Genital movements are not restricted to spermatozoa transfer in a haplogyne spider
Abstract
Internally fertilized animals are characterized by the transfer of their spermatozoa during copulation. However, the duration of copulation is highly variable, which suggests that they may serve for other functions apart from spermatozoa transfer. For example, during copulation, males can stimulate the female by using genitalic movements and/or by positioning their spermatozoa adequately within the female genitalia. In the spider Holocnemus pluchei, males perform strong squeezes and torsion movements with their pedipalps (part of their genitalia actively related to copulation) inside the female genitalia and transfer their spermatozoa during Phase I of copulation. However, the exact moment in which such transfer occurs and its relation to the pedipalp movements are still unknown. Herein, we first have identified the precise moment when the spermatozoa transfer occurs during phase I and its relation to genital movements by interrupting mating couples at the beginning, at middle and at end of this phase. Subsequently, the spermatozoa number remaining in male genitalia and stored in female genitalia were counted. We detected no relation between the number of genital movements and the spermatozoa number present in both, female and male genitalia and body size. As stated above, male genital movements serve another role during copulation in addition to spermatozoa transfer in a context of post-copulatory sexual selection; most likely, they stimulate the female in a cryptic female choice framework.
1 INTRODUCTION
Copulation is the scenario where the spermatozoa transfer occurs and its duration can vary considerably, both within and between species (see Simmons, 2001). In insects, for example, it may last from a few seconds to several days (Mitchell & Mau, 1969; Spielman, 1964), whereas in spiders it may take from a few seconds to almost 20 h (Mammola et al., 2017). Based on the aforementioned, it can be thought that copulation duration could be explained not only by the spermatozoa transfer (Sears et al., 2020; Weggelaar et al., 2018). Increasing the duration of copulation also increases the risks for individuals, for example by exposing them to predators, prevents them from devoting time to other activities, such as foraging, or causes damage to females from male behaviours and transferred substances (Daly, 1978; Edvardsson & Canal, 2006; Simmons, 2001; Watson et al., 1998). So, it is reasonable to assume that long copulation has an adaptive function in sexual reproduction. Indeed, other functions have been proposed to explain the evolution of long copulations, as the transfer of substances that affect the female remating propensity, the manipulation of spermatozoa from previous matings, the placement of spermatozoa in specific regions within the female storage sites, the guarding of a recent mate against other male competitors (Barnett & Telford, 1994; Eberhard, 1996; Edvardsson & Canal, 2006; Simmons, 2001) and/or the female stimulation by male genital or non-genital structures (reviewed in Eberhard, 1991, 1994, 1996; Eberhard & Lehmann, 2019). Related to the last function, it has been proposed that the movements of male genitalia during copulation may have evolved through the so-called cryptic female choice (CFC) and that such movements can be used as a copulatory courtship (Eberhard, 1996, 2010, 2011). Male stimulation can induce a differential use or expulsion of the stored spermatozoa by females, faster oviposition and/or decreased sexual receptivity (Eberhard, 2009) [for a complete and detailed list of the more than 20 possible mechanisms of CFC, see Eberhard (1996)].
To unravel the precise function of genital movements, it is important to differentiate the process of spermatozoa transfer from those processes associated with post-copulatory sexual selection mechanisms. For instance, establishing the exact moment when the spermatozoa transfer occurs allows us to think that male genitalia movements are likely to increase the stimulation of the females in a CFC framework if these movements are not related (at least in their totality) with spermatozoa transfer (see Eberhard & Lehmann, 2019). In other words, detaching these processes allow us to infer with certainty if these genital movements evolved in a context of post-copulatory sexual selection. Nonetheless, in the context of post-copulatory sexual selection, it is not only necessary to be able to dissociate spermatozoa transfer from genital movements, but it is essential to be able to associate these movements with a possible function. Therefore, it is interesting to focus not only on a male-centred perspective, but also on the spermatozoa stored by the females to have a wider holistic view of the copulatory process.
