Vervet monkeys socialize more when time budget constraints are experimentally reduced
Christèle Borgeaud and Béatrice Jankowiak contributed equally to this study.
Abstract
In species that live in stable groups, successful management of time budget (i.e., the proportion of time involved in different behaviours) and social relationships has been proposed to be a key variable affecting individual fitness. Such management is limited by time constraints, which are group size and season dependancy. However, the link between time budget constraints and grooming patterns as a means to service social relationships has never been studied experimentally. Here, we reduced the time constraints of three wild vervet monkey (Chlorocebus aethiops pygerythrus) groups by offering them high quality food for 60 min in the morning. We conducted 10 trials in summer and 10 trials in winter. We found that vervet monkeys indeed reduced the proportion of time spent foraging during the rest of those days compared to control days and increased the proportion of grooming time spread over more bouts. Individuals that did not get access to the food still participated as much in grooming as those that did eat. In accordance with our expectations, social network analysis revealed a larger grooming network on supplementation days: vervet monkeys used their extra time to socialize with more different group members. Furthermore, following our predictions, the effect of provisioning was usually stronger during the winter season (when food was scarce and days shorter) than during the summer, and the number of conflicts decreased during supplementation days. Finally, despite the fact that adults and juveniles of all three groups increased their proportion of time spent grooming during supplementation days, few group and age differences emerged in the way vervet monkeys manage their time budget. Interestingly, some of these differences seem to be independent of group size. In conclusion, vervet monkeys seize the opportunity to increase the proportion of time spent in social behaviours if time budget constraints are reduced.
1 INTRODUCTION
It has long been proposed that living in stable groups leads to selection on individuals to be able to manage their social interactions in a way that benefits their fitness (Kummer, 1967; Harcourt, 1988; Dunbar, 1992; van Hooff & van Schaik, 1992; Harcourt & de Waal, 1992; Engh et al., 2005; Byrne & Whiten, 1988; Whiten & Byrne, 1997; Seyfarth & Cheney, 2003; Connor, 2007; Slocombe and Zuberbuehler, 2007). For example, it is widely accepted that skills involving the development and maintenance of social relationships will have positive effects on an individual's fitness in various species (house mice: Weidt et al., 2008; horses: Cameron et al., 2009; bottlenose dolphins: Frère et al., 2010; reviewed in Silk, 2014). A long-term study on baboons (Papio hamadryas ursinus) yielded positive correlations between the stability and the strength of relationships and females’ measures of fitness such as infant survival and longevity (Silk et al. 2009, 2010; reviewed in Seyfarth & Cheney, 2012). In addition, there is evidence to suggest that acquiring tolerance from a dominant or coalitionary support from an ally improves access to food and/or mates (Borgeaud & Bshary, 2015; Noë, 1992; van Hooff & van Schaik, 1992; de Waal, 1982; de Waal & Harcourt, 1992; Wrangham, 1980).
Time budget (i.e., the proportion of time spent in different behaviours) evaluations have been an important tool for understanding individual time management strategies and the factors that affect investment in social relationships. It is clear that management of social relationships has to be traded off against other important fitness components such as searching for food, escaping predation and resting to reduce physiological stress (van Schaik and van Hooff 1983; Dunbar et al., 2009; Korstjens et al., 2010). However, a currently open question is how individuals adjust their activity budget and especially their management of social relationships if time budget constraints change.
Seasonal variation in the activity budget has been widely reported in primates, including our study species (vervet monkeys: Isbell & Young, 1993) and other species (deers: Pagon et al., 2013; alpine chamois: Brivio et al., 2016; bottleose dolphins: Vermeulen et al., 2015; African elephants: Mramba et al., 2019). During winter (i.e., the dry season in the southern part of Africa), food is scarcer and days are shorter and vervet individuals usually spend a greater proportion of time feeding and less time resting, while time spent moving or involved in social activities may show less consistent patterns (Isbell & Young, 1993; Nakagawa, 2000; Barrett, 2005; but see van Doorn et al., 2010). Such variation might also be sex and age dependent (Canteloup et al., 2019; McFarland et al., 2014; Pereira & Fairbanks, 2002) and correlates with hormonal cycles, pregnancy and the presence of dependant infants (Matsumoto-Oda & Oda, 2001; Silk, 1987).
Besides seasonal variation, access to supplemental food provisioning might have an influence on individuals’ activity budgets. Previous studies on primates indicated that groups with access to human food showed a decrease in time spent foraging and an increase in time spent resting but that time spent in social activities seems less prone to fluctuation (Adhikari et al., 2018; Altmann & Muruthi, 1988; Back & Bicca-Marques, 2019; Fa, 1986; McKinney, 2011; Saj et al., 1999).
In primates, social relationships are usually built up on allogrooming behaviour (Sade, 1972). The time spent grooming correlates positively with group size both within and between species (Dunbar, 1991; Lehmann et al., 2007; Pollard & Blumstein, 2008), which has been interpreted as a result of increased social competition (Schino, 2001; Seyfarth, 1977). Besides time spent involved in social behaviours, another way of looking at how individuals adjust their social relationships when time constraints change is to determine how grooming patterns (i.e., number of grooming bout and network analyses) (Wittig et al., 2008) and conflicts are modified. A study on gelada baboons (Theropithecus gelada) described how females reduced their grooming network during lactation when time devoted to foraging was particularly high (Dunbar & Dunbar, 1988). A similar effect about adjusting network size was found in correlational studies on the link between group sizes and grooming networks: grooming networks are smaller in larger groups, increasing the likelihood of group fission (Dunbar, 1992; Williamson & Dunbar 1999; Watts, 2000; Lehmann et al., 2007). These studies reveal that, under time constraints (e.g., lactation in the case of females) and increased competition over resources, it becomes important to focus on the most valuable partners to ensure their continued support. However, published analyses of grooming patterns are mostly based on correlational data, and only a few studies experimentally manipulated aspects of social patterns (Flack et al., 2006; Fruteau et al., 2009; Madden & Clutton-Brock, 2009). In primate species with a strict hierarchy, the rate of conflicts is usually influenced by seasonality, group size and food scarcity (reviewed in Isbell, 1991). In baboons (Barton & Whiten, 1993), as well as in vervet and patas monkeys (Pruetz, 2015), the number of conflicts is positively correlated with the food scarcity season (i.e., winter in our study site).
