Physiological roles of human interleukin-17 family
Yucong Liu, Ye Ouyang and Wanchun You have contributed equally to this work and share first authorship.
Abstract
Interleukin-17 s (IL-17s) are well-known proinflammatory cytokines, and their antagonists perform excellently in the treatment of inflammatory skin diseases such as psoriasis. However, their physiological functions have not been given sufficient attention by clinicians. IL-17s can protect the host from extracellular pathogens, maintain epithelial integrity, regulate cognitive processes and modulate adipocyte activity through distinct mechanisms. Here, we present a systematic review concerning the physiological functions of IL-17s. Our goal is not to negate the therapeutic effect of IL-17 antagonists, but to ensure their safe use and reasonably explain the possible adverse events that may occur in their application.
1 INTRODUCTION
Since their discovery nearly 30 years ago, IL-17s have been mainly considered as proinflammatory cytokines and shown to play an essential role in the pathogenesis of chronic autoimmune diseases such as psoriasis and ankylosing spondylitis.1, 2 Therefore, drugs targeting IL-17 (e.g. secukinumab) or the IL-17 receptors (e.g. brodalumab) have wide applications in clinic for the treatment of these diseases. In fact, IL-17s have a multitude of vital physiological functions that are still poorly understood.3 An incomplete understanding of IL-17s may lead to irrational drug usage and adverse effects. In clinical trials and real-life applications, it has been observed a significant increase in fungal and upper respiratory tract bacterial infections in patients treated with monoclonal antibodies which block the IL-23-IL-17 pathway.4-7 Additionally, it has been noted that for patients who have been diagnosed with inflammatory bowel disease, the administration of secukinumab or brodalumab as a treatment method resulted in a worsening of intestinal inflammation.6, 8 Moreover, when using brodalumab for the treatment of psoriasis, a small portion of patients arose suicidal thoughts or behaviour, which has been labelled by FDA with a Black Box Warning.9 Therefore, it is of great significance to have a comprehensive understanding of IL-17s and ensure the safe usage of corresponding targeted drugs. In order to have a better understanding of IL-17s and to reasonably explain the phenomena or adverse events that occur during the use of their antagonists, here we review the physiological roles of the IL-17 family (Figure 1) and discuss the promising research points that people are concerned about in this field nowadays.

2 IL-17S AND THEIR RECEPTORS IN HUMAN
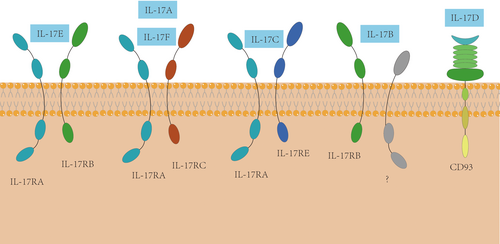
The IL-17 family is composed of IL-17A, IL-17B, IL-17C, IL-17D, IL-17E and IL-17F. Their structures have some relevance. IL-17A and IL-17F, among them, have the closest relationship, since they are often coexpressed on linked gene and are usually coproduced by Type 17 cells. IL-17 receptor (IL-17R) family consists of five receptor subunits, IL-17RA, IL-17RB, IL-17RC, IL-17RD and IL-17RE. IL-17RA is widely expressed with high level in haematopoietic tissue and extensively participating in diverse signalling activities of IL-17s.10, 11 IL-17RC is high in various nonimmune cells. Studies of other receptors are not quite enough. IL-17RB can be expressed by diverse endocrine tissues, kidney, liver and Th2 cells.12, 13 Lung tissues from asthmatic patients and skin lesions from patients with atopic dermatitis have also been found with high level expression of IL-17RB.14 IL-17RD, known as Sef (Similar expression to fibroblast growth factor), has been observed to have three isoforms which is separately hSEF-a, hSEF-b and hSEF-S in human.15 However, expression of hSEF-S has not been extensively characterized. At last, there is few specific studies about IL-17RE and its expression. To be much clearer, expression of each IL-17R isoform have been orderly listed in Table 1.