The most common animal models used for studies about stimulation are the arthropods (Eberhard, 2017). Such is the case of spider's species belonging to the Pholcidae family that have been widely used in post-copulatory sexual selection studies in the last decades (reviewed by Calbacho-Rosa & Peretti, 2015). In these animals, pedipalp movements (i.e., movements of the genitalia) within the female genitalia performed by males during copulation have been associated with female stimulation (Huber & Eberhard, 1997; Peretti & Eberhard, 2010; Schäfer & Uhl, 2002). However, in Holocnemus pluchei and Pholcus phalangioides have been suggested that the pedipalp movements (i.e., the strong squeezes) could also be related to spermatozoa transfer (Calbacho-Rosa et al., 2013; Schäfer et al., 2008; Schäfer & Uhl, 2002). In H. pluchei, males perform two copulatory phases when mating with virgin females. The spermatozoa transfer occurs during Phase I of copulation. The pedipalps move synchronously throughout the copulation, decreasing towards the end of the phase until it stops completely, leading to phase II. These movements include strong squeezes and torsion movements that twist and squeeze the female abdomen (Calbacho-Rosa et al., 2013; Cargnelutti et al., 2018; Huber, 1995). During Phase II, or post-insemination behaviour, where no apparent pedipalp movements are observed and no spermatozoa transfer occurs (Cargnelutti et al., 2018), males may stimulate the female with imperceptible movements or by pressure with the distal part of the procursus (a unique modification of the pedipalp tarsus in pholcid spiders, Huber, 2014) inside the female genitalia (Cargnelutti et al., 2018, 2020). Notwithstanding, it is not clarified yet whether the spermatozoa transfer occurs at the beginning, at the end or throughout all Phase I.
The present paper aims to demonstrate (1) the precise moment when the spermatozoa transfer occurs during the phase I and (2) whether male spermatozoa transfer, and female storage is influenced by pedipalp movements. To achieve these objectives, we interrupt the Phase I at different moments to observe whether there is a difference in the number of spermatozoa in male and female genitalia. We can expect the following scenarios. (1) If spermatozoa are transferred throughout all phase 1, pedipalp movements will be closely related to the primary function of copulation: the spermatozoa transfer. And the numbers of these movements will be related to the amount of spermatozoa transferred. In other words, we would have expected a negative effect of pedipalp movements on the number of spermatozoa remaining in the males; (2) if spermatozoa are not transferred during all this phase, pedipalp movements will have an additional role apart from spermatozoa transfer. And no relation is expected between the number of pedipalp movements and the number of spermatozoa transfer (remaining in males and stored by females), allowing us to investigate other potential functions of the genital movements during copulation.
2 MATERIALS AND METHODS
2.1 Spider collection and rearing
From September to March, 2018–2019, we collected sub-adult males and females of H. pluchei within the campus of the Universidad Nacional de Córdoba, Argentina. We placed collected specimens in cylindrical plastic containers (8 cm wide × 15 cm height) wrapped with paper on the inside (to provide individuals with a surface suitable for web construction) and with a cotton ball soaked in water as a humidity source. Animals were then provided with a 12/12-h light/dark photoperiod. Individuals were fed once a week with adult Drosophila melanogaster and Tenebrio molitor larvae until adulthood and up to three days before were used for experimentation.
2.2 Behavioural recording and size measurements
All interactions were recorded using a stereomicroscope equipped with a Logitech QuickCam® pro 9000 digital camera that allows close-up views of male pedipalp movements during copulation. Adult virgin females were placed individually in containers (8 × 12 cm height) 24 h before males for them to be able to build their webs. After copulation, males and females were euthanized by hypothermia (−20°C) and kept under these conditions for a period no longer than 15 days (Gabel & Uhl, 2013), after which, we proceeded to quantify the sperm. Behavioural events were transcribed from digital videos using J Watcher® 0.9 (Blumstein et al., 2000). The pedipalp movements performed by males during the copulatory phase I were considered as a behavioural variable. For its quantification, we followed the guidelines established in Calbacho-Rosa et al. (2013): each contraction that males perform with their pedipalps, squeezing females' abdomen, followed by pedipalp relaxation and return to their initial posture, was considered a pedipalp movement (Video S1). The length of the tibia-patella segments of the first pair of legs was measured to have an estimate of the body size, as it is common in pholcid spiders (Calbacho-Rosa et al., 2010, 2012, 2019; Huber, 1996; Jakob, 1994). For this purpose, a picture of each individual's tibia-patella segment was taken over a graph paper and under a dissecting microscope (Nikon SMZ 1500), with the same magnification which was later used to measure in the ImageJ software (Schneider et al., 2012). All specimens were deposited in the spider scientific collection of the Laboratorio de Biología Reproductiva y Evolución, Instituto de Diversidad y Ecología Animal, Universidad Nacional de Córdoba, Argentina.