Here, we provide an experimental test on the effects of changes in time budget trade-offs on activities and grooming patterns at the individual level in a study of three groups of wild vervet monkeys. On supplementation days, we offered the groups access to high-quality food (corn) within a single container in order to reduce the need to invest in foraging for the rest of the day. By doing so, we reduced the foraging demand for a majority of the monkeys and asked how they would use this extra time. According to Lehmann et al., (2007), vervet monkeys, which are considered as small-brained primates, already spend more time than expected involved in social behaviours in relation to their cognitive constraints. Therefore, they might not increase their time allocated to grooming in response to food provisioning. However, if there are any trade-offs, then these would be most likely to occur in winter when food is scarce and days are shorter, increasing pressure on time allocated to grooming. We therefore conducted trials both in summer and in winter and predicted that, if the supplementation led to an increase in the proportion of time spent grooming, such an effect should be stronger in winter. Furthermore, we asked whether our manipulation would affect the grooming patterns. Based on published correlational evidence presented above, we predicted that a relaxation on the time budget should lead to an increase of grooming bouts per hour at the group level and in the number of grooming partners at the individual level.
We also predicted that within group competition over resources, and therefore the conflict rate, should decrease on supplementation days. This should be true despite the fact that the food provisioning was clumped, because we only recorded behaviours once the experiment was over. Furthermore, this effect might be stronger in winter than in summer, because food is typically more abundant in summer (Barton, 1990; Barrett, 2005; but see Lee, 1984 for counterexample) and longer daylight hours may generally reduce time budget constraints (Dunbar & Gowlett, 2014). Our major predictions are summarised in Table 1.
| Table of questions and predictions | Influence of: | |
|---|---|---|
| Supplementation | Winter season | |
| Time spent foraging | Decrease* | Increase |
| Time spent grooming | Increase* | Decrease |
| Time spent socializing | Increase | Decrease |
| Number of grooming bouts | Increase* | Decrease |
| Number of grooming partners | Increase* | Decrease |
| Number of conflicts | Decrease* | Increase |
Note:
- Asterisks indicate probable interactions between supplementation and season.
Finally, we also analysed for all individuals that showed variation in participation how having eaten or not affected their grooming behaviour during supplementation days. This is to control for a potential bias of participation in the supplementation in favour of high-ranking individuals that are also usually more involved in grooming interactions (Seyfarth, 1977; Silk et al., 1999; Wubs et al., 2018; see Schino, 2001 for a meta-analysis). Usually, individuals that did not participate were low-ranking individuals who did not have the opportunity to access the corn within the supplementation duration of 1h. We expected that, if individual time budget considerations are key for decisions about grooming, individuals that had access to the food should groom more during days when they were provisioned. In contrast, if increased grooming rates by other group members causes social pressure to which flexible responses are needed, then we expected no difference between individuals that ate or not.
We complemented our main questions on proportion of time spent grooming, grooming networks and aggression with analyses of two further aspects that help to assess the generality of the results. First, we asked whether our three study groups of monkeys would respond in a similar or different way to the food supplementation in their activity budget adjustment. This question is pertinent as previous studies (Borgeaud & Bshary, 2015; Borgeaud et al., 2016; van de Waal, 2018; Canteloup et al., 2019) indicated that vervet groups of the study site behave differently in multiple contexts. Such variation is apparently driven by factors linked to group identity (social interaction patterns, traditions and/or home range characteristics) rather than group size (van de Waal, 2018). Second, because adult and juvenile individuals differ in their physiological needs, in the sense that adults need a higher food intake and need more time spent resting than juveniles, while these ones are usually more involved in playing social behaviours (Pereira & Fairbanks, 2002), we asked whether different age classes are affected differently by our manipulation.
2 METHODS
2.1 Data collection
The study was conducted from January until April 2012 for the summer season and from July until September 2012 for the winter season at the Inkawu Vervet Project, Mawana Game Reserve, KwaZulu Natal, South Africa. Subjects were members of three groups of habituated vervet monkeys (Chlorocebus aethiops pygerythrus), called Baie Dankie (BD), Ankhase (AK) and Noha (NH). The group composition varied between the summer and the winter season as adult and sub-adult males both immigrated and emigrated. Excluding infants, the BD group included 37 individuals (16 adults, 3 sub adults and 18 juveniles) in summer and 36 (17 adults, 2 sub adults and 17 juveniles) in winter, the AK group 26 individuals (10 adults, 3 sub adults and 13 juveniles) and the NH group 24 in summer (13 adults and 11 juveniles) and 27 individuals (16 adults and 11 juveniles) in winter. Data were collected on handheld computers (Palm Zire 22 or TX, Pocket pc HP Travel Companion iPAQ rx5935) with the Pendragon 5.1 software.
Supplementations were conducted once per week over 10 weeks, during both the summer and the winter seasons. For one hour, at the beginning of each day, individuals had free access to 2 kg of dry corn (energetic value: 360 kcal/100g), so that most of the individuals were able to eat. A large container of corn was placed at a single location, allowing the individuals to access it following the group hierarchy. Data collection for supplementation days started once the supplementation was over (i.e., empty container or monkeys not feeding anymore). Because of field or equipment issues, only nine of 10 supplementations were recorded for NH and BD groups during the summer season and only nine were recorded for the AK group in winter. No other food experiments were run during the study.
