Does systemic hydrochlorothiazide increase the risk of developing ultraviolet radiation-induced skin tumours in hairless mice?
Abstract
Hydrochlorothiazide (HCTZ) is a frequently prescribed diuretic that exhibits photosensitizing properties. It is used to treat hypertension and edema. Dermato-epidemiological studies in various populations have linked HCTZ treatment with increased risk of particular types of skin cancer, including malignant melanoma (lentigo subtype), and both basal cell carcinoma and squamous cell carcinoma (SCC). This study investigated whether either of two different doses of HCTZ increased the risk of SCC development in mice exposed to ultraviolet radiation (UVR). A total of three groups of hairless mice were used in this study (total, N = 71). One group received a low dose (0.26 mg/mouse/day) and another group received a high dose (0.52 mg/mouse/day) of HCTZ in their drinking water; a third UVR control group received only tap water. All three groups were irradiated with UVR until the mice developed three tumours that were 4 mm in size. The times to SCC tumour development were recorded. In the low-dose group, the median time to develop an SCC tumour was 170 days; in both the high-dose group and the control group, the median time to develop anexd SCC tumour was 163 days (p ≥ 0.331). In our hairless mouse model, we found that mice treated with UVR plus HCTZ did not develop SCCs more rapidly than mice treated with UVR but not HCTZ.
1 INTRODUCTION
Thiazides were developed as diuretics and antihypertensive drugs in the 1950s.1 Since then, they have been among the most widely used medications globally. Nonetheless, how this group of diuretics affects the risk of particular types of skin cancer remains unclear.
Thiazides such as hydrochlorothiazide (HCTZ) act on kidneys, specifically on the distal convoluted tubules, where HCTZ inhibits the sodium chloride co-transporter.2 This leads to diuresis, which lowers the blood pressure and results in the excretion of potassium via the urine.2 HCTZ also exhibits photosensitizing properties but these are manifested in fewer than 1% of treated patients.3 Various studies have reported the adverse reaction of sunburn among users of thiazide diuretics.4-6 Evidence shows that a photosensitizing reaction following sun exposure may elevate the risk of sunburn and photodamage, increasing the risk of particular types of skin cancer.7
Various photosensitizing drugs, such as the diuretics bendroflumethiazide, bumetanide and furosemide, were evaluated in a large register-based study, and elevated risks of malignant melanoma (MM) and basal (BCC) and squamous cell carcinoma (SCC) were observed.8 Whether HCTZ affects the skin in the same way remains unclear, because various studies have produced conflicting results.6-17
HCTZ is currently being assessed for potentially co-carcinogenic effects in ultraviolet radiation (UVR)-induced skin cancer.6-16 Previously, a significant dose–response relationship between HCTZ use and an elevated risk of SCC has been shown.6, 9-13 A meta-analysis found an association between using thiazide diuretics and an increased risk of all types of skin cancer, including MM.17 Other studies have shown that HCTZ has little or no effect on the risk of developing BCC.6, 12 Jensen et al. suggested that sun-protective measures alone may be insufficient to counteract the carcinogenic potential of HCTZ.11 However, appropriate adjustments may be required for UVR exposure level, Fitzpatrick skin type, and comorbidities, and the possibility that HCTZ may interact with other medications should not be overlooked. Furthermore, the goal of pharmacoepidemiological studies is to evaluate the effects of various treatments in a real-life clinical scenario, but such studies have limitations with regard to completeness, quality, and bias due to confounding.
Drug-induced photosensitivity can occur as photoallergic or phototoxic reactions.18 Although phototoxic reactions are more common, usually dose dependent, and require greater drug exposure than photoallergic reactions, photoallergic reactions are not dependent on a specific dose of the medication or intensity of sun exposure and usually occur hours after the initial exposure as a delayed hypersensitivity reaction.18 Several in vitro studies suggest that HCTZ may trigger drug-induced phototoxic reactions.14, 15, 19 The pathogenesis of phototoxic and photoallergic reactions is different. Phototoxic reactions occur after the activation of the drug or its metabolites by light in viable skin cells, which results in the formation of free radical oxidants, such as reactive oxygen species, and ultimately cell damage.20, 21 Photoallergic reactions do not result in cell damage.22
A recent study found that a cumulative dose of 375 mg HCTZ was safe in healthy adults; however, inflammatory biomarkers were elevated when patients were treated with a combination of ultraviolet B (UVB) and HCTZ.16 Therefore, we investigated whether HCTZ had a co-carcinogenic effect on SCC development in a hairless mouse model. We used a sensitive mouse model that can show a protective effect (i.e., longer time to develop tumours)23 and a co-carcinogenic effect (i.e., shorter time to develop tumours)24 The mice had not been previously exposed to other drugs or UVR.
