEE (Figure 2E).
3.3 O3 and DEE exhibit an additive effect on UV-induced inflammation in human skin and AOX mix topical application is able to moderate the cutaneous inflammatory response
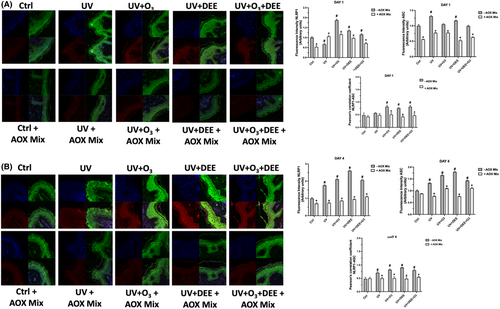
In our previous study, we demonstrated that combined exposure to pollutants could aggravate the oxidative stress levels as well as the inflammatory responses of human skin (i.e. OxInflammation), and that an AOX mix could abrogate this phenomenon.9 To better investigate the link between oxidative stress and inflammation in pollution-induced skin damage, we decided to investigate one of the main inflammatory pathways, the NLRP1 inflammasome, a multiprotein complex whose activation has been correlated to oxidative stress events.33, 34 For this purpose, we performed a double immunofluorescence stain for the inflammasome components NLRP1 and ASC, whose co-localisation normally occurs under inflammasome assembly. As shown in Figure 3A,B, exposure to pollutants was able to induce inflammasome activation by upregulating the expression of NLRP1 and ASC. Furthermore, co-localization of the two proteins occurred especially after the combined prolonged exposure on DAY 4 (Figure 3B). Of note, the topical formulation AOX mix was able to prevent NLRP1/ASC co-localization, suggesting its protective effect against an inflammatory insult.
4 DISCUSSION
It is necessary to take into account that, over the course of our life, we are exposed to a wide range of non-genetic factors that can affect our health. These factors, which are referred to collectively as the “exposome,” can contribute to skin damage, and include infrared radiation, ozone pollution, exhaust emissions, cigarette smoke, transition metals, inadequate nutrition, psychological stress and even lack of sleep.35
The importance of protecting the skin from outdoor stressors such as sunlight is widely recognized, as ultraviolet (UV) light is considered to be the leading cause of skin damage and premature aging.8 However, other outdoor stressors such as O3 and PM have also been shown to affect skin health and compromise its physiologic properties.9, 10
The recent concept of the skin aging exposome has highlighted the noxious effect not only of UV light but also of pollution exposure to cutaneous tissues.35 Urbanization and industrialization have dramatically increased the concentration in the air of toxic compounds such as O3 and PM.
Skin damage caused by sun and environmental exposure is mainly caused by a cascade of reactions involving the generation of reactive oxygen species (ROS), which leads to peroxidation of cellular components, such as lipids, and oxidation of proteins, and nucleic acids and to the depletion of cutaneous antioxidant defence.7 The alteration of the lipid composition of skin can compromise the epidermal barrier, leading to serious skin damage.36 The effects of exposure to outdoor stressors begin earlier in life than many people may realize; signs of aging can already be detected around the age of 18–25.37
Although the common denominator among all the outdoor pollutants can be represented by the induction of oxidative stress, due to the different nature of the stressors, the molecular mechanisms by which those pollutants act on the skin are quite different.7 For instance, O3 is not able to penetrate the skin. However it is a very strong oxidant able to react on carbon–carbon bond with the unsaturated lipids present in the SC to form unstable ozonide that can then decompose into peroxidation products such as aldehydes and hydroperoxide such as hydrogen peroxide.38 The generated oxidation products can contribute to skin irritation and barrier perturbation. Indeed, O3 exposure has been linked to the development and exacerbation of inflammatory skin conditions such as eczema, dermatitis, rushes and so forth.14, 39 Our group and other researchers have shown that O3 exposure can affect skin's oxinflammatory responses and decrease collagen content in several cutaneous models, from 2D, 3D and human subjects.9, 10, 40-42 On the other hand, when PM is in contact with the skin, they lead also to redox reactions.17, 43 The toxicological properties of DEE mainly relay on the transition metal content (i.e. Fe and Cu) and on the PAHs absorbed in the particles and released in the biological systems. PM can catalyse redox reaction when in contact with reducing chemicals, as it has been shown by DEE particles that are able to produce ROS such as hydroxyl radical and superoxide anion.44 In addition, some PM can contain quinone that can eventually form ROS via a redox cycling with biological reductants,45 while the presence of transition metals can generate the Fenton reaction, forming hydroxyl radical. Therefore, PM can produce different types of ROS based on the source and content of the particles.