Data were obtained using scan sampling (Altmann, 1974; Martin & Bateson, 2007) carried out every 30 min. The activity of all visible animals was recorded during a 10-minute window. During this time window, observers were required to move within the group in order to record the behaviour of a maximum number of individuals. To avoid collecting the most conspicuous behaviour, once an individual was spotted, we used the conventional strategy of imposing a 3-second delay before recording the animal's behaviour. As data were collected by multiple observers, an inter-observer reliability test was performed for each observer to prevent any bias. The test reliability was set to a minimum of 80% of agreement between the two observers over the whole data collection (activity budget, distances to shelter, group spread, etc). Behaviour determination was the less subtle in the data collection protocol, and observers had usually a higher average within the activity budget category. However, because those results were not kept in our general database, we cannot provide exact values. Because of dangerous wild animals, new observers, even if properly trained, had to be accompanied for two months initially. As a consequence, some scans were collected with two observers, while only one was entering the data on the single device, limiting the number of scanned individuals. Overall, with one observer, 9.2 monkeys were scanned per 10 minutes window, while 8.3 were scanned with two observers. This suggests that the number of observers did not have any influence on the sampling effort. Observations took place from 06:00 until 18:00 in summer and from 08:00 until 17:00 in winter, which corresponds to the time period where monkeys were active during the day. A total of 24,033 data points were collected with an average of 68 per individual knowing that some were present only during one season. Variation in the number of data points obtained per individual should not affect any conclusions as we do not expect any systematic bias between experimental days and control days and in any case should be controlled for by adding ID as a random factor that controls for identity.
We defined control days as any day of the week, excluding the one following a corn supplementation. Two control days and one supplementation day per week were used for the analyses of time budgets. To prevent any bias as a result of hourly variation in activities, for each control day we matched the starting (i.e., at the end of the supplementation) time of scans of the corresponding supplementation day. This means that the data analysed for control days were only scans that correspond to times after the feeding on supplementation days.
Besides scan data, behaviour sampling (Martin & Bateson, 2007) data on social behaviours were recorded during the whole day. This included the number of grooming bouts and the partners’ identity and the number of agonistic interactions. A grooming bout was defined as two partners interacting, independent of the grooming direction, or as a resumption of grooming after a break of at least 1 min between the two partners. Between scans, observers were also requested to move within the entire group in order to record as many social behaviours as possible. This is important to mention that scans were used to extract the activity budget data which represent the proportion/percentage of records and that we present it as the proportion of time spent in a precise activity throughout the manuscript. On the other hand, behaviour sampling was used to collect data only on the number of social behaviours, such as grooming and conflicts, and the number of grooming partners. This is because conflicts and short-lasting grooming bouts are rather inconspicuous behaviours and can easily be missed during scan data collection.
To run analyses on social behaviours, including the total number of grooming bouts and the number of different partners involved, as well as the number of agonistic interactions, data from both scan and behaviour sampling were considered. We are aware that behaviour sampling data could be subject to certain biases. Indeed, events like grooming might have been difficult to spot while in cover, and activities that took place near the centre of the group could have been more likely to be observed than activities that took place at the periphery. However, we are quite confident that our dataset was not systematically biased to any significant degree. Indeed, grooming bouts often lasted for several minutes or more, which increased the likelihood that they would be observed. Second, the observers were requested to move through the group on a regular basis. Finally, as our analyses were based at the individual level, this should have prevented any bias as the level of boldness/shyness of each individual would remain the same during the whole study period. Behaviour sampling data were recorded during the same time window as scans for each control/supplementation day. Within each group and for each parameter, we controlled for the total number of hours of observation. Throughout the whole study, a total 1438.5 hours of observation were recorded (i.e., winter: 693 hours; summer: 745.5 hours).
2.2 Data analysis
2.2.1 Activity budget
We extracted behaviours falling into five main categories: foraging (searching, reaching, biting, chewing, licking, drinking), moving (walking, trotting, running, climbing, descending, jumping), resting (sitting, lying, autogrooming, self-scratching, sleeping, being vigilant), allogrooming (i.e., grooming hereafter) and other social behaviours (sitting in contact, nursing infant, playing, mouth to mouth, sexual behaviours and agonistic behaviours). Agonistic behaviours were not considered as a separate category within the activity budget and hence were analysed as events (see below). For each individual, we extracted the number of scans recorded in each behavioural category. We collated these numbers for each individual for the Control/Experiment (supplementation) period and for the summer/winter season, allowing us to have 4 data points per individual for each activity (or 2 in the case the individual appeared or disappeared between the two seasons).
Our personal focus was on the general results, that is, potential consequences of supplementary food on average activity budgets, as well as comparisons between winter and summer. Nevertheless, we acknowledge that various more specific questions could be asked, like how different age classes or groups of different sizes would respond to the manipulation. As a consequence, and also with the aim to comply with the STRANGE framework (Webster & Rutz, 2020), we also tested the effect of ‘group identity’ and ‘age’ within our models. However, for a matter of conciseness, these specific results are presented within the supporting information. Although the factor ‘group identity’ in our models cannot completely be interpreted as group size effect as two of the study groups (AK and NH) had a similar size, we sometimes discuss our results in term of group size (c.f. supporting information). This is because AK and NH can be considered as small groups in comparison to the BD group.
Female vervet monkeys were considered as adults once they gave birth to their first offspring, while males were considered as such once they left their natal group to integrate another group. In our analyses, we considered two age categories: adults and juveniles. Sub-adults which were not fully grown-up yet were considered as juveniles.
2.2.2 Grooming patterns and conflicts
We determined the total number of grooming bouts and conflicts per group over the Control/Experiment period and during the summer/winter and weighted each behaviour by the total number of hours of observation of the relevant period and season. For the network analyses, we calculated how many total different grooming partners an individual had over the Control/Experiment period and during the summer/winter and weighted it by the total number of hours of observation of the relevant period and season.
2.3 Statistical analyses
The statistical analyses were carried out using R (version 4.0.2) with the packages lme4, nlme, MuMIn, emmeans and car (Pinheiro et al., 2017). For the activity budget analyses, we used the whole dataset of 24,061 data points, representing 94 different individuals of three different social groups. Because there was no option to analyse compositional data (i.e., the analysis of multiple activities simultaneously) with random factors, we ran a series of generalised linear mixed models (GLMM’s) for each behavioural category separately fitted with the binomial family and the logit link functions. For each individual, we considered the proportion of each activity (by considering a binomial distribution) as the dependant variable. Supplementation (i.e., Control/Experiment), season, age and group identity were all considered as fixed effects, and we also tested for potential interactions between all effects. Each interaction that was integrated within our models is presented in the results part. Individual identity was included as a random factor. Since “moving” and “social” behaviours had a number of outliers, we first checked that they are not influential and then proceeded with these observations included. Although the group and the age effects were analysed within the same models as the activity budget and the season, these two results and the related discussion are presented within the supporting information.