2.3 Experimental design
2.3.1 Spermatozoa transfer during Phase I
Three experimental groups were established to determine whether spermatozoa transfer occurs at the beginning, at the end or continuously during Phase I. To set the time intervals for each group, average duration of Phase I (average duration: 13.11 ± 5.70 min, see Cargnelutti et al., 2018) was divided into 3-min intervals, except for the last one which included the end of Phase I. Although closer time intervals are ideal to get a finer view of the spermatozoa number change, obtaining and manipulating virgin females are not logistically simple. Therefore, there was a first group in which copulation was interrupted 3 min after the beginning of Phase I (females n = 15; males n = 16), a second group in which copulation was interrupted after 6 min (females n = 15; males n = 16) and a third group in which copulation was interrupted 1 min after the end of Phase I (females and males n = 14). In each experimental group, copulation was interrupted by gently touching the mating couple using a fine-tipped brush. The number of spermatozoa in the uterus externus of the females and in the bulbs of the males after copulation was compared among the three experimental groups. It is important to note that we used the remaining spermatozoa of both pedipalps, since in this species there was no asymmetry in the number of spermatozoa between the two bulbs (see Cargnelutti et al., 2018). The experimental design, as well as the spermatozoa counting process, is summarized in Figure 1.
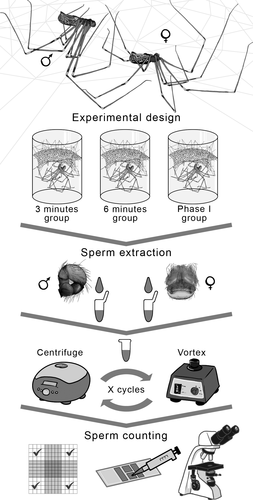
2.3.2 Spermatozoa quantification
For spermatozoa quantification, we apply the technique used in Cargnelutti et al. (2018). The male bulbs were removed from the pedipalps under a dissecting microscope (Nikon SMZ 1500). Each bulb was placed individually in a micro-centrifuge tube with 75 µl of “spider saline solution” (3.26 g NaCl, 0.13 g KCl, 0.30 g CaCl2 + 2H2O, 0.26 g MgCl2 + 6H2O and 250 ml of distilled water) (Albo & Peretti, 2015). The bulbs were crushed using fine-tipped forceps to release the spermatozoa into the solution. The samples were subjected to three vortex cycles for 30 s and three centrifugation cycles at 1075 g for 10 min (Figure 1).
The uterus externus of the females was removed from the opisthosoma under a dissecting microscope and subjected to the same procedure as male bulbs. The only difference was that the female samples were subjected to five vortex cycles of 30 s and five centrifugation cycles of 10 min at 1075 g to facilitate the separation of the sperm, normally agglutinated due to female secretions (Cargnelutti, F pers. obs.). Finally, 10 µl of the sample were placed in a Neubauer chamber to perform the spermatozoa count using a phase-contrast microscope (Nikon Eclipse 50i) (Figure 1). We estimated the total number of spermatozoa at 75 µl (initial volume) using the following equation: Total number of spermatozoa = (75 µl × No. of spermatozoa counted)/0.4 µl.
