Importance of vision in tandem running during colony relocation in an Indian ant
Funding information
All the funding for this study came from Indian Institute of Science Education and Research Kolkata and Engineering Research Board (SERB), India [grant no.: EMR/2017/001457/AS]
Abstract
Visual inputs are important for navigation, and several studies have investigated its importance in ants in the context of foraging. Little is known about the importance of visual cues when the whole colony engages in the goal-oriented task of colony relocation. In this study, we investigated the role of vision for a tandem-running tropical ant Diacamma indicum during colony relocation by impairing the vision in both eyes of all colony members and comparing our findings with ants having unimpaired vision. Colonies relocated successfully under zero-lux conditions and when all colony mates were visually impaired, without compromising their colony cohesion. On examining 265 tandem runs performed by visually impaired ants, we found that they had lower efficiency as compared to ants with normal vision. They traveled along longer routes at lower speeds and often lost their followers, leading to significant increase in transportation time. The absence of visual inputs did not influence the number of tandem leaders that participated nor did it negatively impact their ability to recruit their visually impaired followers from becoming tandem leaders. Visually impaired tandem leaders were not able to minimize their relocation path in an open arena but were capable of doing so in defined paths. Our results suggest that these ants use thigmotactic or proprioceptive cues in order to navigate to their new nest in the absence of visual inputs. Our understanding of tandem-running recruitment would be incomplete until we delineate the role of multiple sensory inputs involved in conducting this mode of recruitment successfully.
Statement of significance
Visually impaired tandem leaders can relocate their nest mates and transfer the information of the relocation path to the colony members.
1 INTRODUCTION
Navigation is a process that enables animals to maintain the path and direction toward the goal (Gallistel, 1990). Animals are known to use several navigational mechanisms during foraging for foods, migration, finding breeding places, and nest relocation. During navigation, animal use inputs from multiple sensory systems (Buehlmann et al., 2020). Vision plays an important role in animal navigation, starting from invertebrates like arthropods to vertebrates. The digger wasp (Philanthus triangulum Fabr.) is known to use visual cue from the objects present near their nest entrance during the homebound journey from foraging (Tinbergen & Kruyt, 1938). While diurnal insects prefer to use polarized light of the sky or position of the sun or terrestrial landmarks (Marshall & Cronin, 2011; Wehner & Müller, 2006), nocturnal insects use celestial bodies like the moon or the milky way for navigation (Dacke et al., 2013; Warrant & Dacke, 2016;). Following Tinbergen's experiment with digger wasp, studies have been conducted on several insects like, hover flies (Collett & Land, 1975), honeybees (Cartwright & Collett, 1983), water striders (Junger & Dahmen, 1991), crickets (Wessnitzer et al., 2008), and fruit flies, and they have showed that visual information is important for navigation to the goal. Almost all of these studies have been conducted in the context of foraging for food.
In addition to visual cues, ants are known to rely on olfactory cues, as well as idiothetic or internal cues which include innate preference for navigation (Freas & Schultheiss, 2018) during foraging as well as relocating their colonies. Various studies have revealed that ants could use both terrestrial and celestial visual cues for navigation. Ants such as Myrmecia pyriformis, Formica japonica, Formica rufra, and Melophorus bagoti use panorama-based navigation where they store a snapshot of the environment during the previous visit (Fukushi, 2001; Narendra et al., 2013; Rosengren & Pamilo, 1978; Wystrach et al., 2011). In addition to the eye, many ant species, such as Cataglyphis sp., Melophorus bagoti, and Myrmecia sp. are known to use ocelli to get the directional information from the celestial cues (i.e., polarized light from the sky, the position of the sun) in general and when their compound eyes were occluded (Fent & Wehner, 1985; Narendra et al., 2011; Narendra & Ribi, 2017; Schwarz et al., 2011). So, it is expected that ants with ocelli can navigate in the absence of terrestrial visual cues.
Nest relocation is a common phenomenon that occurs in social insects. Ant colonies relocate for various reasons; physical damage to their nest is one major reason (McGlynn, 2012). Unlike foraging which involves only a subset of the colony, relocation involves the movement of all the adult members along with brood and the stored resources. Unlike foraging which is a continuous task, relocation is a goal-oriented task that has a defined initiation and termination point. Successful relocation in which all the colony members are recruited to the new nest is important for both the survival and fitness of the colony, and this can be achieved only with efficient navigation. Navigation would require animals to collect information from their environment, store this information, recall and compare this information in order to form optimal routes, and reach the required destination (Freas & Schultheiss, 2018). So, the first step of navigation, that is, the collection of information from the environment, involves several sensory systems of the animals.
The current study has been conducted to examine the importance of vision during colony relocation in Diacamma indicum. This 1 cm long, black, ponerine ant is found in the India, Bangladesh, Sri Lanka, and Japan. Females of this species lack ocelli (Annagiri, 2019). These ants are known to use tandem running exclusively for colony relocation (Kolay & Annagiri, 2015). Tandem running is considered as one of the primitive modes of recruitment where some individuals of the colony have information regarding the target site. Tandem leaders lead their nestmates (followers) to the goal one at a time, with the follower maintaining tactile contact with the leader all along the way (Hingston, 1929; Wilson, 1959). This process does not involve any trail pheromone. Further, they are known to relocate their colony during the day as well as at night (Kaur, 2014) and are known to minimize their relocation path when they encounter a choice between multiple defined paths connecting their old and new nests (Mukhopadhyay et al., 2019) and take relatively straight paths in the natural habitat as well, irrespective of terrains consisting of vegetation or bare patches (Anoop et al., 2021).