2 MATERIALS AND METHODS
2.1 Animals
Hairless, female C3.Cg-Hrhr/TifBomTac immunocompetent mice (total, N = 71) were used. They were 14–20 weeks old at the start of the experiment. All mice were tattooed on the abdomen with successive numbers and divided into three groups. Each group (n = 23–25) was housed in a separate box where the mice had free access to water and standard laboratory food. The animals had a 12-h light/12-h dark cycle with a mean temperature of 23°C–24°C. All the procedures involving animals complied with national guidelines for using laboratory animals. Our facility is subject to minimum annual health monitoring, in accordance with the Federation of Laboratory Animal Science Associations. There were no positive results for any pathogens.
2.2 UVR exposure
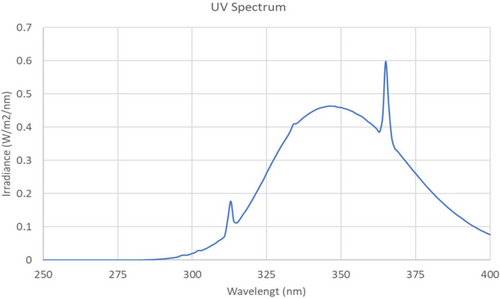
All the groups were irradiated in their boxes from above through wire lids three times each week with 3.5 standard erythema doses for 23 min 11 s.25 The radiation source consisted of one UV6 tube (Waldmann, Wheeling) between five Bellarium-S SA-1-12 tubes (Wolff). UVR emission was measured using a double monochromator (Bentham DM150; Bentham Instruments Ltd) and checked with a dual calibration check source module (Optronic laboratories). In total, 5.9% of the wavelengths emitted by the UVR source were within the UVB range (erythema weighted 89.9%) and the maximum wavelength was approximately 365 nm (Figure 1). The distance between the light source and the mice was adjusted once a month to maintain the desired dose.
2.3 Treatments
The control group (group 1) only received UVR and no treatment with pharmaceutical substances. Group 2 and group 3 received one and two 25-mg HCTZ tablets, respectively (Hydromed®; MediLink A/S, Kgs.). The tablets were dissolved in 390 ml of tap water each day throughout the entire study. A hairless mouse drinks approximately 4 ml of water per day. Thus, group 2 mice received 0.26 mg HCTZ/mouse/day and group 3 mice received 0.52 mg/mouse/day. The animal equivalent dose is 12.3 times higher for mice than for humans.26 Therefore, the doses given to the mice in this study correspond to approximately 50 mg/human/day and 100 mg/human/day, which corresponds to typical doses given to patients.26
2.4 Study design
The animals were stratified according to age into three groups of 23–25 mice. Once a week, mice were examined for tumours. The first four tumours with a diameter of at least 1 mm that developed on each mouse were mapped individually. Tumours were excluded if they did not grow to a size of 4 mm.27 In compliance with all protocols, all mice were sacrificed when they developed three 4-mm tumours or had been in the study for 365 days. Tumours was biopsied from two random mice in each group. The tumours were embedded and stained using haematoxylin and eosin to confirm the SCC diagnosis. Using a Kodak Grey Scale in black light, pigmentation of the skin was quantified on a 20-point categorical scale of arbitrary units. Skin pigmentation was measured on day 14 and then once every month. The mice were also weighed monthly. Statistical analyses were carried out as previously described.24
3 RESULTS
After the first UVR exposures, mild erythema followed by equivalent levels of pigmentation were observed in all three groups. During treatment, no signs of phototoxicity were observed in any of the groups. No significant weight difference was found for any group (p > 0.05). Three mice in group 1 (i.e., the UVR-only control group), four mice in group 2, and three mice in group 3 died before tumour development. None of these differences in the number of deaths in each group were statistically significant (p > 0.05).