2.4 Statistical analyses
To assess whether there were significant differences in the number of spermatozoa stored in the female uterus externus, as well as in the number of spermatozoa remaining in the males bulbs between the experimental groups, we used two Generalized Linear Models (GLMs) with negative binomial distribution. Both GLMs were performed once they were graphically set by using the R package “fitdistrplus” (Delignette-Muller & Dutang, 2015) and analytically set by using Akaike's information criterion. The assessment stated that the negative binomial distribution is the one that best fits our data. The glm.nb function of the R "MASS" package was used (Venables & Ripley, 2002). For both GLMs, the following predictive variables were included: treatment (3 min, 6 min and Phase I), body size of males and females and number of pedipalp movements as a behavioural variable. Note that we could not measure the size of some animals. The reason is that, in this species, leg autotomy is common (Johnson & Jakob, 1999); therefore, the presence of specimens with the absence of some pair of legs (mainly the first pair) is frequent in natural conditions (Johnson & Jakob, 1999; Cargnelutti F pers. obs.). To solve this, some data were imputed (3 data of 46 for males and 1 data of 44 for females) using the nearest K-neighbour function of the R "Bnstruct" package (Franzin et al., 2017). This method replaces the missing value in the data set with the median, considering the K value (K: the number of neighbours used to calculate the median). The cut-off line used as a criterion to impute data is the one suggested by Bennett (2001) where the amount of missing data does not exceed 10% of the total data; this way the results would not be biased (reviewed in Dong & Peng, 2013). The body size of individuals was included in the models since it may have some effect on the amount of spermatozoa produced and/or transferred by males and stored by females (Cargnelutti et al., 2018; Dufresne et al., 2019; O'dea et al., 2014; Whitman, 2008; Wiernasz et al., 2001). For each GLM, the model presents the additive effect of all predictive variables and the effect of the interaction between the treatment and the number of pedipalp movements. These models were subjected to a model selection by means of the R package “MuMIn” (Barton, 2019) using the Akaike information criterion corrected for small samples (AICc). The models are ranked according to their AICc value, and the model with the lowest AICc is the best fitted model. In the model, whose response variable is the number of spermatozoa remaining in the male bulbs, the model selection maintains only the predictive variable number of pedipalp movements (Table S1). However, this variable is not statistically significant (χ2 = 2.717, df = 1, p = .099) so it was removed from the model and only the treatment variable was maintained in it, since it is the main variable to be tested. In the model whose response variable is the number of spermatozoa stored in the female uterus externus; all predictive variables were removed (Table S2). It is interesting to note that, since the interactions between the treatment variable and the pedipalp movements are not maintained in both models, there is no differential effect of the pedipalp movements per treatment. The interaction between the treatment factor and the sizes of the individuals was not added to the final model to avoid over-parameterizing it considering our sample size. Finally, knowing that our response variables (number of spermatozoa remaining in males' bulbs and number of spermatozoa stored in the female uterus externus) did not co-vary with the three experimental groups (see results), we pooled the male and female spermatozoa data of the three experimental groups together and performed a Spearman correlation between these two variables. R version 4.1. (R Core Team) was used for all analyses described.
3 RESULTS
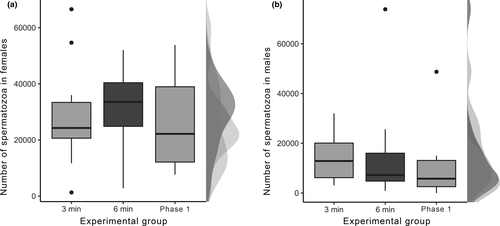
No significant differences were found in both the number of spermatozoa stored by females (χ2 = 0.805, df = 2, p = .669; Figure 2a) and the amount of spermatozoa remaining in the male bulbs among the three experimental groups (χ2 = 1.096, df = 2, p = .578; Figure 2b). Considering the average duration of phase I and the complete duration of the copulation, the spermatozoa transfer represents the 22.88% of the whole phase and 11% of the copulation duration. Neither the size of the individuals nor the behavioural patterns that the individuals perform during copulation explain the variability in the number of spermatozoa found in both genital structures between the treatments (see Tables S1 and S2). Finally, no significant correlation was found between the number of spermatozoa remaining in male bulbs and the number of spermatozoa stored by females (rs = −.168, p = .297).
4 DISCUSSION
Our results show dissociation between the male pedipalp movements during the Phase I of copulation and spermatozoa transfer and shows that spermatozoa transfer occurs in the first 3 min of phase I since no significant differences were found in the number of spermatozoa among the three experimental groups in both females and males. Our findings are consistent with those obtained in a previous research on the species where no correlation was found between the duration of Phase I and the number of spermatozoa remaining in the male bulbs or those in the uterus externus of the females (Cargnelutti et al., 2018).
Another intriguing result is the absence of an effect between the number of pedipalp movements and the number of spermatozoa remaining in the bulbs depending on the treatments.