The importance of vision for tandem running has not been explored in any detail across ant species. Previous studies with another tandem-running species T. albipennis showed that visual cues from the landmarks are used for determining the relocation path (McLeman et al., 2002) and that these ants maintain a continuous retinal image of an horizontal structure that is parallel to their relocation path (Pratt et al., 2001). Vision helped the tandem followers of this species to track the route of the leader enabling them to become leaders themselves. Thus, to become a tandem leader in T. albipennis, an individual must have at least one functional eye (Franklin et al., 2011). This leads us to ask, can tandem-running recruitment occur in poor light conditions or in the dark. This is particularly important as colonies may have to relocate to a new nest whenever an emergency occurs irrespective of the light conditions. Thus, ants would be facing a tradeoff between survival both in terms of maintaining colony cohesion and saving the developing brood inside a sub-optimal old nest vs. taking the risk of moving into a new optimal nest. We wanted to enquire whether lack of visual inputs will influence this tradeoff.
In order to examine whether these ants are capable of relocating their colonies in the absence of visual cues, we specifically asked five questions. We started by investigating whether these ants are able to relocate under complete dark conditions where no visual cues can be perceived. Secondly, we asked whether these ants are able to relocate when all the colony member's vision was impaired. Thirdly, we wanted to know whether ants with completely impaired vision are capable of becoming tandem leaders and whether these leaders displayed a different relocation dynamic as compared to control ants with normal or unimpaired vision. Next, we wanted to know whether these visually impaired ants use an alternate sensory system in the absence of vision. Finally, we wanted to check the effect of impaired vision on the path minimization ability of these ants. All these experiments were conducted inside the laboratory, and both colony-level and individual-level analyses were performed.
2 MATERIALS AND METHOD
Diacamma indicum colonies (n = 48) were collected from Nadia, West Bengal, from July 2018 to December 2019 using the nest flooding method (Kaur, 2014). These ants are not an endangered species and the area from where the colonies were collected is not a protected area. No special permission was required for collecting colonies from this area. Our experiments comply with regulations for animal care in India. After collection, these colonies were brought to the laboratory and maintained following the standard method (Mukhopadhyay et al., 2019) for experiments. All adult females of the colony were given individual identities by marking one or more of their body parts (1st and 2nd thoracic segments and gaster) with non-toxic enamel paint (Testors). Ants were not anesthetized for the purpose of marking; they were held captive with gloved fingers for a short period of time in order to apply the paint. Each colony was used for a single relocation.
2.1 Experimental setups
All relocations were performed in the laboratory in a 60 cm × 90 cm arena with a base constituted by a mixture of sand and soil. For each relocation, the nest containing the colony, referred to as the old nest, was placed in one corner of the arena, and an empty nest (new nest) which was identical to the old nest, was placed at the diagonally opposite corner of the arena (unless mentioned otherwise). To prevent ants from escaping, the walls of the arena were coated with petroleum jelly (Vaseline, Hindustan Unilever Limited). The relocation was initiated by removing the roof of the old nest (Petri plate cover) and placing a white light 15cm above the old nest to motivate the colonies to relocate to the new nest. The whole relocation process was recorded using a video camera (Panasonic, model: HC-V270). Following the last tandem run and all the transfer of all brood items to the new nest, we waited for 30min to ensure that there were no additional transport events before stopping the experiment and considering the relocation as being completed. After each relocation, the sand and soil base of the arena were shuffled thoroughly to avoid the influence of any chemical substances left behind by the previous colony and a gap of at least 12–15 h was given between each relocation experiments.
The overarching aim of this study is to check the importance of vision in D. indicum during tandem running. To address this query, we have conducted four different sets of relocation experiments, which are described below.
2.1.1 Relocation in the dark
This set of relocations was conducted to check whether these ants were able to relocate in completely dark conditions (lower than 1 lux as recorded by Lutron digital lux meter, model: LX101A) when visual cue would not aid the relocation process. Nine colonies having 90 ± 31.54 adults were used for this set of experiments. The 60 cm × 90 cm arena was covered by a 10mm thick plywood in order to ensure complete darkness within this arena. In these experiments, the colony was left undisturbed inside this arena for a night after removal of the roof of the old nest (Petri plate cover). On the following morning, we checked whether the colonies had relocated to the new nest or not by removing the plywood cover of the arena. This set of relocations could not provide us any detail of the relocation process at the individual level as it was not possible to record the relocation process. Only the final result, that is, if colonies relocated or failed to do so, was considered.
Thus, to examine the relocation efficiency in the absence of visual cues, another set of experiments termed as vision-impaired relocations were conducted. The purpose of these experiments was to block the vision of ants in order to prevent them from perceiving any visual cues. This would mimic the zero-lux relocation but would allow us to perform the same under normal light and thus enable us to record the process. We performed three different sets of experiments with colonies containing visually impaired ants to answer three different questions. The details of each of these experiments and their purpose have been described below.