3.1 Photocarcinogenesis
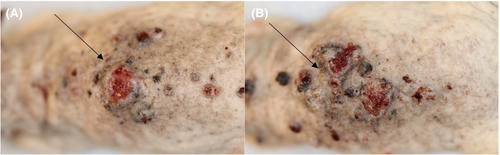
All the mice developed tumours, except for 10 mice that died before tumour development. Microscopic examination of the tumours showed that these were all SCCs (Figure 2). The mice showed no signs of disease during the study that indicated the existence of internal cancer. Time to develop a first SCC tumour did not differ significantly between the mice in group 2 or group 3 and the mice in the control group (170 vs. 163 days, p = 0.753 and 163 vs. 163 days, p = 0.693, respectively; Figure 3 and Table 1). Similarly, time to develop a first SCC tumour did not differ significantly between the mice in group 2 and group 3 (170 vs. 163 days, p = 0.820).

| Group no. | No. of mice (n) | Treatment | Days to 1 tumour median (range) | Days to 2 tumours median (range) | Days to 3 tumours median (range) |
|---|---|---|---|---|---|
| 1 | 25 |
Control |
163 (156–177) |
170 (170–188) |
188 (170–195) |
| 2 | 23 |
0.26 mg HCTZ/mouse/day p-Value* |
170 (142–170) 0.753 |
170 (163–202) 0.982 |
182 (170–202) 0.699 |
| 3 | 23 |
0.52 mg HCTZ/mouse/day p-Value* |
163 (156–170) 0.693 |
177 (170–208) 0.331 |
188 (170–208) 0.964 |
- Note: Interquartile ranges (25th and 75th percentiles) are shown above.
- Abbreviations: HCTZ, hydrochlorothiazide.
- * p-Values marked for each group compared to the control group.
Time to develop a second SCC tumour did not differ significantly between the mice in group 2 or group 3 and the mice in the control group (170 vs. 170 days, p = 0.982 and 177 vs. 170 days, p = 0.331, respectively; Figure 3 and Table 1). Similarly, time to develop a second SCC tumour did not differ significantly between the mice in group 2 and group 3 (170 vs. 177 days, p = 0.423).
Finally, time to develop a third SCC tumour did not differ significantly between the mice in group 2 or group 3 and the mice in the control group (182 vs. 188 days, p = 0.699 and 188 vs. 188 days, p = 0.964, respectively: Figure 3 and Table 1). Similarly, time to develop a third SCC tumour did not differ significantly between the mice in group 2 and group 3 (182 vs. 188 days, p = 0.689).
4 DISCUSSION
We investigated the co-carcinogenic potential of HCTZ using a well-established hairless mouse model, which can develop skin pigmentation and SCCs when exposed to UVR. We found that HCTZ did not accelerate SCC development in UVR-exposed mice. Our results contrast with those reported in recent dermato-epidemiological publications.6, 9-13, 28-31 Neither dose of HCTZ was phototoxic or co-carcinogenic in the hairless mice. The term co-carcinogenesis is used to describe a treatment that is not carcinogenic when administered alone but has that potential when combined with another treatment, such as exposure to UVR.32 The hairless mouse model has been successfully used to test the photoprotective effects of black tattoos, clobetasol propionate, and photodynamic therapy,23, 33, 34 as well as the photocarcinogenic effects of X-rays and PUVA (combined oral methoxypsoralen with ultraviolet A [UVA]).24, 35 We used a UVR source consisting of both UVB and UVA. The UV spectrum of this source is shown in Figure 1 and the UV spectrum of HCTZ has been published previously.36 HCTZ exhibits minor absorption at 290–350 nm, but no absorption above 350 nm. A treatment of 3.5 standard erythema doses was given three times per week and is equivalent to approximately 35 min exposure to a clear midday sky in July in Copenhagen, Denmark.37
As described earlier, approximately 1% of patients who receive HCTZ exhibit photosensitivity.3 It is unclear whether this is the same 1% of patients who also exhibit an elevated risk of developing skin cancer later at a more advanced age. The mice in this study that received HCTZ did not develop more erythema than the control mice. Therefore, we believe there was no increase in photosensitivity, perhaps explaining why no increase in skin cancer risk was observed. The focus of this study on mice was to investigate the photocarcinogenic potential of HCTZ using time until tumour development as the endpoint because this would be problematic in humans. In contrast, we believe that photosensitivity is better tested on humans.