To test how actual participation (eating high-quality food) affected subsequent behaviour, we ran a generalised linear mixed model with a binomial distribution and considered the number of scans of grooming behaviour as the dependent variable. We fitted the models with the binomial family and logit link functions. We compared the grooming activity budget of individuals that sometimes had access to the food and sometimes not. We took into consideration only individuals that showed a variation of participation and for which we had at least 10 scan data points for participation and 10 scan data points for non-participation during each season. Considering only the individuals that varied in participation was important to test if those who did not eat still modified their behaviour. This represented 19 individuals over the three groups and a total of 1118 data points. This included 63 grooming points and 604 other behaviours data points for individuals that participated and 54 grooming points and 397 other behaviours for individuals that did not participate. The factor eating or not, the season and the group identity were considered as fixed effects and the identity of the individual as a random factor.
To test the effect of the provisioning on the total number of grooming bouts (N = 1129) and conflicts (N = 181), we ran a series of linear mixed models (LMM’s) and considered the number of log-transformed grooming bouts and conflicts as the dependant variables. We considered supplementation, season and group identity as fixed effects. We also tested for a potential interaction between supplementation and season effects. We considered session (i.e., number assigned to each control/supplementation per week) as a random factor. To analyse the effect of the provisioning on the number of the different grooming partners (i.e., degree), we analysed each group separately with the help of linear mixed models. This is because we found a very strong group variation in our analyses (cf. Results part). The arcsine-transformed total number of different grooming partners for each individual within each group was the independent variable. We considered supplementation and season as fixed effects and tested for an interaction between these effects. We considered the individual identity as a random factor.
Full models contained all possible fixed effects and two by two interactions between each fixed effect. Because full models would provide results for a large number of fixed effects and interactions, we ran a series of model selection in order to present the most valuable results. During the process of selection, we kept all the fixed effects that were of interest for our study (i.e., even if not significant) but we removed the interactions of lower order that were not significant. As a consequence, the proportion of variance explained by our models was very variable. For each selected model, we inspected diagnostic plots and ran a Shapiro-Wilk normality test. Except within the activity budget analyses (see below), we found no evidence of violation of the assumptions of normality or homogeneity of residuals. To assess the robustness of our final models, we ran a series of comparisons between our models and a null model in which we only kept the random factor. Because our models contained multiple levels and interactions, the results presented in the Results section are P values and items of the Analysis of Deviance Table computed with the final selected models (Type II Wald chi-square tests; “Anova” function from the package “car” (Fox et al., 2012). This function allows to extract a single P value for each factor even if this one contains a multiple number of contrasts and levels. To compute pairwise comparisons between the various levels, we ran a series of post-hoc analyses with the help of the “emmeans” function and package (Lenth & Lenth, 2018). We also calculated the marginal R² (R²M; i.e., variance explained by fixed factors) and conditional R² (R²C; i.e. variance explained by fixed and random factors) for all our final models following Nakagawa and Schielzeth (2017) using the MuMin package (Barton & Barton, 2015).
2.4 Ethics statement
The study was approved by the relevant authority, Ezemvelo KZN Wildlife, South Africa. Individuals were habituated to observers and regularly involved in non-invasive experiments since 2010.
3 RESULTS
3.1 Activity budget in function of supplementation, season, group and age
The proportion of explained variance varied considerably among the fitted models (delta R²: foraging: R²M = 0.67; R²C = 0.80; grooming: R²M = 0.23; R²C = 0.68; social behaviours: R²M = 0.48; R²C = 0.54; moving: R²M = 0.25; R²C = 0.66 and resting: R²M = 0.48; R²C = 0.74).
3.1.1 Effect of supplementation and season on activity budget
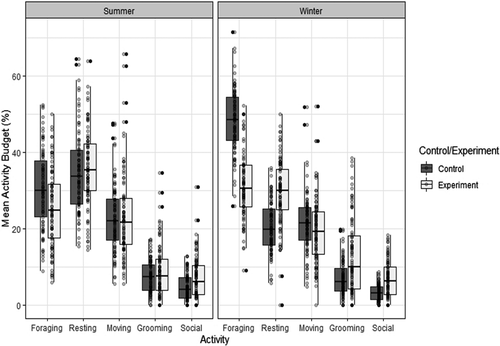
On supplementation days, individuals spent a smaller proportion of time foraging than on control days (GLMER: χ2 = 318.28; DF =1; p < 0.001; Figure 1; Table 2 and Table 3). During the winter season, individuals generally spent proportionally a larger proportion of time foraging than during the summer season (χ2 = 425.13; DF =1; p < 0.001). Furthermore, we found a significant interaction between the supplementation and the season effects in that the reduction in the proportion of time spent foraging was particularly high in winter on supplementation days (χ2 = 70.59; DF =1; p < 0.001).