2.1.2 Vision-impaired relocation in the open arena
The aim of this experiment was to examine the efficiency of the relocation process and tandem leaders, in the absence of vision. Nine colonies having 92 ± 36.92 adults were used in this set of relocations which was also referred to as the test relocation. All the members of the colonies were blinded using non-toxic enamel paints (Testors) on both their compound eyes. We used the same enamel paints which were used for marking the individual ants, to manipulate their vision. Unlike many other ants, D. indicum does not have ocelli (Annagiri, 2019); thus, covering their compound eyes with paint would completely impair their vision. A small drop of non-toxic enamel paint was taken on the tip of a dissection pin and carefully applied to both the eyes of the ant (Figure 1B). In order to apply this impairment, ants were not anesthetized. They were held captive between two gloved fingers, and the paint was applied on their compound eyes carefully avoiding the antenna and mouth parts. After blinding all the adult females of the colony, they were allowed to rest for 10–12 h. Two hours prior to the relocation, all members were examined to confirm that they had not groomed off the paint. If any colony member was found to have removed a part of the paint from their compound eye, this member was repainted to ensure that all the ants in the colony were visually impaired at the start of the experiment. Only a small number of ants were found to have removed a part of the paint from their eye (less than 5% of the colony). Further, all members of the colony including those in the control received the same paint on different parts of their body and thus they can be considered as sham control for the paint and handling of ants. At the end of 48 h, we found that almost all the ants had removed the paint from their eyes and that our blinding process was only a temporary event.

Another ten colonies with 94 ± 38.67 adult females were used in control relocation, where colony member were not visually impaired by putting paint on their eyes. The abiotic factors like the arena dimensions, light, old/new nest, and distance between them were identical between this control set and the vision-impaired relocation. The initiation of the relocation was also identical with the old nest's roof being removed and a white light being placed above them, but note that visually impaired ants may not have perceived this light in a similar manner as control ants. All the other procedures regarding the collection of data were the same across the test and control sets. The colony sizes in vision impaired and control relocations were comparable (Mann Whitney test, N = 9, N2 = 10, p = .97). The arena was divided into several grids of 10 cm × 10 cm, using thin white cotton threads that were placed 5 cm above the arena floor, and each grid was labeled uniquely and enabled us to track the path taken by tandem leaders during their transports. As these threads were well above the floor of the arena on which ants were traveling, this did not impact the relocation process in any way. A video recorder (Panasonic, model: HC-V270) was placed above the arena to track the relocation path of the ants for both vision impaired and control relocation. A second video recorder was placed at the top of the new nest to record the behavior of the ants at new nest site.
2.1.3 Importance of tactile (or thigmotactic) cue from the walls of the arena:
This set of experiment was conducted to check whether D. indicum were guided by the arena walls to locate the nest position during relocation in vision-impaired condition. This set of relocations were conducted with visually impaired ants in a 185 cm × 145 cm arena (larger arena). The procedure to manipulate the ants’ vision, initiate relocation, and record data in this set of experiments was identical to what has been described in experimental setup section except for one point. The old nest was placed at one corner of the arena while the new nest was placed at a distance of 90 cm (similar to the previous experiments) from the old nest, but this time we placed the new nest in the middle of the arena keeping a minimum distance of 60cm from the arena wall. This placement would remove any tactile or thigmotactic cues from the arena walls that the ants might use to navigate to the new nest. Colonies (n = 10) having 82 ± 22.9 adult females were used in this relocation setup.
2.1.4 Path minimization in vision-impaired ants
This set of experiments was conducted to check whether the visually impaired ants are capable of minimizing their path during relocation. Relocations were conducted in a defined path setup instead of the open arena setup used in the previous experiments. A wooden bridge (design adopted from Goss et al., 1989), which had a combination of four paths with three different lengths including two bifurcated decision points, was used (for details see Mukhopadhyay et al., 2019 (fig S1). The relocation procedure was similar to what was done in the previous experiments. Colonies were placed in a box at one end of the bridge, and the roof of their nest was removed to initiate colony relocation. Individuals would have to travel through defined paths in order to reach their new nest which was identical to the old nest, except that it contained an intact roof. Individuals could take either the short arm (SS path) at both of the decision points which would be the shortest route or they could take the long arm (LL path), making it the longest possible route between the nests. If ants followed the walls of the path, they would end up taking an intermediate length path that is a short followed by long arm (SL path) or a long arm followed by short arm (LS path) to reach their new nest. A video recorder was placed at the midpoint of the bridge, where it can cover both the decision points and record the relocation process. Eight colonies with 94 ± 11.49 adults were used for this set of relocation experiment. The number of individuals present this set of relocations was comparable to the number of individuals of the colonies that had been used in combined path relocation (99.56 ± 27.80) experiments in Mukhopadhyay et al., 2019 (Mann–Whitney U test, N1 = 8, N2 = 9, p = .62).
2.2 Behavioral observation
Behavioral observation was conducted using real-time data collection and video recorders. Information regarding the time at which colony was placed inside the arena, the time at which 1st individual of the colony discovered the new nest, the time at which the first tandem run reached the new nest (designated as the start of transport) and the last tandem run reached the new nest (designated as the end of transport) were recorded for each relocation except for the relocations in the dark setup. Based on these inputs, discovery time (time between placing the colony in the arena and the first individual discovering the new nest), latency (time between the first individual discovering the new nest and the first tandem pair reaching the new nest), and transportation time (time between start and end of transportation) were calculated.