In addition, the ability of UV radiation to induce cutaneous oxidative damage has been well demonstrated, especially for UVA. Indeed, while UVB radiations, which are normally absorbed by the epidermis, lead to the formation of pyrimidine dimers in DNA and consequent mutations (i.e. direct mechanism), UVA which are able to penetrate more in deep the skin reaching the dermis, lead to the production of ROS able to damage the DNA and attack other biomolecules such as protein and lipids (i.e. indirect mechanism).46 In particular, due to their longer wavelength, UVA1 radiations (340–400 nm) are able to penetrate deeper into the dermis compared to UVA2 (320–340 nm) and UVB, reaching the subcutaneous layer where they can affect different cell types including infiltrating T cells and B lymphocytes, fibroblasts, dendritic cells, and immature mast cells. UVA radiation can also induce cellular release of free iron and heme that can then participate to the Fenton's reaction. The results of these reactions can either be the formation of superoxide radical anion •O2– (i.e. type 1 reaction) or of single oxygen 1O2 (i.e. type 2 reaction), which is particularly reactive towards biomolecules. •O2– can undergo dismutation resulting in H2O2 production involved in the Fenton reaction in presence of Fe2+, resulting in hydroxyl radical formation (HO•), that is extremely reactive towards biomolecules as well.47
The majority the studies showing the different mechanisms involved in skin damage have been performed taking into consideration only one pollutant. Therefore, taking into account the lack of studies evaluating the effects of combined exposure of UV light, O3, and PM, the present work is aimed to investigate whether these outdoor stressors can act synergistically in inducing skin damage. UV light is one of the strongest outdoor stressors; therefore, we focused our efforts in understanding the synergy between this outdoor stressor and the other two most toxic anthropogenic environmental air pollutants, PM and O3.
We were able to show that K10, which forms a keratin pair with Keratin 1 and it is localized in the stratum spinosum and granular layer,48 was clearly affected by the pollutants, especially at day 4 and this effect was much more evident respect to the only UV treated skin. These data confirm the additive effect of the multipollutant exposure. These results are in line with previous work in which a specific anti-pollutant technology was able to prevent K10 loss in a co-culture skin model.49 Also in our case, the topical application of the AOX mix was able to prevent the decrease of K10 and avoid the eventual structure modification induced by outdoor stressors. The same trend was noticed also for desmocolin-1 and claudin-1, proteins involved in maintaining skin barrier properties. Kim et al. was able to demonstrate that the PAHs present in the particles were able to affect the expression of both proteins, suggesting that the ability of the skin to absorb such liposoluble compounds leads to barrier perturbation and can make the skin more penetrable by outdoor substances including pathogens.50 These data are in line with the ability of pollutants to affect the “inside-out” skin barrier provided by tight junctions.17 It is likely that PM and pollution in general are able to disrupt the skin barrier function, by modulating or even degrading the TJs, such as Desmocolin-1 and Claudin-1, as shown in our experiments. Similar mechanism has been suggested for other organs directly exposed to the outdoor stressors such as lung25 and intestine,51, 52 confirming the detrimental effect of pollution on human health.
In animal and ex-vivo models, PM exposure decreased the expression of structural barrier proteins such as the keratins loricrin and filaggrin.53 The same has been suggested also for O3 and UV. In addition, a study by Ferrara et al. has shown the additive effect of O3 and UV on skin damage, demonstrating the decrease of structural proteins involved in maintaining the skin barrier.10 In porcine skin, deterioration of the skin barrier by PM due to disrupted TJs led to increased skin permeability,53 making the skin even more susceptible to damage.