| Activity | Effect (Contrast) | Level of interacting covariable | Estimate | SE | z ratio | LCL | UCL | P value |
|---|---|---|---|---|---|---|---|---|
| Feeding | ||||||||
| glmer(cbind(Feeding, Resting+Grooming+Social+Moving) ~ Season * (Ctrl. Exp +Age + Group) + (1|Individual), family=binomial | ||||||||
| Supplementation | Control-Experiment | 0.505 | 0.03 | 16.671 | 0.446 | 0.564 | <0.001 | |
| Season | Summer-Winter | −0.524 | 0.031 | −16.813 | −0.585 | −0.463 | <0.001 | |
| Season =Summer | Control-Experiment | 0.251 | 0.045 | 5.52 | 0.162 | 0.34 | <0.001 | |
| Season =Winter | Control-Experiment | 0.759 | 0.04 | 18.934 | 0.681 | 0.838 | <0.001 | |
| Grooming | ||||||||
| glmer(cbind(Grooming, Resting+Feeding+Social+Moving) ~ Ctrl. Exp * (Season +Group) + (Season * Group) + Age + (1|Individual), family=binomial | ||||||||
| Supplementation | Control-Experiment | −0.365 | 0.049 | −7.43 | −0.462 | −0.269 | <0.001 | |
| Season | Summer-Winter | −0.205 | 0.05 | −4.11 | −0.303 | −0.107 | <0.001 | |
| Season =Summer | Control-Experiment | −0.122 | 0.074 | −1.641 | −0.268 | 0.024 | 0.1 | |
| Season =Winter | Control-Experiment | −0.608 | 0.064 | −9.465 | −0.734 | −0.483 | <0.001 | |
| Social | ||||||||
| glmer(cbind(Feeding, Resting+Grooming+Social+Moving) ~ Season * (Ctrl. Exp +Age + Group) + (Ctrl. Exp * Group) + (1|Individual), family=binomial | ||||||||
| Supplementation | Control-Experiment | −0.583 | 0.064 | −9.105 | −0.708 | −0.457 | <0.001 | |
| Season | Summer-Winter | 0.057 | 0.069 | 0.834 | −0.077 | 0.192 | 0.4 | |
| Season =Summer | Control-Experiment | −0.355 | 0.088 | −4.019 | −0.529 | −0.182 | <0.001 | |
| Season =Winter | Control-Experiment | −0.81 | 0.09 | −8.961 | −0.987 | −0.633 | <0.001 | |
| Moving | ||||||||
| glmer(cbind(Moving, Resting+Feeding+Social+Grooming) ~ Group * (Ctrl. Exp +Age + Season) + (Age * Season) + (1|Individual), family=binomial | ||||||||
| Supplementation | Control-Experiment | 0.054 | 0.034 | 1.567 | −0.013 | 0.12 | 0.12 | |
| Season | Summer-Winter | 0.116 | 0.033 | 3.5 | 0.051 | 0.181 | <0.001 | |
| Resting | ||||||||
| glmer(cbind(Moving, Resting+Feeding+Social+Grooming) ~ Ctrl. Exp * (Season +Group) + (Age * Season) + (1|Individual), family=binomial | ||||||||
| Supplementation | Control-Experiment | −0.313 | 0.031 | −10.22 | −0.373 | −0.253 | <0.001 | |
| Season | Summer-Winter | 0.518 | 0.031 | 16.602 | 0.457 | 0.579 | <0.001 | |
| Season =Summer | Control-Experiment | −0.0789 | 0.043 | −1.854 | −0.162 | 0.005 | 0.06 | |
| Season =Winter | Control-Experiment | −0.5467 | 0.044 | −12.516 | −0.632 | −0.461 | <0.001 | |
| Participation | ||||||||
| glmer(cbind(Grooming, Other) ~ (Participation +Season + Group) + (1|Individual), family=binomial | ||||||||
| Participation | Eaten-Not eaten | 0.198 | 0.204 | 0.968 | −0.203 | 0.598 | 0.33 | |
| Season | Summer-Winter | −0.479 | 0.25 | −1.914 | −0.97 | 0.012 | 0.06 | |
| Group | AK-BD | −0.520 | 0.417 | −1.251 | −1.497 | 0.456 | 0.42 | |
| AK-NH | −0.512 | 0.409 | −1.251 | −1.471 | 0.447 | 0.42 | ||
| BD-NH | 0.008 | 0.345 | 0.024 | −0.801 | 0.817 | 0.99 | ||
Note
- All fixed effects but only significant interactions are presented. Significant results are indicated in bold.
- Abbreviations: BD, Baie Dankie; LCL, Lower control limit; NH, Noha; SE, standard error; UCL, Upper control limit. Monkey groups: AK, Ankhase.
| Social patterns | Effect (Contrast) | Level of interacting covariable | Estimate | SE | df | t ratio | LCL | UCL | P value |
|---|---|---|---|---|---|---|---|---|---|
| Number of conflicts | |||||||||
| lmer(Conflict) ~ Group +Ctrl. Exp * Season + (1|Session) | |||||||||
| Supplementation | Control-Experiment | 0.294 | 0.131 | 98.3 | 2.246 | 0.034 | 0.553 | 0.03 | |
| Season | Summer-Winter | 0.008 | 0.131 | 98.8 | 0.061 | −0.252 | 0.268 | 0.95 | |
| Season =Summer | Control-Experiment | −0.141 | 0.187 | 98.4 | −0.753 | −0.513 | 0.231 | 0.45 | |
| Season =Winter | Control-Experiment | 0.728 | 0.182 | 98.2 | 3.995 | 0.367 | 1.09 | <0.001 | |
| Group | AK-BD | 0.103 | 0.161 | 98.3 | 0.641 | −0.279 | 0.485 | 0.7978 | |
| AK-NH | −0.111 | 0.159 | 98.2 | −0.07 | −0.39 | 0.368 | 0.99 | ||
| BD-NH | −0.114 | 0.16 | 98.3 | −0.711 | −0.496 | 0.268 | 0.76 | ||
| Number of grooming bouts | |||||||||
| lmer(Grooming) ~ Ctrl. Exp * Season +Group + (1|Session) | |||||||||
| Supplementation | Control-Experiment | −0.453 | 0.107 | 99.2 | −4.236 | −0.665 | −0.24 | <0.001 | |
| Season | Summer-Winter | −0.397 | 0.107 | 99.8 | −3.704 | −0.61 | −0.18 | <0.001 | |
| Group | AK-BD | 0.137 | 0.131 | 99.2 | 1.047 | −0.175 | 0.449 | 0.55 | |
| AK-NH | 0.242 | 0.131 | 99.2 | 1.847 | −0.07 | 0.554 | 0.16 | ||
| BD-NH | 0.105 | 0.131 | 99.2 | 0.801 | −0.207 | 0.417 | 0.7 | ||
| Number of grooming partners | |||||||||
| lmer(Degree)) ~ Ctrl. Exp * Season + (1|Individual) | |||||||||
| NH Group | Supplementation | Control-Experiment | 0.003 | 0.014 | 69.2 | 0.185 | −0.026 | 0.031 | 0.85 |
| Season | Summer-Winter | 0.019 | 0.016 | 81.9 | 1.178 | −0.013 | 0.051 | 0.24 | |
| BD Group | Supplementation | Control-Experiment | −0.047 | 0.013 | 90.5 | −3.737 | −0.072 | −0.02 | <0.001 |
| Season | Summer-Winter | 0.019 | 0.015 | 119 | 1.321 | −0.009 | 0.048 | 0.19 | |
| Season =Summer | Control-Experiment | −0.018 | 0.017 | 90.5 | −1.017 | −0.052 | 0.017 | 0.31 | |
| Season =Winter | Control-Experiment | −0.076 | 0.018 | 90.5 | −4.203 | −0.113 | −0.04 | <0.001 | |
| AK Group | Supplementation | Control-Experiment | −0.059 | 0.012 | 70.1 | −5.032 | −0.083 | −0.04 | <0.001 |
| Season | Summer-Winter | −0.102 | 0.013 | 76.3 | −7.985 | −0.128 | −0.08 | <0.001 | |
| Season =Summer | Control-Experiment | −0.035 | 0.017 | 70.1 | −2.086 | −0.068 | −0 | 0.04 | |
| Season =Winter | Control-Experiment | −0.084 | 0.017 | 70.1 | −5.03 | −0.117 | −0.05 | <0.001 | |
Note
- All fixed effects but only significant interactions are presented. Significant results are indicated in bold.