Data were also collected at the level of individual ants for different parameters across all the relocations expect for the relocations in the dark setup. All ants who came out from the disturbed old nest and explored the arena were designated as explorers. We noted the identities of the explorers for the open arena relocation experiments as well as on different paths in case of defined path relocation setup.
Once transportation started, the identity of the tandem leader, the follower, initiation time, initiation site, end time, and termination site was noted for each tandem pair. Based on these inputs, we calculated the number of primary leaders (who acquired information regarding the path to the new nest by exploring the arena themselves) and secondary leaders (leaders who acquired the information regarding the path to the new nest as a follower of a tandem run). Tandem runs, which were initiated from the old nest with a particular leader-follower pair and terminated at the new nest as the same pair, were considered as successful tandem runs. Only the successful tandem runs were considered for further analysis. If the initial leader-follower pair got separated from each other (denoted as pair interruption) on the way to the new nest or if the lost follower was taken by another leader (denoted as leader switch), they were designated as unsuccessful tandem run. The percentage of successful tandem runs was calculated for both vision impaired and control relocations conducted in the open arena. The number of unique leaders was noted at every 10% time interval of the total transportation time by keeping track of the identity of leaders. From the video recording, the number of tandem runs that occurred at every 10% time interval of total transportation time was also noted. Based on these data, percentage leader recruitment and percentage of transportation progression over time were calculated and the relationship between this was analyzed to examine the manner in which leaders got recruited into the relocation, both in control and vision-impaired relocation.
Paths of individual tandem runs were decoded from the video recordings for open arena setups for both vision impaired and control relocation. The arena was divided into several grids (length 10 cm and width 10 cm). Each grid was given a number and an alphabet, in order to identify every grid uniquely within the arena. The path traveled by the leader with her follower from the old nest to the new nest for each transport was reconstructed manually by tracking the grids through which they traversed by following the video recordings and voice recordings. Automatic tracking was not possible because the unique identity of these ants was not decipherable from the video reliably and this was compounded further whenever several ants gathered at a given location. By plotting the x and y coordinates (unique id of the grids) over time, we were able to track the movement of tandem runs and use this for our analysis. Based on these entries, path length (length of the path traveled from the old to new nest), path efficiency (which is the deviation of the actual path traveled from the shortest distance between the old and new nests), and speed (distance traveled per unit time) of each tandem run were calculated Using a tracking code developed by (Paul, 2019). Path efficiency was calculated by dividing the shortest distance from the old to new nests with the actual path traveled by a given tandem leader with her follower. This was calculated for every tandem runs. The values of path efficiency can range from 0 to 1, with 1 being the most efficient. A total of 879 unique successful tandem runs (265 runs from vision-impaired setup and 614 runs from control setup) were considered for these analyses.
While tracking the tandem runs, we noted whether the tandem leaders and followers were walking along the walls of the arena. If leaders were observed touching the walls of the arena for at least 5 successive grids (about 50 cm) as they progressed to the new nest, we noted them as using tactile cues. We later calculated the percentage of tandem runs that followed the arena walls for both control and vision-impaired relocations. The path lengths of the first and final tandem runs of individual leaders who performed a minimum of 2 complete tandem runs in each relocation were calculated and compared. The number of transport and the number of unique tandem leaders that emerged in every relocation were noted. The number of tandem runs led by individual leaders was recorded. The manner in which these tandem runs were distributed among the leaders of the colony was compared across control and treatment relocations. For each colony, the tandem leader who had performed the highest number of tandem runs was denoted as Maximum tandem leaders (Max TL). The percentage of tandem runs performed by Max TL was also calculated for both vision impaired and control open arena relocation.
In the minimization experiment with defined path, we recorded the tandem-running data in the same manner as described in the previous section However, in this setup, the paths preferred for transportation were recorded by considering the choice of tandem leaders at the two different decision points. We recorded the path taken by individual leaders and combined this to get information at the colony level. Individual leaders that performed a minimum of two complete transports were considered for individual-level analysis.
2.3 Statistical analysis
Statistical tests were performed using StatistiXL (version 2.0) and R (version 4.1.0). Two-tailed non-parametric tests, generalized least-squares model (GLS), generalized linear model (GLM), and generalized linear mixed-effect model GLMM, package glmmTMB; (Brooks et al., 2017) were used for all the analysis. For pairwise comparison, emmeans package version 1.6.1; (Lenth, 2021) was used. The details of each model have been presented as supplementary data. A value of p < .05 was considered as the cutoff value for statistical significance. Unless mentioned otherwise, mean ± standard deviation values of all parameters have been presented.
3 RESULT
3.1 Relocation in the dark
All colonies (n = 9) that were placed in the zero-lux arena relocated successfully. All the adult members and brood of the colony were transported into the new nest.
3.2 Effect of vision impairment on colony relocation in the open arena
By masking the ants’ eye, we were able to create the same effect as zero lux, but this allowed us to decipher how the process of relocation proceeded in the absence of visual inputs. We performed experiments with all the ants in the colony being normal (control) and all members being visually impaired and contrasted our findings at the level of the colony and at the level of tandem leaders.