| Receptors20 | Expression of receptors in human tissue |
|---|---|
| IL-17RA | Vascular endothelial cells, peripheral T cells, B cell lineage, fibroblasts, bone marrow stromal cells, bone marrow monocytes and lungs10, 11, 159 |
| IL-17RB | Diverse endocrine tissues, liver and kidney12, 13 |
| IL-17RC | Nonimmune cells of the prostate, kidney, thyroid and joints160, 161 |
| IL-17RD (including three isoforms) | Epithelial tissues, brain, endothelial cells, kidney, bone, spinal cord, skeletal muscle, heart, nerves for hSEF-a; thyroid, testes, brain and endothelial cells for hSEF-b; hSEF-S has not been extensively characterized15, 162-164 |
| IL-17RE | Th17 cells, keratinocytes, colonic epithelial cells and skin nerve fibers66, 91, 94, 165 |
It has been verified that different receptor subunits of IL-17s frequently combine into diverse receptor complexes activating a number of different downstream effector signalling pathways (Figure 2). For example, IL-17RA can combine with IL-17RC to form the IL-17R, through which homodimers and heterodimer of IL-17A and/or IL-17F can induce the signals.13 In addition, recently a study concerning IL-17D in mouse model found CD93 can be a receptor of IL-17D.16 Even so, there is still a small amount of indeterminacy, which have been flagged in Figure 2 by question mark.
3 IL-17S ASSIST WITH THE CLEARANCE OF EXTRACELLULAR PATHOGENS
There is mounting evidence indicating that IL-17s contribute to protection against bacteria, fungi, viruses and other microorganisms. When extracellular pathogens invade, on the one hand, IL-17s stimulate the generation of antimicrobial peptides in epithelial cells (e.g. β-defensin and mucin)17-19 and mediate the release of inflammatory mediators (e.g. acute phase proteins, prostaglandins, matrix metalloproteinases and nitric oxide)20-24 to constitute the first line of defence in the body. On the other hand, IL-17s vigorously promote the expression of proinflammatory cytokines (e.g. IL-6, IL-8 and TNF-α)25-27 and chemokines (e.g. macrophage inflammatory protein-2, monocyte chemokine-1, CXCL-8, CXCL-1 and CXCL-10).20, 21, 26, 28-30 Under the influence of these proinflammatory cytokines and chemokines, myeloid cells, especially neutrophils, are prominently driven to the sites of infection or tissue damage where they recognize and eliminate pathogens. It is worth noting that innate IL-17-producing cells in the mucosa, such as natural killer (NK) cells, lymphoid-tissue inducer-like cells and γδ T cells, are characterized by responding quickly to mucosal barrier damage and strategically recruiting neutrophils within 4–8 h after exposure to ensure timely clearance of pathogens in vitro. Due to this ability, innate IL-17-producing cells are always considered as sentinel cells of the immune system.31, 32 In addition, IL-17s increase the levels of granulocyte-macrophage colony-stimulating factor (GM-CSF) and GM-CSF receptors to stimulate the production of granulocyte in defence against infectious agents.29, 33
Firstly, IL-17s play a protective role in host defence against fungal pathogens. The importance of IL-17s in preventing candidiasis has long been recognized in human and mouse experiments. In humans, people with genetic defects in the IL-17 signalling pathway exhibit a heightened susceptibility to Candida in the skin and mucous membranes. For example, chronic mucocutaneous candidiasis occurs primarily in patients with IL-17A mutations, autosomal dominant IL-17F deficiency or defects in the Th17 signalling pathway.34-37 Interestingly, a similar phenomenon can be observed in animal studies whereby mouse models with IL-17 deficiency, have a severely impaired defence reaction in skin and mucous membranes to Candida albicans infection, confirming the involvement of IL-17 in the resistance to Candida albicans.38, 39 Furthermore, IL-17 has been confirmed to defend mice against other fungal pathogens, including Cryptococcus neoformans,40, 41 Pneumocystis carinii,42 Aspergillus fumigatus,43 Coccidioides posadasii44 and Histoplasma capsulatum.44 These findings provide a compelling rationale for illustrating the importance of IL-17s in protecting from the attack of fungal infections.