The absence of this effect could be explained whether the spermatozoa transfer did not occur continuously during the first 3 min of Phase I. This has been suggested in relation to other spiders, in which spermatozoa transfer may occur rapidly during copulation (e.g., Knoflach, 2004; Linn et al., 2007; Schneider et al., 2005; Snow & Andrade, 2004). This lack of association between spermatozoa transfer and pedipalp movements supports the possible evolution of the pedipalp movements in a post-copulatory sexual selection framework (Schäfer & Uhl, 2002). However, it is difficult to determine which mechanism is responsible for the evolution of this behaviour (see below the possible mechanisms involved in pedipalp movement evolution).
Also, we did not find a correlation between the number of spermatozoa remaining in the male bulbs and those stored by the females. Considering that females only store a percentage of the total amount transferred by males, this result is not surprising (Cargnelutti et al., 2018). We do not know why females store only 20% of the total amount transferred by males (but see Cargnelutti et al., 2018). However, this result does not rule out that the transfer occurs within the first 3 min of phase I since no differences in spermatozoa remaining between treatments were observed in males. Interestingly, there is a possible bimodality in the number of spermatozoa stored by females in the phase I group. In this group, two spermatozoa storage peaks similar to the 3-min and 6-min groups can be observed. We are not sure if these peaks are associated with alternative storage tactics implemented by the females. However, since we do not find differences between the 3-min and 6-min groups, our results are still robust.
In our study model, as well as in other pholcids, it has been suggested that pedipalp movements evolved by CFC (reviewed in Calbacho-Rosa & Peretti, 2015). There are also other possibilities involved, like sperm competition and sexual conflict. We can rule out that pedipalp movements have a function related to sperm competition in the context of our work mainly because we work with virgin females. Although it is possible, following the logic of Barnett and Telford (1994), that males use their genitalia (i.e., the pedipalps) to position their spermatozoa in some regions of the uterus externus of the females, and thus increase their reproductive success, is unlikely. Mainly, because the males who copulate later can easily access, with their pedipalps, to the regions of the uterus externus where the spermatozoa are deposited and remove it (Calbacho-Rosa et al., 2013). In fact, this species paternity is biased towards the last male that copulates (Kaster & Jakob, 1997). Also, explain this pattern from a sexual conflict perspective it is complicated. The theory of sexual conflict predicts that the area near to the sensory organs (i.e., the region of the female abdomen where the pedipalps would exert pressure during their movements) should show previous signs of sexual conflict, such as structures that prevent the males from contacting the females (review in Eberhard, 2015). Such evidence has not been reported for H. pluchei. So, female stimulation is the more feasible explanation for pedipalp movement evolution.
An interesting fact that could support the latter is that males that copulate with mated females perform the same pedipalp movements that those that copulate with virgin females. The only difference is that in the copulations with mated females these movements are not restricted to a copulatory phase and are performed throughout their entire copulation (Calbacho-Rosa et al., 2013). By doing that, males could stimulate mated females for a longer period to favour the use of their spermatozoa to fertilize the eggs. In this species, males and females can copulate several times with different mates in a reproductive period (Cargnelutti, 2020; Dutto et al., 2011). There is no evidence that spermatozoa transfer process may be different during copulation with virgin or mated females. Considering that males also remove rival spermatozoa from the female uterus externus in mated females (Calbacho-Rosa et al., 2013), the combination of both strategies could give these males a substantial advantage against the first mating males. However, the males that copulate with virgin females may have strategies to avoid a total loss of paternity (see Cargnelutti et al., 2018, 2020). Another interesting aspect that could help to elucidate whether CFC contributed to the evolution of pedipalp movements is whether males modulate this behaviour considering female characteristics. This has not been tested in H. pluchei; however, preliminary studies show that males vary the number of pedipalp movements in successive copulations with different virgin females considering their own size, not the size of the females with which they copulate (Cargnelutti, 2020).
Other evidence in favour of the CFC hypothesis is the presence of different sizes setae in the male pedipalps of various species (Huber, 2000, 2011) that brushes the abdomen of females during pedipalp movements (Stefani et al., 2012). However, in our model species there are no studies that have explored this possible female stimulation mechanism (Calbacho-Rosa & Peretti, 2015).