3.2.1 Overall relocation dynamics
All the 19 colonies used for these relocations relocated successfully. We found that colony size had an influence on the discovery time, but blinding did not significantly affect it. The discovery time for the vision impaired (18.22 ± 10.59 min) and control (12 ± 7.04 min) setups was comparable (GLM, t = −0.35, p = .19; for details see table S1; Figure 2A). Latency was influenced by the colony size and discovery time but the vision impairment of ants' eyes did not affect it. Latency for vision-impaired and control relocation setups were 20.11 ± 20.76 min and 17.9 ± 8.62 min, respectively (GLM, t = 0.11, p = .77, for details see table S2; Figure 2B). The percentage of individuals who became explorers in both vision-impaired (5.62 ± 3.51) and control (5.75 ± 4.57) relocation setups were not significantly different (Mann–Whitney U test, N1 = 9, N2 = 10, p = .90). The percentage of individuals who became leaders in vision-impaired and control setups was 13.67 ± 2.99 and 15.07 ± 4.27, respectively, and this was also not significantly different (Mann–Whitney U test, N1 = 9, N2 = 10, p = .59).
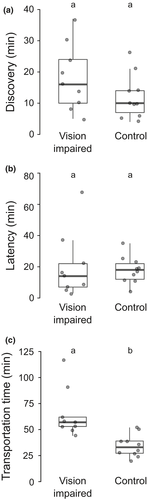
The transportation time was significantly higher in vision-impaired relocation experiments as compared to the control relocation experiments. This result was influenced by colony size, and the condition of ants' eye. The average transportation time for vision-impaired relocation setup (64.88 ± 23.7 min) was approximately two times higher on average as compared to control relocations (35.2 ± 10.59 min) (GLM; t = −0.46, p < .01, for details see table S3; Figure 2C). The percentage of tandem runs performed by Max TLs was compared across vision-impaired (28.83 ± 17.34) and control (26.71 ± 14.38) relocations and was found to be not significantly different (Mann–Whitney U test, N1 = 9, N2 = 10, p = .7). The percentages of primary and secondary leaders in both vision impaired and control relocations were also not significantly different (Table S4).
The percentage of successful tandem runs in vision-impaired relocations (41.73 ± 11.78) was significantly lower than control relocations (80.14 ± 6.44) (Mann–Whitney U test, N1 = 9, N2 = 10, p < .01). The percentage of tandem runs that touched the arena walls in vision-impaired relocation (88.36 ± 8.56%) before reaching the new nest was significantly higher than control relocation (25.17 ± 14.08%) (Mann–Whitney U test, N1 = 9, N2 = 10, p < .01).
3.2.2 Recruitment efficiency
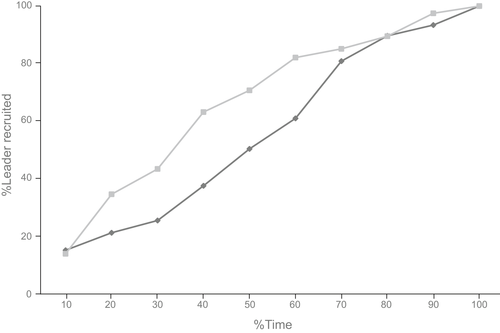
The extended transportation time in vision-impaired ant relocation can be a result of lower relocation efficiency. In order to analyze this further, we investigated two parameters, the manner in which recruitment of leaders occurred over relocation time and the manner in which transportation progressed over time relocation. Either lower rate of leader recruitment or lower performance of tandem runs by these leaders or a combination of these two factors can cause a decrease in relocation efficiency. A generalized least-squares model (GLS) was used to compare both these factors. The leader recruitment progress in vision-impaired and control relocation was significantly different (GLS, t = −2.55, p = .011, for details see table S5; Figure 3). Vision-impaired relocations consistently showed a slightly higher recruitment of leaders as compared to control relocations, even though the percentage of individuals who became leaders was not significantly different. Vision-impaired leaders were recruited earlier in the process as compared to unimpaired leaders control relocations. The progress of transports over time in control and vision-impaired relocation did not significantly differ (GLS, t = 1.06, p = .29; see table S6).
3.2.3 Efficiency of individual tandem run
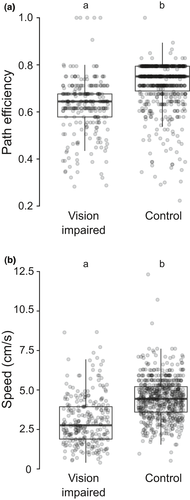
The delay in transportation time in vision-impaired relocation could be due to slower tandem runs by visually impaired leaders. In order to examine this, we conducted further analysis on the efficiency of tandem runs. The efficiency of the individual tandem run was determined by three parameters—the length of the path traveled from the old nest to the new nest, path efficiency, and the speed of the tandem run. A total of 879 tandem runs from 19 colonies across the vision-impaired and control relocations were successful as the same leader and follower pair reached the new nest, having started from their old nest. The length of the path traveled by vision-impaired tandem leaders was significantly higher than that taken by ants with regular vision in the control experiment. The average path length of tandem runs in vision-impaired (n = 265) and control (n = 614) setup was 133.35 ± 31.42 cm and 114.69 ± 24.86 cm, respectively (GLMM; z = −7.43, p < .01; for details, see table S7). Note that the minimum path length between two nests is 90 cm. If tandem leaders traveled about 90 cm to reach the new nest, they would get path efficiency value close to 1; however, if they take highly meandering path to the new nest, they would have path efficiency values closer to 0. The path efficiency of individual tandem run in the vision-impaired (0.63 ± 0.12) setup was significantly lower than the path efficiency in the control (0.72 ± 0.09) setup (GLMM; z = −0.98, p < .0001, for details see table S8; Figure 4A). Average speed of individual tandem runs in vision-impaired relocation (3.40 ± 4.45 cm/s) was significantly slower than in control relocations (4.46 ± 1.28 cm/s) (GLMM; z = −6.69, p < .0001, for details see table S9; Figure 4B).