Secondly, IL-17s play a crucial role in effectively countering the virus. We take interstitial keratitis (SK) as an example. SK is an immuno-inflammatory lesion of corneal tissue caused by herpes simplex virus-1 infection.45 Experiments in mice with SK have revealed that neutrophils are responsible for virus clearance in early infection.46 However, how do neutrophils migrate to infection sites? Studies have shown that IL-17A can modulate granulopoiesis via GM-CSF production and induce the secretion of the major chemoattractant. It is for this reason that IL-17A promotes increased infiltration and survival of neutrophils at the infection sites.26, 33, 47 Not only does IL-17A exert an influence on removing the virus, but also IL-17D is essential for resistance against viruses. More interestingly, the production of IL-17D can be directly induced by the nuclear factor erythroid-derived 2-related factor 2 (Nrf2).48 During vaccinia virus (VV) and murine cytomegalovirus (MCMV) infection, the Nrf2-IL-17D regulatory axis is activated so that IL-17D can promote NK cell recruitment to combat the virus.48 The knockout of IL-17D in mice led to an observable increase in susceptibility to VV and MCMV, indicating IL-17D is required for optimal antiviral response.48 Additionally, it has been confirmed in the H5N1 virus mouse model that IL-17 can make a tremendous difference in virus clearance by driving B cells to the sites of infection.49 Compared with wild-type mice, IL-17 knockout mice showed a predominantly reduced frequency of B cells in the lung, accompanied by decreased expression of CXCR5 in B cells.49 In this perspective, IL-17s do play a significant role in mediating B cell recruitment. Meanwhile, the mice exhibited more pronounced lung immunopathology, substantial weight loss and markedly increased mortality, which indicated a critical role for IL-17 in protection against the highly pathogenic H5N1 influenza virus infection.49 These studies have documented that IL-17 is involved in a defensive function against viruses through distinct mechanisms.
Thirdly, regarding bacterial invasion, the body mainly upregulates the expression of IL-17A and IL-17F genes in the IL-17 family to maintain microbial homeostasis.28, 50 IL-17A is the primary inflammatory mediator in the IL-17 family31 and has been proven to play a pivotal part in the immune response to various bacteria, such as Streptococcus pyogenes,51 Staphylococcus aureus,52 Escherichia coli53 and others. Moreover, similar to IL-17A with regards to amino acid sequence and chemokine-inducing function, IL-17F can also resist bacterial invasion.54 In the IL-17F-deficient mouse models, Ishigame et al. observed a severe impairment in the defence response when controlling infection caused by extracellular pathogens (e.g. Staphylococcus aureus and Citrobacter rodentium).10
Finally, aside from the roles mentioned above, IL-17E participates in defence against intestinal parasites. During parasitic infections, IL-17E is able to strengthen type 2 immune response via upregulating Th2 cytokines expression. As a result, Th2 cytokines, such as IL-4, IL-5 and IL-13, recruit and activate effector cells (e.g. eosinophils) to remove intestinal parasites timely.55-59
4 IL-17S MAINTAIN EPITHELIAL INTEGRITY
In addition to microbial host defence, IL-17 has also been shown to be strongly associated with the integrity of epithelial tissues and wound healing. IL-17A can induce keratinocytes to exhibit moderate proliferation60, 61 and increase keratin 17 expression, which promotes keratinocyte migration.62 More interestingly, the latest research has demonstrated that IL-17A regulates hypoxia tolerance of the damaged epithelium in mouse models. Konieczny et al. reported in Science that skin resident γδ T cells are the main population at regeneration sites in the wound-edge epithelium where they provide IL-17A to activate hypoxia-inducing factor (HIF1α).63 Furthermore, they proposed the importance of IL-17A-dependent metabolic reprogramming for re-epithelialization. Specifically, they suggested that the IL-17A-HIF1α axis induces a glycolytic metabolic program in epithelial cells. By this means, epithelial cells can provide energy to facilitate cell migration, which is indispensable for skin wound healing.63 These findings overturn the long-held belief that hypoxia alone is sufficient to induce HIF1α production and thus mediates metabolism.64
In recent years, there has been increasing evidence that IL-17E, a unique cytokine of the IL-17 family, also engages in tissue repair. When the epithelial tissue is damaged, epithelial cells upregulate IL-17E expression. In human primary keratinocytes, Borowczyk et al. observed that IL-17E, in one respect, induces keratinocyte proliferation by significantly enhancing metabolic activity and proliferation rate, which is essential for the replacement of damaged cells.62 In another respect, IL-17E can increase the expression of loricrin, filaggrin, desmoglein 1, desmocollin 1 and other differentiation markers, thus promoting the terminal differentiation of keratinocytes.62 On the contrary, IL-17A inhibits terminal keratinocyte differentiation. In addition, IL-17E can upregulate the expression of keratins related to skin healing, especially keratin 6, facilitating keratinocyte migration.62 Notably, compared with IL-17A, IL-17E increases keratinocyte displacement and velocity more significantly, which is beneficial for wound closure.62 Surprisingly, IL-17E has been demonstrated to be more substantial than IL-22 in enhancing keratinocyte migration.62 In the in vitro scratch assay model, the rate of wound closure was significantly faster in wounds treated with IL-17E than in those treated with IL-22 or IL-17A.62 In conclusion, IL-17E can repair the damaged epithelial layer and participate in the continuous renewal of the epithelial layer by promoting the proliferation, differentiation and migration of wound-edge cells.