- Abbreviations: BD, Baie Dankie; df, degree of freedom; LCL, Lower control limit; NH, Noha; SE, standard error; UCL, Upper control limit. Monkey groups: AK, Ankhase.
On supplementation days, individuals spent a larger proportion of time grooming (GLMER: χ2 = 29.25; DF =1; p < 0.001; Figure 1; Table 2 and Table 3). Furthermore, individuals spent more time grooming during the winter than during the summer season (χ2 = 9.34; DF =1; p = 0.022). The positive effect of supplementation on the increase of the proportion of time spent grooming was stronger during the winter than during the summer season (χ2 = 24.43; DF =1; p < 0.001).
Generally, individuals spent a larger proportion of time involved in social behaviours during supplementation days than during control days (GLMER: χ2 = 118.11; DF =1; p < 0.001; Figure 1; Table 2 and Table 3), while individuals were less social during the winter than during the summer season (χ2 = 10.76; DF =1; p = 0.001). The positive influence of supplementation was stronger during the winter season (χ2 = 13.26; DF =1; p < 0.001).
Supplementations did not have any influence on the proportion of time spent moving (GLMER: χ2 = 1.31; DF =1; p = 0.25; Figure 1; Table 2 and Table 3), and individuals generally spent a larger proportion of time moving during the winter than during the summer (χ2 = 11.31; DF =1; p < 0.001).
Proportionally the proportion of time resting was larger during summer than during the winter season (GLMER: χ2 = 364.25; DF =1; p < 0.001; Figure 1; Table 2 and Table 3). In addition, we found that on supplementation days, individuals spent a larger proportion of time resting (χ2 = 94.08; DF =1; p < 0.001), and we found an interaction between the supplementation and the season effects, with supplementations leading to a higher increase in the proportion of resting time during winter (χ2 = 59.28; DF =1; p < 0.001).
3.1.2 Variation between groups and age categories in the activity budget
Our general findings are that groups, even the AK and NH groups that were of similar size, behaved differently in some activities and that these differences are emphasised by the seasonal variation and also by other potential parameters that we discuss within the supporting information. However, all groups generally reacted similarly to the supplementation by decreasing their proportion of time spent foraging and, in contrast, increasing the proportion of time spent grooming and resting (Figure S1; Table S1).
Adults and juveniles differed in their proportion of time spent involved in activities, but not in grooming. These differences were also influenced by the seasonal variation. However, supplementation influenced the two age categories in a similar way, in the sense that, individuals decreased the proportion of time foraging and increased the proportion of time spent grooming, socializing and resting (Figure S2; Table S1). We also discuss these results within the supporting information.
3.2 Effects of participation in foraging experiment on grooming activity
Our model did not fit very well with our data (R²M = 0.13; R²C = 0.42), but this is probably because we integrated all the fixed factors of interest. For the individuals that sometimes ate from the provided food and sometimes not, there was no effect of participation on the proportion of time spent in grooming interactions (GLMER: χ2 = 0.94; DF =1; p = 0.33; Table 2 and Table 3), no season effect (χ2 = 3.66; DF =1; p = 0.06) and no group effect (χ2 = 1.89; DF =2; p = 0.39).
3.3 Number of conflicts
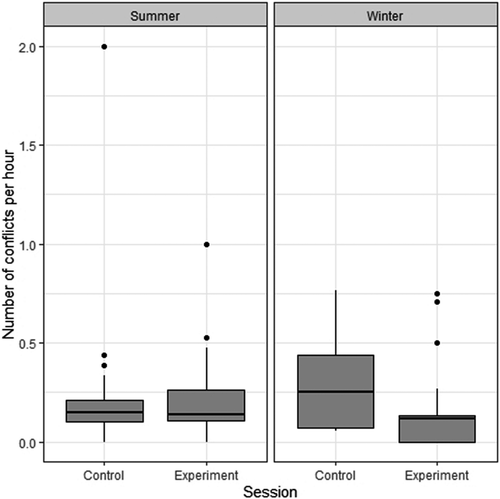
Generally, during supplementation days, there were less conflicts than during control days (LMER: χ2 = 5.46; DF =1; p = 0.02; R²M = 0.13; R²C = 0.15; Figure 2; Table 3 and Table 4). There was generally no difference between season (χ2 = 0.009; DF =1; p = 0.92) or between groups (χ2 = 0.61; DF =2; p = 0.74). However, there was a strong interaction between the supplementation and the season effects (χ2 = 11.07; DF =1; p < 0.001), indicating that the decrease of conflicts during supplementation days was stronger in winter.