3.3 Importance of tactile (or thigmotactic) cue from the walls of the arena
In vision-impaired relocations in the open arena presented in the previous section, it was found that almost 90% of the tandem runs followed the arena walls by physically touching them before entering the new nest. So, in this set of relocations, the new nest was placed away from the arena walls. Out of 10 colonies, only one colony relocated successfully to the new nest (Binomial test, p < .01). The remaining nine colonies did not even discover the new nest in 3 h, which is around 2.8 times longer than the total relocation time of control relocations. These colonies scattered away from the roofless old nest and were seen in small clusters near the corner of the arena and elsewhere, but no adults or brood reached the new nest within 3 h.
3.4 Path minimization in vision-impaired ants
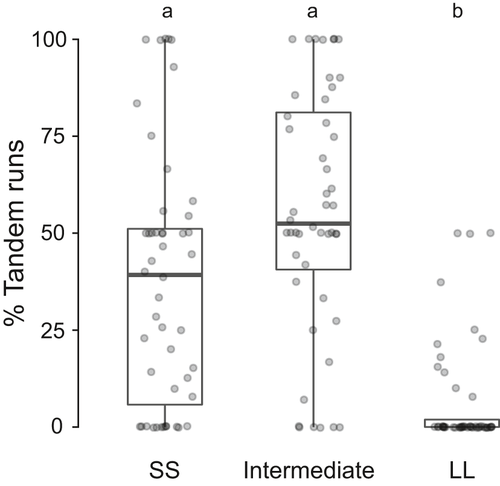
This set of experiments were performed to check the impact of visual impairment on path minimization when a combination of paths with different lengths was provided. The relocation dynamics of these relocations with vision-impaired ants were compared with relocations using the same setup where the ants were not manipulated, as described in Mukhopadhyay et al. (2019). On comparing control relocation with visually impaired ant relocations, we found that several relocation parameters namely discovery, latency, transportation time, percentage explorers, and percentage leaders were not significantly different for both sets of experimental setups (Table S10). From the vision-impaired relocation in the open arena, we found that the visually impaired ants followed the walls of the arena during tandem running. In the case of path minimization (defined path) relocation, if ants followed the walls of the bridge, then they are expected to take the path with intermediated length, as the bridge was designed in such a manner to avoid right or left bias (fig. S1). However, this is not what we found in the path minimization relocations with visually impaired ants. Results at the colony level and individual tandem leaders’ level showed that the SS and intermediate path were preferred over the LL path. The details of the colony level analysis have been given as table S11. The percentage of tandem runs that traveled along the shortest (SS- 33.82 ± 16.73) path was comparable to the percentage of tandem runs that followed intermediate (SL and LS- 60.06 ± 11.57) paths and both of these were significantly higher than the longest (LL- 6.12 ± 6.95) path. For analysis at the level of individual leaders, only the leaders who had done at least two transports from the old to the new nest successfully were considered. Of the 48 such tandem leaders, five used the shortest path, seven leaders used either of the intermediate paths exclusively and 36 leaders used all the four available paths for transportation. None of these leaders used the longest path only for transporting the colony. They showed a significant preference for the shortest and intermediate paths over the longest paths along their outward journey. Among the 48 tandem leaders, 37.79 ± 32.77% of their tandem runs occurred on the SS path, 55.49 ± 31.59% on intermediate path, and 6.71 ± 14.04% on the LL path (Friedman test, chi-square = 42, df = 2, p = 0.05; post hoc. Wilcoxon paired-sample test with Bonferroni's correction, SS versus intermediate, p = 0.04; SS versus LL, p < .01; intermediate vs. LL, p < .01 (following Bonferroni's correction for a significant difference, p should be lower than 0.016); Figure 5). In control (Mukhopadhyay et al., 2019), individual leaders (n = 77) showed equal preference for SS (55.53 ± 35.64%) and intermediate (40.07 ± 33.44%) path while the preference for LL (4.39 ± 10.81%) path was significantly lower Friedman test, chi-square =68.9, df =2, p < .01; post hoc. Wilcoxon paired-sample test with Bonferroni's correction, SS versus intermediate, p = .03; SS versus LL, p < .01; intermediate vs. LL, p < .01 (following Bonferroni's correction for a significant difference, p should be lower than 0.016).