5 IL-17S ARE INVOLVED IN BARRIER SURFACE PROTECTION
So far, more and more studies have demonstrated that IL-17s are critical cytokines for regulating barrier defence against various attacks from the external environment. For instance, IL-17C is predominantly expressed in mucosal tissues, such as the gastrointestinal tract,65-67 respiratory tract68, 69 and skin barrier,70, 71 and plays a protective role at these sites. IL-17E, a noteworthy “barrier surface” cytokine, is widely distributed throughout the body, including the brain,12 the respiratory tract12, 56 and the intestinal tract,56 and serves as an alarm to immune cells of tissue damage.72
First, take the respiratory mucosal barrier as an example. IL-17 induces substantial gene expression in airway epithelial cells. On the one hand, to preserve the integrity of the physical airway barrier, IL-17 increases the expression of adhesion molecules, such as ICAM-173, 74; on the other hand, IL-17 can mediate the gene expression of antibacterial molecules (including CCL20, DEFB4, MUC5B/AC, S100A7, S100A8, etc.)75 and chemokines/cytokines (including CXCL1, CXCL2, IL-6, IL-8, KC, etc.)29, 33, 76-78 in airway epithelial cells. In addition to acting on airway epithelial cells, IL-17A can directly act on airway smooth muscle (IL-17RA and IL-17RC positive) to enhance the contractile response. This has been confirmed in both mice and humans.79
In the lung, early IL-17A production is derived from innate immune cells such as γδ T cells in the respiratory mucosa.32 For instance, γδ T cells produce IL-17A rapidly in the early stage to control infection with Mycobacterium tuberculosis32, 80 and Mycobacterium bovis bacille Calmette-Guérin (BCG).81 Moreover, in the mouse model, IL-17A produced by γδ T cells has been confirmed to cooperate with BCG-infected macrophages to induce integrin IFA-1 and its ligand ICAM-1 expression, promoting mature granuloma formation.81 The mature granuloma is an aggregation of immune cells that facilitates the rapid decline of infection and thwarts the dissemination of pathogens.81, 82 Through these mechanisms, IL-17A can sustain respiratory mucosal homeostasis. However, except for innate immunity, Th17 cells are the major source of IL-17A in adaptive immune responses.83, 84 In human, IL-17 produced by Th17 cells has been confirmed to regulate lung microvascular endothelial cells (LMVEC) to secrete chemokines and cytokines. Interestingly, IL-17A does not have the capacity to increase the production of GM-CSF, CXCL1, CXCL5 and CXCL8 in LMVEC directly. Instead, it has a strengthening effect on macrophage-derived IL-1β and TNF-α-induced GM-CSF, CXCL1, CXCL5 and CXCL8 production. More interestingly, unlike IL-17A, IL-17F significantly inhibits the above TNF-α-induced production.85-87 In addition to regulating chemoattractant production in LMVEC, IL-17A depends on p38 MAPK to promote endothelial activation via medicating the expression of endothelial adhesion markers such as E-selectin, VCAM-1 and ICAM-1 in human LMVEC.88 The release of chemoattractant and the expression of endothelial adhesion markers are both responsible for neutrophil adhesion and migration to the site of infection, enhancing the inflammatory response.88 Therefore, IL-17 has the ability to safeguard the microvascular barrier located in the lung. Although innate and adaptive immunity differ in many respects, studies have shown γδ T cells facilitate activated CD4+ T cells to generate IL-17 and amplify Th17 responses.89 This phenomenon indicates that IL-17 may serve as an essential link between innate and adaptive immunity.