Recently, the Food and Drug Administration has approved label changes to HCTZ describing a small risk of non-melanoma skin cancer.38 The increased risk was mostly for SCC, with one additional case per 16 000 patients per year.38 Furthermore, various studies have reported that HCTZ can have dose–response dependent co-carcinogenic effects.6, 9-13 Jensen et al. demonstrated an elevated risk of developing MM and SCC tumours on chronically sun-exposed sites in patients taking oral HCTZ combined with amiloride.6
A study by Pedersen et al. that involved 102 366 participants showed that the percentage of skin cancers attributable to the use of HCTZ was approximately 0.6% for BCCs and 9.0% for SCCs.13 No relationships were found between the use of other diuretics or hypertensives and BCCs or SCCs.13 Furthermore, a dose–response relationship was found between the use of HCTZ and skin cancer, regardless of prior or present use of amiloride.13 Results published by Eworuke et al. and Schneider et al. described a similar dose-dependent increase in risk for developing SCC, but not BCC or MM.10, 12 Eworuke et al. reported that HCTZ users tended to live in areas with high levels of UVR, which might have had an impact on their study outcome.10 Compared to the results published by Pedersen et al., Eworuke et al. observed smaller effect estimates for both BCC and SCC when patients had 50 mg or more of cumulative exposure to HCTZ.10, 13 However, when HCTZ–amiloride combination products are excluded from the results reported by Pedersen et al., the findings are more similar to those described by Eworuke et al.10, 13
Lecaros-Astorga et al. reported a tendency toward a relationship between HCTZ treatment and SCC incidence; however, the relationship was not significant.39 Furthermore, Kristensen et al., found that many other photosensitizers did not elevate the risk of skin cancer in epidemiological studies.40 It is therefore intriguing that a relationship between HCTZ use and SCC has been shown in previous studies.6, 7, 9-13, 28-31, 40
A recent short-term randomized placebo-controlled trial by Götzinger et al. found that a daily dose of 25 mg HCTZ for 15 days did not result in photosensitivity to UVA or UVB radiation. Furthermore, no thymidine dimers were detected in the urine of any participants.16 Because no thymidine dimers were detected in the placebo group, it remains unclear whether HCTZ can influence the production of thymidine dimers.16 Furthermore, only repaired thymine dimers are excreted in the urine.16 Therefore, unrepaired DNA damage may occur in the skin that is not detected in urinary assays.
Clearly, one limitation of our study is that it was necessary to use an animal model. However, a major advantage of using hairless mice is that they can be kept in a carefully controlled environment. Humans are exposed to innumerable substances throughout their lives and, consequently, the co-carcinogenicity of HCTZ may be difficult to quantify. Another limitation of our research was that we did not investigate any biomarkers. Biomarkers such as cyclobutane pyrimidine dimers may provide information regarding photocarcinogenic potential before any malignancies are visible.
In our hairless mouse model, we found that mice treated with UVR plus HCTZ did not develop skin tumours (SCCs) more rapidly than mice treated with UVR but not HCTZ.
AUTHOR CONTRIBUTIONS
Original draft preparation of the manuscript, C.M.L. and H.C.W. Review and editing of the manuscript, C.M.L., H.C.W. and R.N.A. Supervision, C.M.L. All the authors have read and agreed to the final published version of the manuscript.
ACKNOWLEDGEMENTS
We thank animal caretaker Catrine Fischer Goldschmidt for helping with the animals. The research was done as a part of the Danish Research Center for Skin Cancer (https://vfhk.org/en) and the Skin Cancer Innovation Clinical Academic Group (SCIN CAG), Greater Copenhagen Health Science Partners (GCHSP).
FUNDING INFORMATION
The work was funded by the Copenhagen University Hospital, Bispebjerg and Frederiksberg.
C.M.L is funded by an unrestricted grant from the Lundbeck Foundation (R307-2018-3318).
CONFLICT OF INTEREST
None declared.
CONSENT FOR PUBLICATION
All the authors hereby consent to publication of the work.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