In order to get a more comprehensive picture of path minimization, we wanted to find out whether leaders minimized their path in the open arena relocations as well. On comparing the length of the path of first and final tandem run for individual leaders in case of control relocation (n = 107) in the open arena relocations (fig. S2), we found that the average path length of final tandem run (110.05 ± 13.46cm) was significantly lower than the first tandem run (135.88 ± 46.85 cm) of an individual leader (GLMM, z = 4.44, p < .01; pairwise comparison with emmeans, t = - 6.62, p < .01; table S12). Whereas, in case of vision-impaired relocation (n = 52), the average path length of first tandem run (136.96 ± 36.84 cm) and the final tandem run (139.41 ± 31.03 cm) of an individual leader was statistically comparable (GLMM, z = 4.44, p < .01; pairwise comparison with emmeans, t = 0.31, p = .76; table S12). The average path length of the first tandem run of an individual leader in control and vision-impaired relocation experiment was statistically comparable (GLMM, z = 4.44, p < .01; pairwise comparison with emmeans, t = - 0.04, p = .97; table S12) while the average path length of the final tandem run of an individual leader in control relocation was significantly lower than vision-impaired relocation (GLMM, z = 4.44, p < .01; pairwise comparison with emmeans, t = 5.36, p < .01; table S12).
4 DISCUSSION
Whenever the nest quality gets compromised, colonies relocate to a comparatively good quality shelter (McGlynn, 2012). Unlike foraging where a subset of the colony is involved, relocation involves movement of the whole colony along with brood and reproductive individual as well as stored resources. Successful relocation is crucial for the survival and fitness of the colony (Visscher, 2007). Thus, it is essential that the relocation process be optimized to occur across a wide range of conditions. Under normal conditions, inputs from different sensory systems like vision, olfactory, and tactile would be involved in conducting this process successful.
In the current study, we investigated the importance of vision in the process of relocation, in D. indicum that uses only tandem running for recruitment to the new nest. This is the first study on ant navigation during relocation using colonies in which all ants were visually impaired. This study showed that in the absence of vision these ants performed tandem runs and successfully relocated to their new nest unlike T. albipennis (Franklin et al., 2011). Visually impaired tandem leaders relied on thigmotactic cues to locate the position of the new nest, when the nests were along the walls of the arena, but failed to relocate when this such cues were eliminated. To date, navigation in vision-impaired ants has been investigated only in T. albipennis in the context of relocation. When both eyes of T. albipennis were occluded, they were mostly incapable of becoming tandem leaders and leading a follower, as only two leaders with both eyes blinkered were observed to perform less than 4% of the tandem runs within the colony (Franklin et al., 2011). In contrast, both eyes visually impaired D. indicum ants became tandem leaders and performed tandem runs. The total number of leaders, the manner in which they functioned, was comparable to normal ants in control experiments. However, the manner in which tandem leaders were recruited was significantly different with more leaders starting earlier as compared to visually unimpaired ants. The organization of colony relocation was comparable with similar percentage of leaders participating in both vision-impaired and control relocations, indicating that visually impaired ants were equally capable of tandem running. This difference would be due to multiple mutually non-exclusive reasons. We can speculate these reasons, but further experiments will be required to confirm the same. T. albipennis lives in rock crevices where light penetration inside the nest is probably higher, and they may depend more on visual cues to perform different activities both inside and outside the nest. On the contrary, D. indicum lives in subterranean nests with an average depth of 10.59 ± 4.48 cm (Bhattacharyya & Annagiri, 2019) where the light penetration probably is very low and they may be adapted to very low light conditions. It is also possible that visual cues play a role in D. indicum but they have alternate methods including proprioceptive cues to comprehend their environment and synergistically perform critical functions. Also, D. indicum uses only tandem running throughout the recruitment of their nestmates to the new nest unlike Temnothorax albipennis, Camponotus sericeus, and Pachycondyla tesserinoda that perform a combination of tandem running and carrying of adults during relocation (Franklin, 2014) making tandem running particularly important for this species. Hence, it is possible that these ants have adopted to perform tandem running across a whole range of light conditions.
Even though vision-impaired D. indicum relocated their colonies successfully, they did so at a cost of additional time. The transportation time was longer by two times as compared to control colonies. This result is similar to previous experiments with D. indicum which showed that visual landmarks at the nest entrance enabled colonies to complete transportation faster as compared to nests without visual landmarks at the entrance. When visual landmarks were removed midway through the relocation, colonies continued to relocate, but they took approximately 1.5 times longer, indicating that these ants do not rely entirely on the visual inputs for relocation (personal observations, 2016–2019). In the current experiment, we wanted to understand the reasons that caused visually impaired colonies to take additional time to complete transportation.
One of the important reasons was the number of successful tandem runs. In the case of the vision-impaired relocations, almost 80% of the tandem runs were interrupted during transportation. The high number of incomplete or interrupted tandem runs in the vision-impaired relocations indicates that maintaining the tandem pair cohesion requires visual inputs. This decreased the overall efficiency of transportation, as lost followers had to be located and tandem run to the new nest mostly by other leaders. In most of the cases, we found that once the followers got separated from their leaders, they were taken by other returning leaders who were coming from the new nest toward the old nest within five minutes of being lost. However, not a single nestmate was lost and all colony members were eventually brought to the new nest. Another factor that adds to the increased transportation time was the decrease in the efficiency of individual tandem runs, by analyzing three parameters, such as the path length traveled, path efficiency, and speed of individual tandem runs. The path length was longer, the path efficiency was lower and the speed of tandem running was lower in the case of visually impaired ants as compared to control ants, delaying the overall transportation.