Next, take the gastrointestinal barrier as an example. Helicobacter pylori (H. pylori) infection is a global concern. Studies found that, compared with the healthy control group, the IL-17C (a novel innate-like cytokine) mRNA level was upregulated about 4.5 times in the gastric mucosa infected with H. pylori.90 By contrast, the mRNA level of other IL-17 family members did not change significantly in human infected with H. pylori.90 As a growing body of evidence proves that IL-17C binds to the IL-17RA/IL-17RE receptor complex to activate downstream signalling pathways and regulate the expression of genes encoding innate immunity-related proteins such as cytokines, chemokines and antimicrobial peptides,65, 66, 91, 92 this phenomenon suggested that H. pylori might stimulate the gastric epithelial cells to up-regulate IL-17C expression. However, the physiological role of IL-17C in H. pylori-positive gastric epithelium remains unclear.90
IL-17 also mediates the intestinal barrier's stability through various mechanisms. First and foremost, IL-17 promotes the formation of tight junctions to limit excessive permeability and maintain the integrity of the intestinal mucosal mechanical barrier.93 In the mouse model with dextran sodium sulphate (DSS)-induced colitis, a colonic epithelial cell line, YAMC, expresses high levels of IL-17RE mRNA and tight junction proteins (including occludin, claudin-1 and claudin-4) after the treatment of IL-17C.94 Interestingly, in this mouse model, Reynolds et al. also observed that in addition to IL-17RE, YAMC cells also express IL-17RA and IL-17RC.94 Further, not only IL-17C, but other IL-17 family members (excluding IL-17F) can also induce the expression of tight junction proteins,94 which provides a convincing basis for illustrating the importance of the IL-17 family in maintaining the stability of intestinal epithelial cells.
Moreover, it has become increasingly apparent in recent years that gut microbiota plays a role in maintaining the mucosal barrier.95, 96 Studies have indicated that the pathology of DSS-induced colitis in mice is aggravated in the absence of IL-17, suggesting that IL-17 is protective in this model,97, 98 while another study has discovered that IL-17 has a pathological effect on the intestinal tract.99 Why does IL-17 exhibit two opposite effects? Differences in the gut microbiome may contribute to the inconsistent results.
How does IL-17 play a protective role in the gut? Research has revealed that dysregulated gut microbiota triggers regulatory T cells (Tregs)-derived fibroblast growth factor2 (FGF2) expression during colitis.100 FGF2 collaborates with IL-17 to induce genes for repairing damaged intestinal physical barrier, which can restrain microbiota outgrowth and protect against colitis pathology.100 As a result, FGF2 or IL-17 deficiency makes it hard to fully ameliorate epithelium injury and repair epithelium integrity, finally leading to worse pathology and more severe microbiota outgrowth in the mouse model.100 In addition to synergizing with FGF2 in the colon, IL-17 can also induce antimicrobial peptides (AMP) to play an essential role in the ileum, especially alpha-defensin.101 In the ileitis of mice, Brabec et al. found that when the IL-17 receptor is defective, the ileum of mice not only shows decreased antibacterial ability characterized by decreased production of alpha-defensin, but also unfolds significantly reduced diversity of the microbiota.102 This suggests that IL-17 may maintain the ecological balance of gut microbiota by promoting the expression of antimicrobial peptides. Through these mechanisms, IL-17 can maintain a healthy balance of intestinal flora and thus protect the principal function of the barrier. Interestingly, recent researches suggest that the commensal flora of the gut can also influence the production and reaction of IL-17. Symbiotic bacteria, specifically segmented filamentous bacteria, adhere to the colonic epithelium, and promote the differentiation and maturation of Th17 cells,103, 104 maintaining gut homeostasis and the intact barrier. By contrast, another symbiotic bacteria in the gut, Bacteroides fragilis, inhibits the development of Th17 cells.105 These suggest a close relationship between intestinal flora and intestinal immune balance.