In addition, a proper skin barrier is maintained also thanks to the ability to prevent tissue dehydration and Aquaporin 3 plays a key role in this pathway.32 It was not surprising to observe that exposure to multipollutants clearly induced this protein loss with an additive trend at day 1. AQP3 is essential not only to maintain homeostatic hydration of the skin, but also to properly regulate keratinocytes proliferation and differentiation.32
The loss of AQP3 could be a consequence of the peroxidation products induced by the pollutants; as suggested in previous work by Pecorelli54 where the formation of 4HNE, a peroxidation product, is able to covalently bind to AQP3 leading to its ubiquitination and degradation. The ability of the AOX mix to prevent this process lies in precluding the formation of ROS by inducing endogenous cellular antioxidant defence through the NRF2 pathway.55
The tight link between oxidative stress and inflammation, i.e. OxInflammation,56 has been clearly shown to be involved in skin condition.9 A relatively new inflammatory mechanism is represented by the inflammasome pathway. Previous work has confirmed the involvement of the inflammasome activation in pollution damage.10 Also in our case, the exposure to the different pollutants synergistically increased the inflammasome activation measured as NLRP1/ASC binding. The inflammatory process triggered by the exposome can further contribute to skin aging through a process referred to as “inflammaging,” which is directly correlated to the altered redox homeostasis of the tissue. In addition, this process may also exacerbate other inflammatory conditions such as eczema, psoriasis or acne.15, 57
In previous work, we confirmed that the combination of outdoor stressors induced oxidative damage, as determined by 4HNE levels and HO-1 expression, the latter involved in the cellular defensive system controlled by Nrf2.9 Furthermore, our data are in line with the increased inflammatory responses previously shown by the increase in NFκB and COX2 levels.9
The antioxidant technology used in this study is commercially available and composed of 15% ascorbic acid, 1% alpha tocopherol and 0.5% ferulic acid. Previous studies have shown the ability of the same technology to improve skin defensive response to outdoor damage.9, 55 The efficiency of this formulation can partially derive from the fact that ferulic acid, an hysroxycinnamic acid, protects L-ascorbic and α-tocopherol, by serving as a sacrificial substance.58
It is important to mention that although so far limited, there is some literature that supports the synergistic and/or additive damaging effects of different pollutants on the cutaneous tissue. In the paper by Ferrara et al., the authors showed how O3 and DEE in combination with UV act synergistically in inducing skin damage by increasing levels of oxidative stress related markers (i.e. 4HNE, HO-1) and inflammatory molecules such as COX2 and NF-κB.9 Furthermore, using ex vivo skin explants, the same authors demonstrated that O3 could implement UV-mediated skin oxinflammation damage by affecting the levels of several oxidative stress (4-HNE, Nrf2, HO1, Ahr) and inflammatory markers (NF-kB, COX2, NLRP1), leading to altered expression of protein related to skin structure and differentiation.10 Besides these works, only a few other studies have evaluated the possible interaction between different pollutants. For example, Marrot's group demonstrated the deleterious synergy between pollution and sunlight on skin tissue, showing how polycyclic aromatic hydrocarbons could induce a huge toxic stress on the skin, when exposed to long UVA wavelengths.18, 59 Therefore, although it is not easy to simulate a real-life/daily and chronic exposure to simultaneous pollutants in the laboratory, the above-mentioned works clearly indicate that airborne pollution, particularly if chronic, may compromise cutaneous homeostasis and exacerbate sunlight-induced skin damage, contributing to the onset and progression of several skin conditions.
Finally, although skin health can be improved by diet,60 the daily use of topical applications to prevent pollution-induced skin damage is still strongly recommended, as confirmed by the use of specific combinations of cosmeceutical formulations mixture that has previously been shown to also protect against single pollutants in preclinical and clinical studies.13, 41, 55
AUTHOR CONTRIBUTIONS
FF, GV: Conceptualization, methodology, investigation, data curation, writing—original draft preparation. AP: conceptualization, investigation, supervision, writing—review and editing. EP, AC: Methodology, investigation, data curation. SW, HC, GV: Investigation, funding acquisition: All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGEMENTS
The authors thank SkinCeuticals for research support.
FUNDING INFORMATION
The authors thank Skinceuticals for partial support.
CONFLICT OF INTEREST STATEMENT
The authors declared no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