Although the percentage of individual became tandem leaders was not influenced by the impairment of vision of these ants, the recruitment of the leaders was significantly higher in visually impaired relocation. At midpoint of transportation time, 70% of all leaders were recruited in visual impaired relocation while in control experiment only 50% of the total leaders were recruited. On the contrary, progression of transport of colony members over time was not significantly different across both the relocation setups, which indicates that to maintain the pace of transportation over time, visually impaired colonies recruited higher number of leaders. In addition, the performance of Max. tandem leaders was also not influenced by the impaired vision.
In D. indicum, we found that they rely on thigmotactic cues from the walls along their route. Visually impaired tandem leaders were seen frequently touching the walls while they tandem run significantly more often than control tandem leaders. Subsequently visually impaired leaders took a longer route into their new nest as they followed the walls of the arena while control leaders took more direct routes to the new nest. When thigmotactic cues along the route were eliminated by placing the new nest away from the walls, we found that visually impaired ants were not able to locate the new nest nor relocate into them, clearly indicating that thigmotactic cues acted as a backup for blind tandem leaders to conduct both exploration of the arena and recruitment.
On examining the path minimization or the ability to increase their path efficiency with experience, we found that control tandem leaders showed that they are capable of doing so. The last tandem run was 1.2 times more efficient as compared to the first tandem run they performed in an open arena relocation. However, such an improvement was not seen in tandem leaders with impaired vision. They continued to have comparable path efficiency across the first and last tandem run, in open arena relocations. We found that the average path length of the first tandem run of individual leaders in the control and vision-impaired relocations in the open arena was statistically comparable, whereas the average length of the final tandem run of control relocation was significantly lower than the vision-impaired relocation. Thus, in both cases, the leaders initiated the transportation with similar efficiency but, visually impaired leaders did not make corrections to their path in order to reduce their path length and hence the transport faster over time. As a result, it led to faster transportation time and total relocation time. Following the walls certainly impacted the path minimization of visually impaired ants and possibly imposed restrictions, so we investigated the ability of those ants to minimize their path in a separate set of defined path experiments. D. indicum tandem leaders are known to minimize their path when given a choice between different defined paths. Ants with fully functional eyes preferred the shortest path when they were provided with a wooden bridge having a combination of paths of three different lengths. The design of the bridge was such that if the ants followed the walls of the bridge, they would end up taking the intermediate path (Mukhopadhyay et al., 2019). However, in the case of visually impaired ants in the current study when they were given the same choice, we found that they selected the shortest path and the intermediate path with equal frequency in the current study, similar to control ants. If thigmotactic cues were the only factor, we would not have expected this result. Thus, ants with impaired vision are using a combination of thigmotactic cues and some other means to achieve path minimization on facing defined paths. We expect them to be using path integration to achieve path minimization but additional experiments are required to confirm this hypothesis. In order to fully understand these results and see whether this finding is unique to D. indicum, we would need additional experiments with other tandem-running species like T. albipennis in which ants with both eye impaired have access to thigmotactic cues.
Also, tandem running is considered to be a unique recruitment process as it is considered to be the first example of teaching among invertebrates (Franks & Richardson, 2006) with tandem leaders teaching followers the route to their destination. In T. albipennies where this was first investigated, ants with both of their eyes impaired were unlikely to conduct tandem runs and hence were not able to pass on information regarding their new nest to other nestmates (Franklin et al., 2011). But in D. indicum ants with both their eyes impaired performed tandem runs and with equal propensity as control ants. Further, several of their followers became tandem leaders in turn. These recruited leaders were termed secondary leaders in this study and we found that the percentage of secondary leaders that emerged in both vision-impaired and control relocations were comparable. Thus, visually impaired D. indicum leaders were able to transfer the knowledge of the new nest and its route to their visually impaired followers, in the presence of thigmotactic cues. This allows us to conclude that, vision per se is not required for leaders to teach and for followers to learn the route, other sensory inputs can serve as a backup for this process to occur.
In conclusion, we found that D. indicum is capable of performing the goal oriented, essential task of relocation when deprived of visual inputs. They continued to use tandem-running recruitment and relocated to the new nest without compromising their colony cohesion, but at the additional cost of time. Visually impaired tandem leaders used thigmotactic cues in order to perform tandem runs at lower speeds, with lower path efficiency and frequently lost their followers leading to an increase in their transportation time. Tandem leaders with impaired vision were able to recruit other members to the task and the work organization mostly remained unaltered. Performing manipulative experiments involving thigmotactic inputs would be the next step toward understanding tandem-running recruitment. Further, we would have to understand not only the individual contribution by different sensory modalities but also how this information is integrated in order to perform the behavior of tandem running.
ACKNOWLEDGMENTS
We thank Mr. Basudev Ghosh for his assistance in the collection and maintenance of D. indicum colonies. We thank Mr. Subhasis Halder for processing the pictures of visually impaired ants. We thank Ms. Sandhya S who joined the laboratory as summer trainee and helped to take data during vision-impaired relocation experiments. We thank Dr. Bishwarup Paul for his valuable inputs in statistical tests. We thank two reviewers for their inputs which has helped us improve this manuscript.
CONFLICT OF INTERESTS
The authors declare no competing interest.
ETHICS APPROVAL
We have conducted all our experiments in accordance with the guidelines that are applicable to working with the model organism in our country. We have collected colonies from areas close to human habitation that is not part of any protected areas or forests. Colonies were maintained in the laboratory with ad libitum food and water and after the experiment they were released back in the habitat from which they were collected.