6 IL-17S IN NEUROCOGNITIVE FUNCTION
Over the past few years, increasing evidence suggests that IL-17 has neuromodulatory functions and is fundamental in regulating cognitive processes. According to Kalil Alves de lima, γδ 17 T cells are the potential source of IL-17A in the central nervous system (CNS) of mice under homeostasis and IL-17RA expression is mainly observed in cortical glutamatergic neurons located within the medial prefrontal cortex (mPFC).106 Remarkably, the mPFC has been shown to be involved in processes of emotion, decision-making, social cognition and psychopathology.107
IL-17A signalling can regulate anxiety-like behaviours in mice. This may be related to its ability to drive appropriate neurotransmitter release from presynaptic terminals of excitatory glutamate neurons in the mPFC.106 In addition, IL-17 is associated with autism spectrum disorder (ASD)-like behaviour. Choi et al. constructed a model of maternal immune activation (MIA) by injecting poly (I: C) into pregnant mice in their study. They observed the Th17 cells/IL-17 signalling pathway was initiated in inflammation-infected mothers. Subsequently, the offspring of MIA mice exhibited IL-17A mediated increase in irregular brain structures and impaired fetal brain development which were mediated by increased IL-17A, thereby causing autism ASD-like behaviour.108 Interestingly, Kalish et al. found that this neurodevelopmental manifestation, similar to humans,109 occurred only in male offspring of MIA mice. A possible signalling mechanism was proposed: increased IL-17A could induce endoplasmic reticulum stress in the cortex of offspring during maternal immune activation. Thereupon it activated a sex-biassed integrated stress response via the pERK and eIF2α pathways, thereby blocking protein synthesis in the cortex of male offspring. Ultimately, it may result in abnormal embryonic neurodevelopment and behavioural deficits.110 In contrast to IL-17A in the fetus, increased IL-17A does not cause ASD-like behaviour in adult offspring of MIA mice but ameliorates social behavioural defects through direct effects on neuronal activity in the CNS.111
Besides, the IL-17 axis is involved in the neuroimmune crosstalk in the CA1 region of the mouse hippocampus.112 In mouse models, IL-17 promotes the production of glial brain-derived neurotrophic factor (BDNF) in the hippocampus under physiological circumstances. BDNF effectively regulates the synaptic plasticity of neurons in the hippocampus, consequently augmenting short-term memory and cognitive functions.113 Consistent with this, it was shown in another study that IL-17A might help maintain normal memory.114 Conversely, under pathological conditions, such as multiple sclerosis112 and Alzheimer's disease,115 IL-17A overexpression in mouse models of multiple sclerosis adversely affects glutamatergic synaptic plasticity by stimulating IL-17RA and p38 MAPK, which in turn contributes to disruptions in hippocampal long-term potentiation and associated cognitive impairments.112 Inevitably, IL-17A in serum and cerebrospinal fluid increases in aging mice, potentially fostering cognitive aging by instigating neuroinflammation and causing damage to hippocampal theta oscillations.116
In addition, IL-17A is involved in perioperative neurocognitive deficits. Specifically, in the PND mouse models, surgery may lead to an increase in IL-17 in the serum and hippocampus, subsequently stimulating IL-17A receptor expression in the hippocampus and MMP-2/MMP-9 production. As a result, the tight junctions linking endothelial cells within the blood–brain barrier may be hydrolyzed, leading to the compromised blood–brain barrier and subsequent cognitive decline.117
Moreover, the link between IL-17 and depression has long been of interest.118-120 Several studies have shown that depression patients have higher levels of IL-17.121-123 In mouse models, it was observed that Th17 cells were predominantly clustered in the PFC and the hippocampus, which are brain regions linked to depression.124 The abnormal inflammatory response triggered by IL-17 may be involved in the progression of depression.125 However, the precise mechanism still remains unknown.
Collectively, the multifaceted nature of IL-17A is evident in its role in modulating neurobehavioral functions and cognitive processes. In particular, it often exhibits the opposite effects. The conflicting outcomes appear to rely on different conditions, involving the organism's state and the IL-17A level. Additionally, the possibility that IL-17A may be subject to synergistic and/or antagonistic effects with other molecules should be considered. Therefore, it is crucial to undertake further research to identify precise mechanisms for these phenomena.