| Model | Random effect | Variance | Std. deviation |
|---|---|---|---|
| Feeding | Individual | 0.041 | 0.202 |
| Grooming | Individual | 0.223 | 0.512 |
| Social | Individual | 0.048 | 0.218 |
| Moving | Individual | 0.105 | 0.324 |
| Resting | Individual | 0.072 | 0.269 |
| Participation | Individual | 0.221 | 0.47 |
| Number of grooming bouts | Session | 3.87E−10 | 1.97E−05 |
| Residual | 0.326 | 0.571 | |
| Number of conflicts | Session | 0.012 | 0.109 |
| Residual | 0.482 | 0.694 | |
| Number of grooming partners (AK) | Individual | 0.007 | 0.082 |
| Residual | 0.004 | 0.06 | |
| Number of grooming partners (BD) | Individual | 0.005 | 0.074 |
| Residual | 0.006 | 0.075 | |
| Number of grooming partners (NH) | Individual | 0.006 | 0.081 |
| Residual | 0.005 | 0.073 |
3.4 Number of grooming bouts and number of partners
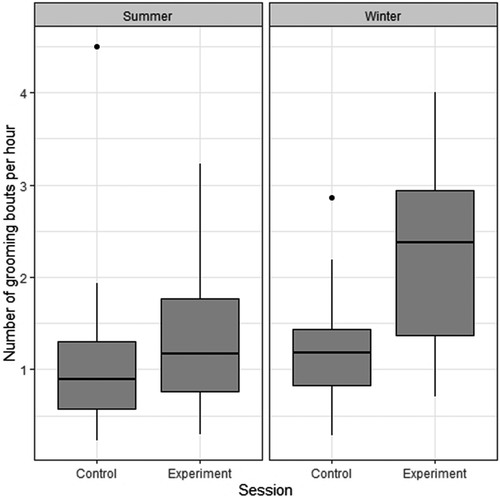
The number of grooming bouts per hour was generally higher on supplementation days than on control days (LMER: χ2 = 18.16; DF =1; p < 0.001; R²M = 0.25; R²C = 0.25; Figure 3; Table 3 and Table 4) and also higher during winter than during the summer season (χ2 = 13.73; DF =1; p < 0.001). This effect was similar in all three groups (χ2 = 3.43; DF =1; p = 0.18), and there was no significant interaction between supplementations and season effects (χ2 = 2.14; DF =1; p = 0.14).
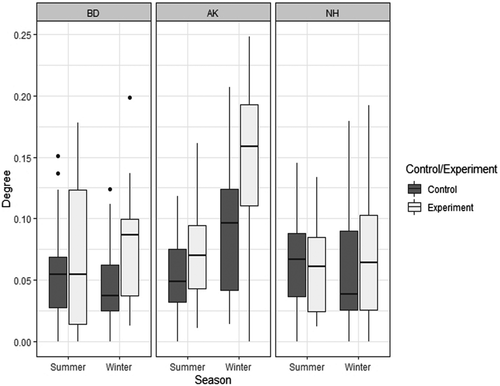
When looking at how the provisioning influenced the number of grooming partners throughout the study, we noticed a strong group difference (LMER: χ2 = 13.66; DF =2; p = 0.001): individuals of the AK group generally had a higher numbers of different partners than the BD and the NH groups. As a consequence, we analysed the groups separately (Figure 4; Table 3 and Table 4). The proportion of variance explained by our models was very variable across groups (AK: N = 752; R²M = 0.26; R²C = 0.74; BD: N = 676; R²M = 0.07; R²C = 0.53; NH: N = 522; R²M = 0.01; R²C = 0.56). In the AK group, we found that provisioning generally increased the number of grooming partners (χ2 = 25.32; DF =1; p < 0.001) and that in winter the number of grooming partners was higher than in summer (χ2 = 64.23; DF =1; p < 0.001). We also found an interaction between supplementation and season effects (χ2 = 4.33; DF =1; p = 0.04), indicating that the increase in the number of grooming partners was stronger in winter. In the BD group, we found that provisioning also increased the number of grooming partners (χ2 = 13.27; DF =1; p < 0.001) but there was no season effect (χ2 = 1.77; DF =1; p = 0.18). Again, we found a significant interaction between supplementation and season (χ2 = 5.43; DF =1; p = 0.02), indicating that, as with AK, the effect of provisioning was stronger in winter. In contrast to the other groups, provisioning did not have any influence on the number of grooming partners in the NH group (χ2 = 0.018; DF =1; p = 0.89), nor did the season (χ2 = 1.41; DF =1; p = 0.24).
4 DISCUSSION
We had asked how wild vervet monkeys respond to a temporary reduction of foraging constraints by adjusting their time budget, focussing on social behaviours in general such as the number of grooming bouts, the number of grooming partners and the number of conflicts. Our results confirm some key theoretical predictions, most notably that there are time constraints on social behaviour, that these time constraints are proportionally most important during periods of low food availability and/or short daylight hours, such as during winter, and that a decrease in the time constraint leads to an increase in the proportion of time spent socializing, as well as the number of grooming bouts and an enlargement of the grooming network. Below we discuss each of these results in more detail. A discussion of differences between groups and between age categories can be found in the online supporting information, as these factors were not the central focus of our study.
4.1 Effects of food provisioning on time budgets
The fact that food provisioning consistently caused a reduction in foraging activities shows that our manipulation worked as intended. In contrast to other studies on subsidised monkey groups (Altmann & Muruthi, 1988; Fa, 1986; Saj et al., 1999), we found that the free time was not only used for resting but also to increase social activities. Following our predictions, the effect was particularly strong during winter, when food is scarcer and daylight shorter (Dunbar & Gowlett, 2014), and individuals usually spend a larger proportion of time travelling and foraging, with less time allocated to social behaviours (Adeyemo, 1997; Baldellou & Adan, 1998; Isbell & Young, 1993). A relaxation of foraging constraints seems to influence other social behaviours in a similar way to grooming. However, these results are more complex to interpret as some behaviours such as mating and nursing show strong seasonal variation.