In other pholcids species such as P. phalangioides and Physocyclus globosus, the first male pedipalp movements in a double copulation experiment have been associated with increased male paternity (Peretti & Eberhard, 2010; Peretti et al., 2006; Schäfer & Uhl, 2002). However, the mechanism behind this pattern is still under discussion. Only in P. globosus CFC has been suggested as the primary mechanism (Peretti & Eberhard, 2010). In fact, females of P. globosus have internal stretching receptors, which could sense this stimulus (Huber & Eberhard, 1997). In other spiders such as Micrathena gracilis (Araneidae), the second pedipalpal insertion plays an active role in the amount of spermatozoa stored by females without being involved in spermatozoa transfer (Bukowski & Christenson, 1997; Eberhard & Huber, 2010). The function of pedipalp movements remains unclear. In fact, to our knowledge, there are no detailed studies in spiders that investigate the effect of such movements on the dynamics of spermatozoa once inside the female. Only one study analysed the impact of such behaviour on the spermatozoa viability inside the females without finding significant results (Cargnelutti et al., 2020).
There are also examples of insects that coincide with our results and suggest a similar explanation concerning genital movements. For example, in tsetse flies (Glossina sp.) it has been documented that the rhythmic movements made by the male genitalia within the female are supposed to have evolved by CFC (Briceño & Eberhard, 2015) and this behaviour triggered the ovulation, the spermatozoa storage and the intention to remating (Briceño & Eberhard, 2009; Eberhard & Lehmann, 2019). Also, for the Morsitans subgenus of tsetse flies (Glossina), the setae located in the genitalia of males may stimulate the female by rubbing her abdomen during the rhythmic movements of the male genitalia (Briceño & Eberhard, 2015; Briceño et al., 2007; Eberhard & Lehmann, 2019).
Additionally, it has been proposed that spider pedipalp insertions in the female genitalia can be used by females to assess male “vigour” (Eberhard, 1996; Huber & Eberhard, 1997). Nonetheless, it has not been investigated whether males who are more vigorous benefit from getting a higher paternity.
Considering that spermatozoa transfer occurs in the first 3 min of phase I, and the pedipalp movements decreased to the end of it (Calbacho-Rosa et al., 2013) and are no related to spermatozoa transfer, it is possible that males use their pedipalp movements in different ways during phase I.
For example, to place their spermatozoa in other regions of the female genitalia (e.g., uterus internus), or to stimulate the female to mobilize the spermatozoa to access her eggs. However, the absence of interaction between pedipalp movements and the treatments on the amount of spermatozoa stored by the females goes against this possibility. This means that the number of pedipalp movements that males perform in each experimental group does not differentially affect the spermatozoa in females' uterus externus. This may be because, in theory, fertilization occurs in the same place where spermatozoa are stored (Calbacho-Rosa et al., 2013; Herberstein et al., 2011; Huber, 1995). Following this reasoning, males should not have to use pedipalp movements to affect the storage and fertilization process by mobilizing spermatozoa, for example, to the uterus internus, mechanically or by stimulating females to perform this process.
Finally, there is no influence of the male and female size on the number of spermatozoa remaining in the male bulbs and stored inside the female uterus externus. Notably, the pattern found in females differs from those reported in other work on the species (Cargnelutti et al., 2018). This contradictory result evidence the need of raising analysis with a higher sample number and greater statistical power. By doing that, we can obtain more robust patterns. In conclusion, our research detaches the pedipalp movements from the spermatozoa transfer process and provides evidence in favour of a mechanism of post-copulatory sexual selection on genital movements, setting the basis for future questions regarding this topic.
ACKNOWLEDGMENTS
We thanks German Gonzalez for his help with statistical analysis and Maximiliano Tourmente, Jackelyn Kembro, Nelson Ferreti and Alex Córdoba-Aguilar for their useful comments to a previous version of this paper. We also thanks Manuel Sosa for his help with the figures. Thanks to the contributions of three anonymous reviewers. Financial support was provided by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Fondo para la Investigación Científica y Tecnológica (FONCYT) and Secretaría de Ciencia y Tecnología de la Universidad Nacional de Córdoba (SECYT).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.