7 IL-17S IN ADIPOCYTE ACTIVITY AND ADIPOCYTE THERMOGENESIS
It has been observed for a long time that mice with IL-17R or IL-17A deficiency experience symptoms of being overweight,126 which suggests that IL-17s modulate adipocyte activity. γδ T cells are the predominant producers of IL-17 in adipose tissue.126, 127 Much like IFN-γ and IL-6,128, 129 IL-17 exhibits antiadipogenic properties and has been observed to be upregulated in obese individuals.130-132 Studies showed that in mouse-derived 3 T3-L1 adipocytes or human bone marrow mesenchymal stem cells (Hbm-MSCs), IL-17A not only inhibited adipocyte differentiation, but also promoted differentiated lipolysis.126, 133-135 This effect has been proven to be partly achieved by elevating PGE2 levels through the amplified expression of cyclooxygenase (COX)-2.134 Notably, the inhibition of adipose tissue expansion by COX-2 occurred via a paracrine mechanism mediated by PGE2.136
Furthermore, the stimulation of IL-17A also results in a corresponding elevation of IL-6 and IL-8 levels in adipocytes from a Hbm-MSCs culture.134 They have previously been confirmed to impede the differentiation of preadipose tissue while promoting the process of lipolysis.137, 138 Not only that, a considerable reduction of transcription factors is associated with the promotion of adipocyte generation and maturation, including PPARγ, C/EBP-a, FABP4, CIDEC and PLIN1.135, 139 Concurrently, several anti-obesity transcription factors, like KLF2 and KLF3, are upregulated.133, 139 These transcription factors occupy an instrumental position in inhibiting adipocyte differentiation by IL-17.
Aside from its role in adipocyte adipogenesis, IL-17 also plays a critical part in controlling the thermogenesis of adipose tissue.127 In brown adipose tissue (BAT) of mouse models, IL-17, working in conjunction with TNF, can stimulate stromal cells to produce IL-33 under thermoneutral conditions.127, 140 Remarkably, the role of IL-33 in regulating thermogenesis in adipose tissue has been demonstrated.141 Different from thermoneutral conditions, IL-17A and TNF directly instigate adipocytes to enhance the manifestation of thermogenic genes, such as Ucp1, Dio2, Cidea and IL-33 when exposed to low temperatures.127, 140 However, a noteworthy point is that the inhibition of IL-17A signalling will not diminish adipose tissue thermogenesis in a diet-induced obesity mouse model, but instead elevate the processes of thermogenesis and white adipose tissue browning. This outcome is particularly pronounced in high-fat diet mice.142 In fact, researchers have varying opinions on the role of IL-17 in adipocyte thermogenesis. Hu et al. emphasized that it is IL-17F that plays a crucial role in adaptive thermogenesis, but not IL-17A. Their studies have illustrated that in mouse models, IL-17F elicits the expression of TGF-β 1 in adipose tissue when it binds to the IL-17RC receptor, subsequently stimulating sympathetic innervation and thermogenesis in BAT.143 It is worth noting that BAT thermogenesis relies on histone deacetylase 3, an enzyme that requires interaction with nuclear receptor coblockers (NCoRs). In mice lacking NcoRs, the IL-17 inflammatory pathway is activated, resulting in BAT adapting to thermogenesis by stimulating sympathetic innervation.144 Collectively, both IL-17A and IL-17F are implicated in the process of adipose tissue thermogenesis and are indispensable in regulating the temperature.
8 IL-17 AND BONE METABOLISM
In addition to blood lipid homeostasis, researches have demonstrated that IL-17 is significant for bone metabolism. On one side, IL-17A promotes the development of human mesenchymal stem cells into osteoblasts, while the absence of IL-17A or its receptors results in osteoporosis.145 After experimenting with mice, it is believed that this phenomenon is linked to increased leptin synthesis, promoting differentiation of marrow stem cell and calcification.146 On the other side, clinical human studies show Th17 cells participate in the disease process of middle ear cholesteatoma by expressing IL-17, and are closely related to cholesteatoma bone destruction,147 which indicates the potential connection between IL-17 and osteoclasts.
In fact, IL-17 can affect osteoclast formation and activity through multiple mechanisms. Firstly, IL-17 has been found to induce cells to express RNAKL directly or stimulate cells to release IL-1, IL-6, TNFα, PGE2 and other factors to promote the production of RANKL.148 Mice lacking RANKL will not be able to form osteoclasts and thus cause severe osteosclerosis. Additionally, IL-17 can hamper the expression of osteoclastogenesis inhibitory factor. As a result, IL-17 can increase osteoclast activity, induce osteoclast development, and thus promote bone resorption. However, it has been discovered that IL-17 causes osteoblasts to express GM-CSF when 1, 25 (OH) 2-Vit D3 is produced. This inhibits the synthesis of RANK from osteoclast precursor and ultimately prevents the creation of osteoclasts. To conclude, IL-17 can induce the general differentiation of osteoclasts and leading to bone resorption activity mediated by osteoclasts, but it can prevent the development of osteoclasts under the impact of certain external conditions.