During mid-summer, when days are longer (i.e., maximum 3h of photoperiod difference), we also collected data over a longer period than during the winter season as monkeys were active over a longer period (i.e., about 3h per day). Because the animals are under less time pressure during summer months as a result, the proportion of time devoted to activities will change. Activities such as foraging that have a fixed upper limit (there is only so much foraging an animal needs to do to meet its nutritional requirements) might be expected to decrease as a proportion of the day, making more time available for resting and grooming which in principle have no biological upper limit. This is, indeed, what we find, both in terms of percentage of time (Figure 1) and in terms of absolute number of hours devoted to each activity—although resting time exhibits a much larger summertime increase in both relative and absolute time than grooming and other social interaction do. For example, for grooming behaviour, in terms of absolute number of hours (13 hours during the summer season versus 10 hours during the winter), our proportions represent for control and provisioning days respectively 0.75 hours versus 0.88 during summer and 0.68 hours versus 1.27 during winter. Controlling for day length within the models would have been only possible if individual budget would have been analysed at the daily level but such data set would have contained multiple zeros and increased the complexity of data analyses. Furthermore, data exploration revealed no correlational trend between any activity budget and day length averaged at a monthly level. However, our main concern is not with the seasonal differences in activity budgets but the within-season change in response to provisioning. Our findings show that vervets are highly flexible in their behaviour and can adapt their activity budget as a function of the availability of time and food. We suggest that this makes sense in an environment where seasonal variation has a strong impact on food availability (Canteloup et al., 2019). Generally, vervet monkeys in our study seem to be able to find the right balance between their physiological and social needs.
4.2 Time budget constraints and potential social facilitation
Our analyses on a subset of individuals that only sometimes participated in the supplementations yielded no effect of eating high-quality food on their grooming behaviour compared to the rest of the group. This means that these individuals adjust their time budget according to the activities of other group members rather than their own needs. As groups spread out up to 100 meters while foraging (long term database), and the time spent moving was not significantly different between control and supplementation days: individuals that did not have access to the corn could have successfully foraged while travelling but did not. This suggests a strong group pressure over social time budget. As differences in activity budgets are known to cause group to split (for example, in herding ungulates: Dunbar & Shi, 2008), our observations suggest that the synchronisation of non-participating individuals helps to promote group cohesion. Nevertheless, food-deprived individuals could try to forage in the vicinity or rest while well-fed individuals groom and, thereby, still maintain group cohesion. Thus, the general observed increase in grooming activities is in line with a previous study demonstrating that vervet monkeys show strong group conformity in the choice of food sources (van de Waal et al., 2013) and with similar effects in respect of the timing of resting bouts in the highly social Quelea weaver bird (Dunbar & Crook, 1975). We do not imply any complex cognitive mechanism; it could simply be that social facilitation makes individuals that had not eaten high-quality food engage in the same behaviours as those that had consumed high-quality food. Copying the majority or the most frequent behaviour of one's group might be of great benefit and the best strategy for group integration or managing one's social relationships (Claidière & Whiten, 2012), especially in social species with a strong hierarchy such as vervet monkeys.
4.3 Effects of food provisioning on conflicts and grooming patterns
Similar to a previous study (Barton, 1990), our results indicate that, on control days, group competition over resources is stronger during the scarce season, although such results contrast with other studies where within group competition is weaker during the scarce season (Lee, 1984; Wrangham, 1980). Proximity is not a likely explanation for our results on aggression because, first of all, we collected data on behaviours once the supplementation was over and once the monkeys resumed their natural behaviours. Second, group spread after the supplementation (c.f. supporting information) was rather similar between control and experimental days. Furthermore, the recorded higher levels of grooming during experimental days cannot be a consequence of higher aggression levels leading to more reconciliation. Reconciliation, that is, affiliative post-conflict behaviour between the former opponents (e.g., grooming, sitting in contact, etc. in primates) (de Waal & Roosmalen, 1979), mostly occurs within the first few minutes after a conflict (Arnold & Aureli, 2007; Fraser & Aureli, 2008), including in vervet monkeys (Seyfarth & Cheney, 1989). Therefore, conflicts during provisioning would have caused reconciliation before our data collection started.
During supplementation days, individuals increased the size of their grooming network by socialising with more partners than on control days, and this effect was even stronger in the winter season, during which all groups show the same tendency. Apparently, winter imposes a time constraint on vervet monkeys that leads to similar effects as those reported for lactating gelada females, who reduced the number of grooming partners as well as time spent allogrooming as their time budgets came under pressure to increase foraging time as lactation demand increased with infant age (Dunbar & Dunbar, 1988). This result supports the hypothesis that the observed reduction in the number of grooming partners in large groups is indeed due to time constraints (Dunbar, 1991; Lehmann & Dunbar, 2009). Increasing the quality and the quantity of its social relationships within a group might benefit an individual. In primates, multiple studies indicated that maintaining strong and stable relationships with different group members increased infant survival and longevity (Silk et al., 2003, 2009, 2010), promoting the diffusion of information, for example, about food resources or predator recognition, which are of high importance for survival (Sueur, 2012; Claidiere et al., 2013; Duboscq et al., 2016; Watson et al., 2017). One can imagine, that if foraging constraints are naturally reduced, social relationships within a group might improve. Knowing that more tolerant species usually have a higher network efficiency and that this also positively correlates with cognitive abilities (Pasquaretta et al., 2014), an increased quality and quantity of relationships might be beneficial at the group and individual level.
In conclusion, our study provides experimental evidence that vervet monkeys experience time budget constraints on their social/grooming activities that are influenced by the proportion of time devoted in other activities. These constraints are even stronger during periods of food limitation and shorter daylight. Most importantly, when extra time becomes available, vervet monkeys use the extra grooming time to improve the group cohesion and potentially their own fitness by interacting with more partners. To our knowledge, this is the first time that such results are shown experimentally.
ACKNOWLEDGMENTS
The authors would like to thank particularly Kerneels van der Walt for permission to conduct the study on his land and Erica van de Waal and Albert Driescher for their support. The authors thank all the students and volunteers who helped out with the data collection. This study was financed by the Swiss National Science Foundation (Sinergia: CRSI33_133040). Open Access Funding provided by Universite de Neuchatel.