All these studies indicate that IL-17 is involved in human bone metabolism. Further research is needed to fully understand the complex impact of IL-17 on osteoblasts, osteoclasts and bone metabolism.
9 THE ASSOCIATION BETWEEN IL-17 AND TUMOURS
IL-17s are found to be highly expressed in tumour tissue (e.g. gastric cancer,144 lung cancer,149-152 prostate cancer153 and breast cancer154) and affect tumour cells. The widespread consensus is that IL-17s mainly promote tumour cell proliferation by enhancing angiogenesis to provide ‘nutrient supply pathways’ for tumour cells. Meanwhile, it is also found that IL-17s can play a similar role by inhibiting tumour cell apoptosis and regulating immune response to tumour cells. More importantly, antiapoptotic effects can develop resistance to anticancer drugs in tumour tissue, ultimately accelerating tumour growth.153 In addition, IL-17s can upregulate the expression of matrix metalloproteinases,155 which can damage barrier mucosa156 and facilitate cancer progression.
Nevertheless, there are still studies suggesting the inhibitory effect of IL-17s on tumour cells. Studies have shown that IL-17 expression levels in colon cancer are higher than in colon adenomas and other nontumour tissues. Cox regression analysis shows that IL-17+ colon cancer patients have a higher overall survival rate,157 which indicates IL-17 is associated with a good prognosis in some cancers. Another research has shown that knocking out the IL-17 gene can rapidly worsen the condition of tumour-bearing mice, with the tumours enlarged and the number of infiltrating IFN-γ + NK cells and T cells in the internal and adjacent lymphatic systems decreased.158 The reduction in immune cells is not conducive to the immune response to tumours, which suggests the inhibitory effect of IL-17s on tumours.
In summary, the effect of the IL-17 family on tumours has two sides, which may vary depending on the site of tumour formation or other influence actors.
10 CONCLUSION
The physiological effects of IL-17s are diverse, and large areas of unknown territory are waiting for researchers to explore. We believe that clarifying the following questions will be helpful to thoroughly understand the physiological roles of IL-17s and the potential adverse reactions that may occur during the targeted blockade of IL-17s in inflammatory therapy. (1) The exact distribution and quantity density of IL-17s and their receptors should be clarified in various organs, tissues and cells. (2) The quantitative relationship between IL-17 receptors on cell surface and corresponding IL-17 cytokines around them should be elucidated under physiological and pathological conditions. (3) What are the differences in effectiveness and safety between antagonizing IL-17 receptors and antagonizing IL-17 cytokines? (4) Which organs outside the skin can IL-17 antagonists reach at a therapeutic dose during routine treatment? (5) Will long-term use of IL-17 antagonists affect the physiological functions of IL-17s, and which functions are most likely to be affected?
With the aim of not denying the important role of IL-17 antagonist therapeutics for inflammatory diseases, but rather to guide the use of IL-17 antagonist therapeutics more safely and effectively, we summarized the complex biological activity and the changes of physiological effects of IL-17s here. Physiological effects of IL-17s are independent of the proinflammatory effects and need to be observed and investigated during the long-term use of IL-17 antagonists. Only by further clarifying the physiological role of IL-17s can the clinical applications of IL-17 antagonists be more standardized and safer.
AUTHOR CONTRIBUTIONS
Z.S. conceived and designed the study. Y.L., Y.O. and W.Y. searched the literature and wrote the manuscript. W.L., Y.C., X.M. and Z.S. critically revised the manuscript. W.L., Y.C. and X.M. provided constructive suggestions throughout the writing process. All authors have read and approved the final version of the manuscript and the publication. All materials related to our study are available from the corresponding author.
ACKNOWLEDGEMENTS
This work was supported by National Natural Science Foundation of China (No. 82273537, 82073444).
FUNDING INFORMATION
National Natural Science Foundation of China (No. 82273537, 82073444).
CONFLICT OF INTEREST STATEMENT
The authors declare no potential conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data availability is not applicable to this review article as no new data are created or analysed in this review article.




